Oxidative Stress and Cytotoxicity Induced by Co-Formulants of Glyphosate-Based Herbicides in Human Mononuclear White Blood Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. Cell Treatment
2.4. Cytotoxicity Assessment
2.5. Measurement of Intracellular Oxidative Stress
2.6. Statistical Analysis
3. Results
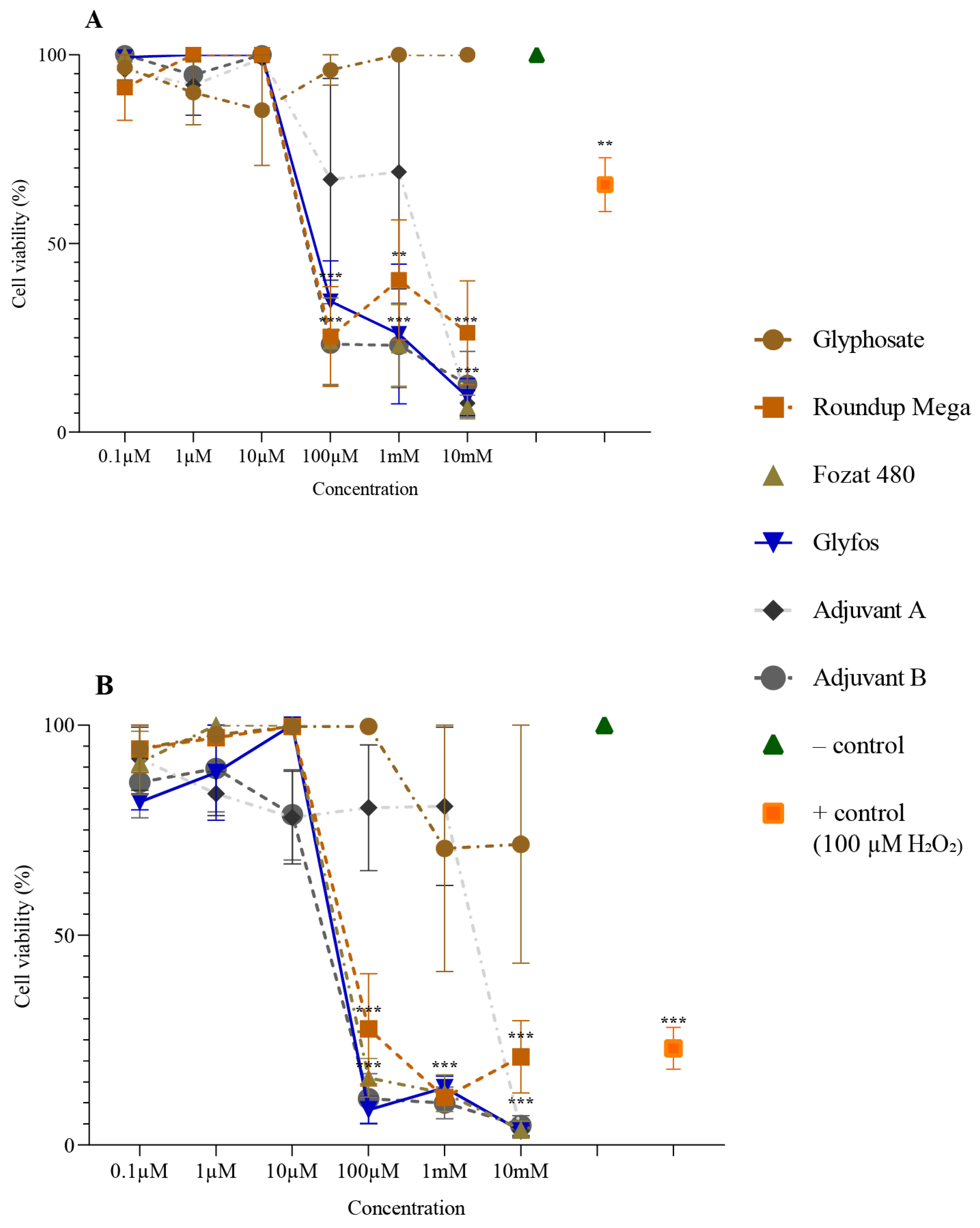
3.1. Cytotoxicity
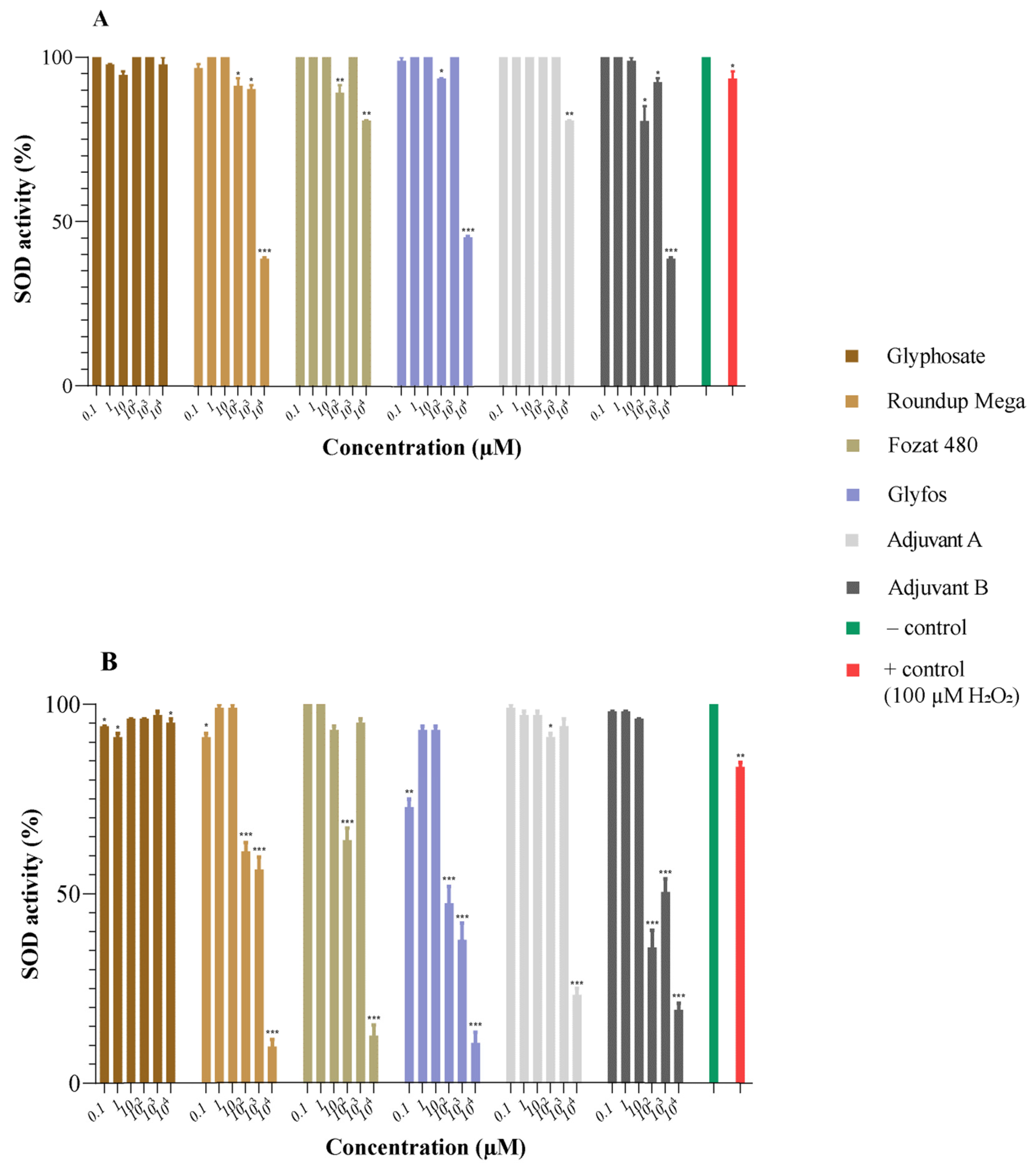
3.2. Superoxide Dismutase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perry, E.D.; Ciliberto, F.; Hennessy, D.A.; Moschini, G.C. Genetically Engineered Crops and Pesticide Use in U.S. Maize and Soybeans. Sci. Adv. 2016, 2, e1600850. [Google Scholar] [CrossRef] [PubMed]
- Benbrook, C.M. Trends in Glyphosate Herbicide Use in the United States and Globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; la Cecilia, D.; Tang, F.H.M.; McBratney, A. The Global Environmental Hazard of Glyphosate Use. Sci. Total Environ. 2020, 717, 137167. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Li, H.; Li, H.; Wang, L.; Lyu, J.; Yang, L.; Tong, S.; Yu, Q.J.; Ruan, H.D.; Atabila, A.; et al. Exposure Routes and Health Risks Associated with Pesticide Application. Toxics 2022, 10, 335. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Cox, C.; Surgan, M. Unidentified Inert Ingredients in Pesticides: Implications for Human and Environmental Health. Environ. Health Perspect. 2006, 114, 1803–1806. [Google Scholar] [CrossRef]
- Gandhi, K.; Khan, S.; Patrikar, M.; Markad, A.; Kumar, N.; Choudhari, A.; Sagar, P.; Indurkar, S. Exposure Risk and Environmental Impacts of Glyphosate: Highlights on the Toxicity of Herbicide Co-Formulants. Environ. Chall. 2021, 4, 100149. [Google Scholar] [CrossRef]
- Cserhati, T. Alkyl Ethoxylated and Alkylphenol Ethoxylated Nonionic Surfactants: Interaction with Bioactive Compounds and Biological Effects. Environ. Health Perspect. 1995, 103, 358–364. [Google Scholar] [CrossRef]
- Defarge, N.; Takács, E.; Lozano, V.L.; Mesnage, R.; de Vendômois, J.S.; Séralini, G.E.; Székács, A. Co-Formulants in Glyphosate-Based Herbicides Disrupt Aromatase Activity in Human Cells below Toxic Levels. Int. J. Environ. Res. Public Health 2016, 13, 264. [Google Scholar] [CrossRef]
