MicroRNA miR-212-5p Regulates the MEK/ERK Signaling Pathway by Targeting A-Raf proto-oncogene serine/threonine-protein kinase (ARAF) to Regulate Cowshed PM2.5-Induced NR8383 Apoptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. PM2.5 Sample Collection
2.3. Cell Culture
2.4. CCK8 Cell Viability Assay
2.5. Plasmid Construction and Cell Transfection
2.6. Determination of Calcium Ion Concentration
2.7. Determination of Reactive Oxygen Species (ROS) Level
2.8. JC-1 Detection of Mitochondrial Membrane Potential
2.9. Real-Time Quantitative PCR
2.10. Dual-Luciferase Reporter Assay
2.11. Flow Cytometry
2.12. Western Blotting
2.13. Statistical Analysis
3. Results
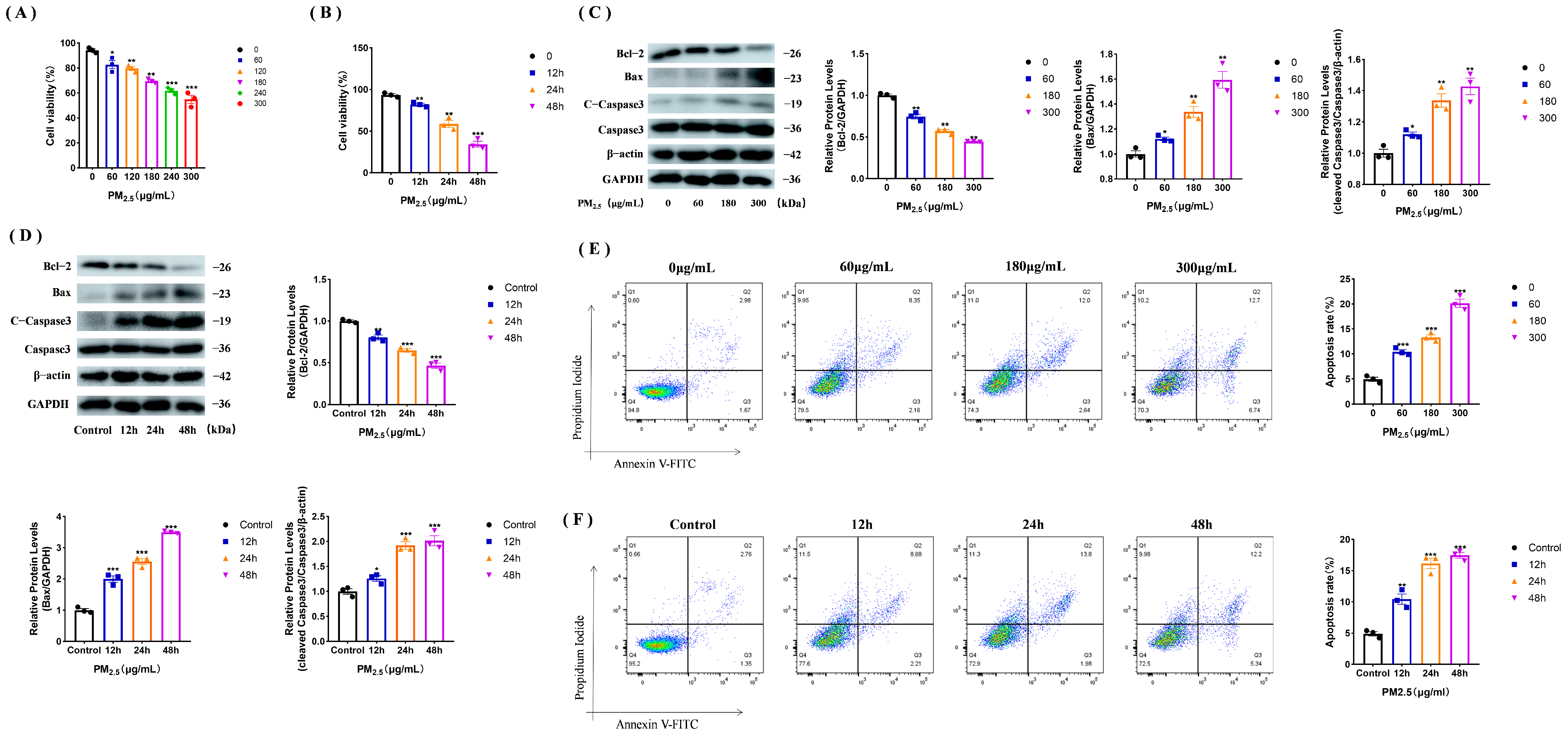
3.1. PM2.5 Causes Apoptosis in Rat NR8383 Cells
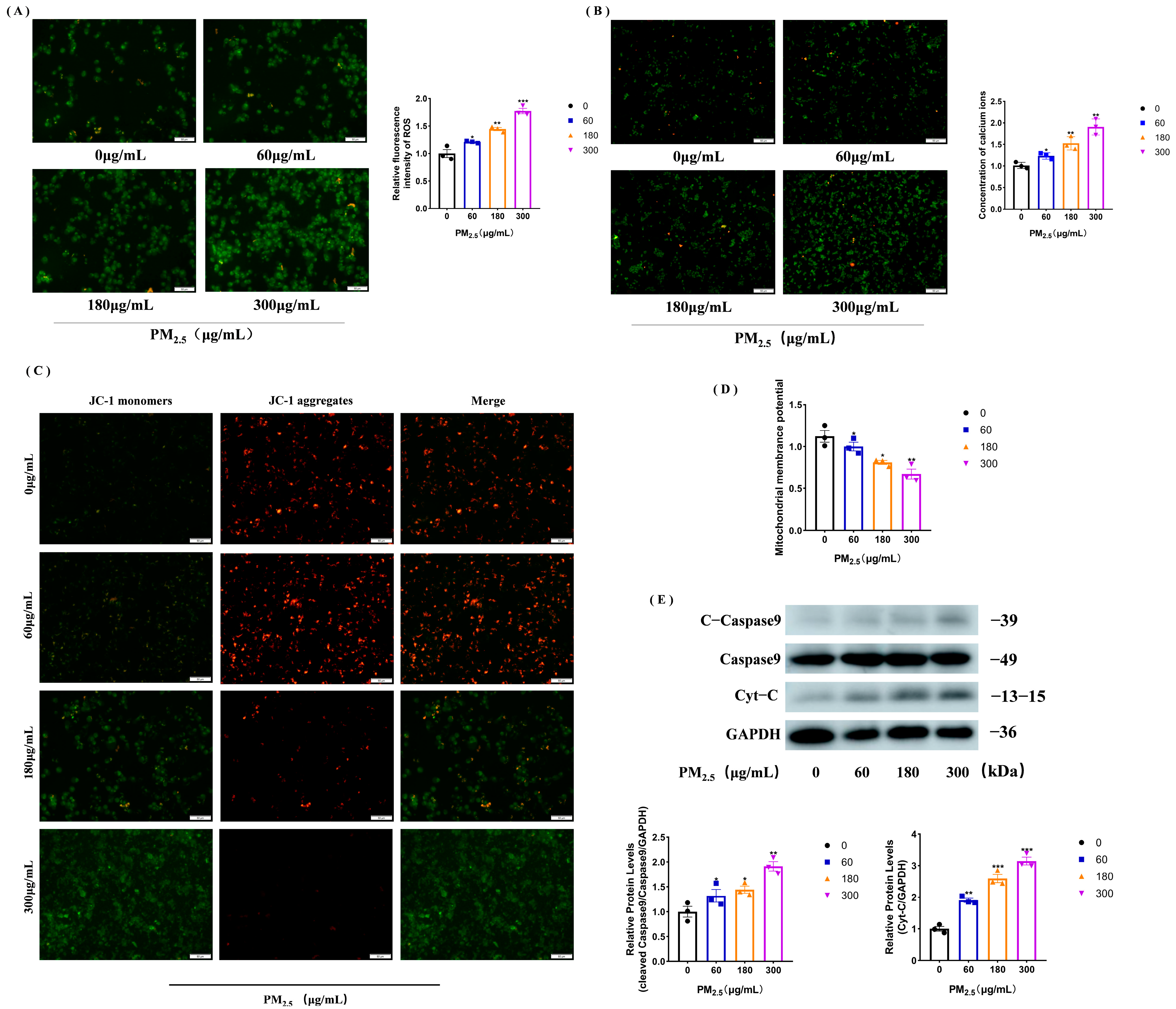
3.2. PM2.5 Increases Calcium Ions and ROS to Trigger Mitochondrial Damage