- Mesnage, R.; Teixeira, M.; Mandrioli, D.; Falcioni, L.; Ibragim, M.; Ducarmon, Q.R.; Zwittink, R.D.; Amiel, C.; Panoff, J.M.; Bourne, E.; et al. Multi-Omics Phenotyping of the Gut-Liver Axis Reveals Metabolic Perturbations from a Low-Dose Pesticide Mixture in Rats. Commun. Biol. 2021, 4, 471. [Google Scholar] [CrossRef]
- Webster, T.M.U.; Santos, E.M. Global Transcriptomic Profiling Demonstrates Induction of Oxidative Stress and of Compensatory Cellular Stress Responses in Brown Trout Exposed to Glyphosate and Roundup. BMC Genomics 2015, 16, 32. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Silva, T.L.; Andreani, T.; Silva, A.M. Glyphosate vs. Glyphosate-Based Herbicides Exposure: A Review on Their Toxicity. J. Xenobiot. 2022, 12, 21–40. [Google Scholar] [CrossRef]
- Mesnage, R.; Ferguson, S.; Brandsma, I.; Moelijker, N.; Zhang, G.; Mazzacuva, F.; Caldwell, A.; Halket, J.; Antoniou, M.N. The Surfactant Co-Formulant POEA in the Glyphosate-Based Herbicide RangerPro but Not Glyphosate Alone Causes Necrosis in Caco-2 and HepG2 Human Cell Lines and ER Stress in the ToxTracker Assay. Food Chem. Toxicol. 2022, 168, 113380. [Google Scholar] [CrossRef]
- Székács, A.; Darvas, B. Re-Registration Challenges of Glyphosate in the European Union. Front. Environ. Sci. 2018, 6, 1–35. [Google Scholar] [CrossRef]
- Mañas, F.; Peralta, L.; Raviolo, J.; Ovando, H.G.; Weyers, A.; Ugnia, L.; Cid, M.G.; Larripa, I.; Gorla, N. Genotoxicity of Glyphosate Assessed by the Comet Assay and Cytogenetic Tests. Environ. Toxicol. Pharmacol. 2009, 28, 37–41. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Reszka, E.; Woźniak, K.; Jabłońska, E.; Michałowicz, J.; Bukowska, B. DNA Damage and Methylation Induced by Glyphosate in Human Peripheral Blood Mononuclear Cells (In Vitro Study). Food Chem. Toxicol. 2017, 105, 93–98. [Google Scholar] [CrossRef]
- Koller, V.J.; Fürhacker, M.; Nersesyan, A.; Mišík, M.; Eisenbauer, M.; Knasmueller, S. Cytotoxic and DNA-Damaging Properties of Glyphosate and Roundup in Human-Derived Buccal Epithelial Cells. Arch. Toxicol. 2012, 86, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Luaces, J.P.; Rossi, L.F.; Chirino, M.G.; Browne, M.; Merani, M.S.; Mudry, M.D. Genotoxic Effects of Roundup Full II® on Lymphocytes of Chaetophractus Villosus (Xenarthra, Mammalia): In Vitro Studies. PLoS ONE 2017, 12, e0182911. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, E.; Sicińska, P.; Michałowicz, J.; Woźniak, K.; Reszka, E.; Huras, B.; Zakrzewski, J.; Bukowska, B. The Mechanism of DNA Damage Induced by Roundup 360 PLUS, Glyphosate and AMPA in Human Peripheral Blood Mononuclear Cells—Genotoxic Risk Assessement. Food Chem. Toxicol. 2018, 120, 510–522. [Google Scholar] [CrossRef]
- Mladinic, M.; Berend, S.; Vrdoljak, A.L.; Kopjar, N.; Radic, B.; Zeljezic, D. Evaluation of Genome Damage and Its Relation to Oxidative Stress Induced by Glyphosate in Human Lymphocytes in Vitro. Environ. Mol. Mutagen. 2009, 50, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Antoniou, M.N. Ignoring Adjuvant Toxicity Falsifies the Safety Profile of Commercial Pesticides. Front. Public Health 2018, 5, 361. [Google Scholar] [CrossRef] [PubMed]
- Gasnier, C.; Dumont, C.; Benachour, N.; Clair, E.; Chagnon, M.C.; Séralini, G.E. Glyphosate-Based Herbicides Are Toxic and Endocrine Disruptors in Human Cell Lines. Toxicology 2009, 262, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.; Tessema, R.A.; Budnik, L.T.; Ádám, B. Comparative Cyto- and Genotoxicity Assessment of Glyphosate and Glyphosate-Based Herbicides in Human Peripheral White Blood Cells. Environ. Res. 2019, 179, 108851. [Google Scholar] [CrossRef]
- Fuselier, S.G.; Ireland, D.; Fu, N.; Rabeler, C.; Collins, E.S. Comparative Toxicity Assessment of Glyphosate and Two Commercial Formulations in the planarian Dugesia japonica. Front Toxicol. 2023, 5, 1200881. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, F.; Mandrioli, D.; Gnudi, F.; Scheepers, P.T.J.; Silbergeld, E.K.; Belpoggi, F.; Dinelli, G. Comparative Evaluation of the Cytotoxicity of Glyphosate-Based Herbicides and Glycine in L929 and Caco2 Cells. Front. Public Health 2021, 9, 643898. [Google Scholar] [CrossRef] [PubMed]
- Tóth, G.; Háhn, J.; Radó, J.; Szalai, D.A.; Kriszt, B.; Szoboszlay, S. Cytotoxicity and Hormonal Activity of Glyphosate-Based Herbicides. Environ. Pollut. 2020, 265, 115027. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, B.; Michalowicz, J.; Pieniazek, D.; Sicinska, P.; Duda, W. Superoxide Dismutases and Their Inhibitors-the Role in Some Diseases. Curr. Enzym. Inhib. 2006, 2, 379–397. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of Superoxide Dismutase, Catalase and Glutathione Peroxidase in Cultured Cells and Tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef]
- Landis, G.N.; Tower, J. Superoxide Dismutase Evolution and Life Span Regulation. Mech. Ageing Dev. 2005, 126, 365–379. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Pintó-Marijuan, M. Free Radicals, Oxidative Stress and Antioxidants. Encycl. Appl. Plant Sci. 2016, 1, 16–19. [Google Scholar] [CrossRef]
- Mesnage, R.; Ferguson, S.; Mazzacuva, F.; Caldwell, A.; Halket, J.; Antoniou, M.N. Cytotoxicity Mechanisms and Composition of the Glyphosate Formulated Herbicide RangerPro. bioRxiv 2021, 2021.11.18.469091. [Google Scholar] [CrossRef]
- Maldonado-Reina, A.J.; López-Ruiz, R.; Romero-González, R.; Martínez Vidal, J.L.; Garrido-Frenich, A. Assessment of Co-Formulants in Marketed Plant Protection Products by LC-Q-Orbitrap-MS: Application of a Hybrid Data Treatment Strategy Combining Suspect Screening and Unknown Analysis. J. Agric. Food Chem. 2022, 70, 7302–7313. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, Y.; Cheng, J.; Xu, W.; Xu, Z.; Gao, J.; Tao, L. Adjuvant Contributes Roundup’s Unexpected Effects on A549 Cells. Environ. Res. 2020, 184, 109306. [Google Scholar] [CrossRef]
- Bednářová, A.; Kropf, M.; Krishnan, N. The Surfactant Polyethoxylated Tallowamine (POEA) Reduces Lifespan and Inhibits Fecundity in Drosophila Melanogaster- In Vivo and in Vitro Study. Ecotoxicol. Environ. Saf. 2020, 188, 109883. [Google Scholar] [CrossRef]
- OECD. Screening Information Dataset (SIDS) Initial Assessment Profile (SIAP). October 2006. Available online: https://hpvchemicals.oecd.org/ui/handler.axd?id=8e660767-927e-40d7-ae67-d04c7a9e786a (accessed on 17 October 2023).
- European Chemical Agency (ECHA). Chemical Registration Dossier. Available online: https://echa.europa.eu/de/registration-dossier/-/registered-dossier/14910/11/?documentUUID=6b474032-0399-439f-a86b-c66c63a88f82 (accessed on 25 September 2023).
- Brausch, J.M.; Smith, P.N. Toxicity of Three Polyethoxylated Tallowamine Surfactant Formulations to Laboratory and Field Collected Fairy Shrimp, Thamnocephalus Platyurus. Arch. Environ. Contam. Toxicol. 2007, 52, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Bernay, B.; Séralini, G.E. Ethoxylated Adjuvants of Glyphosate-Based Herbicides Are Active Principles of Human Cell Toxicity. Toxicology 2013, 313, 122–128. [Google Scholar] [CrossRef]
- Lopez, O.; Hernandez, A.; Rodrigo, L.; Gil, F.; Pena, G.; Serrano, J.; Parron, T.; Villanueva, E.; Pla, A. Changes in Antioxidant Enzymes in Humans with Long-Term Exposure to Pesticides. Toxicol. Lett. 2007, 171, 146–153. [Google Scholar] [CrossRef]
- Sule, R.O.; Condon, L.; Gomes, A.V. A Common Feature of Pesticides: Oxidative Stress—The Role of Oxidative Stress in Pesticide-Induced Toxicity. Oxid. Med. Cell. Longev. 2022, 2022, 5563759. [Google Scholar] [CrossRef]
- Hayashi, Y. Overview of Genotoxic Carcinogens and Non-Genotoxic Carcinogens. Exp. Toxicol. Pathol. 1992, 44, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Gillezeau, C.; Van Gerwen, M.; Shaffer, R.M.; Rana, I.; Zhang, L.; Sheppard, L.; Taioli, E. The Evidence of Human Exposure to Glyphosate: A Review. Environ. Health Glob. Access Sci. Source 2019, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.; Arena, M.; Auteri, D.; Binaglia, M.; Castoldi, A.F.; Chiusolo, A.; Colagiorgi, A.; Colas, M.; Crivellente, F.; De Lentdecker, C.; et al. Peer Review of the Pesticide Risk Assessment of the Active Substance Fat Distillation Residues. EFSA J. 2023, 21, 7811. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and Health Effects of the Herbicide Glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef]
- Kongtip, P.; Nankongnab, N.; Phupancharoensuk, R.; Palarach, C.; Sujirarat, D.; Sangprasert, S.; Sermsuk, M.; Sawattrakool, N.; Woskie, S.R. Glyphosate and Paraquat in Maternal and Fetal Serums in Thai Women. J. Agromed. 2017, 22, 282–289. [Google Scholar] [CrossRef]
- Aris, A.; Leblanc, S. Maternal and Fetal Exposure to Pesticides Associated to Genetically Modified Foods in Eastern Townships of Quebec, Canada. Reprod. Toxicol. 2011, 31, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Connolly, A.; Coggins, M.A.; Koch, H.M. Human Biomonitoring of Glyphosate Exposures: State-of-the-Art and Future Research Challenges. Toxics 2020, 8, 60. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makame, K.R.; Masese, S.N.; Ádám, B.; Nagy, K. Oxidative Stress and Cytotoxicity Induced by Co-Formulants of Glyphosate-Based Herbicides in Human Mononuclear White Blood Cells. Toxics 2023, 11, 976. https://doi.org/10.3390/toxics11120976
Makame KR, Masese SN, Ádám B, Nagy K. Oxidative Stress and Cytotoxicity Induced by Co-Formulants of Glyphosate-Based Herbicides in Human Mononuclear White Blood Cells. Toxics. 2023; 11(12):976. https://doi.org/10.3390/toxics11120976
Chicago/Turabian StyleMakame, Khadija Ramadhan, Sylvia Nyambeki Masese, Balázs Ádám, and Károly Nagy. 2023. "Oxidative Stress and Cytotoxicity Induced by Co-Formulants of Glyphosate-Based Herbicides in Human Mononuclear White Blood Cells" Toxics 11, no. 12: 976. https://doi.org/10.3390/toxics11120976
APA StyleMakame, K. R., Masese, S. N., Ádám, B., & Nagy, K. (2023). Oxidative Stress and Cytotoxicity Induced by Co-Formulants of Glyphosate-Based Herbicides in Human Mononuclear White Blood Cells. Toxics, 11(12), 976. https://doi.org/10.3390/toxics11120976






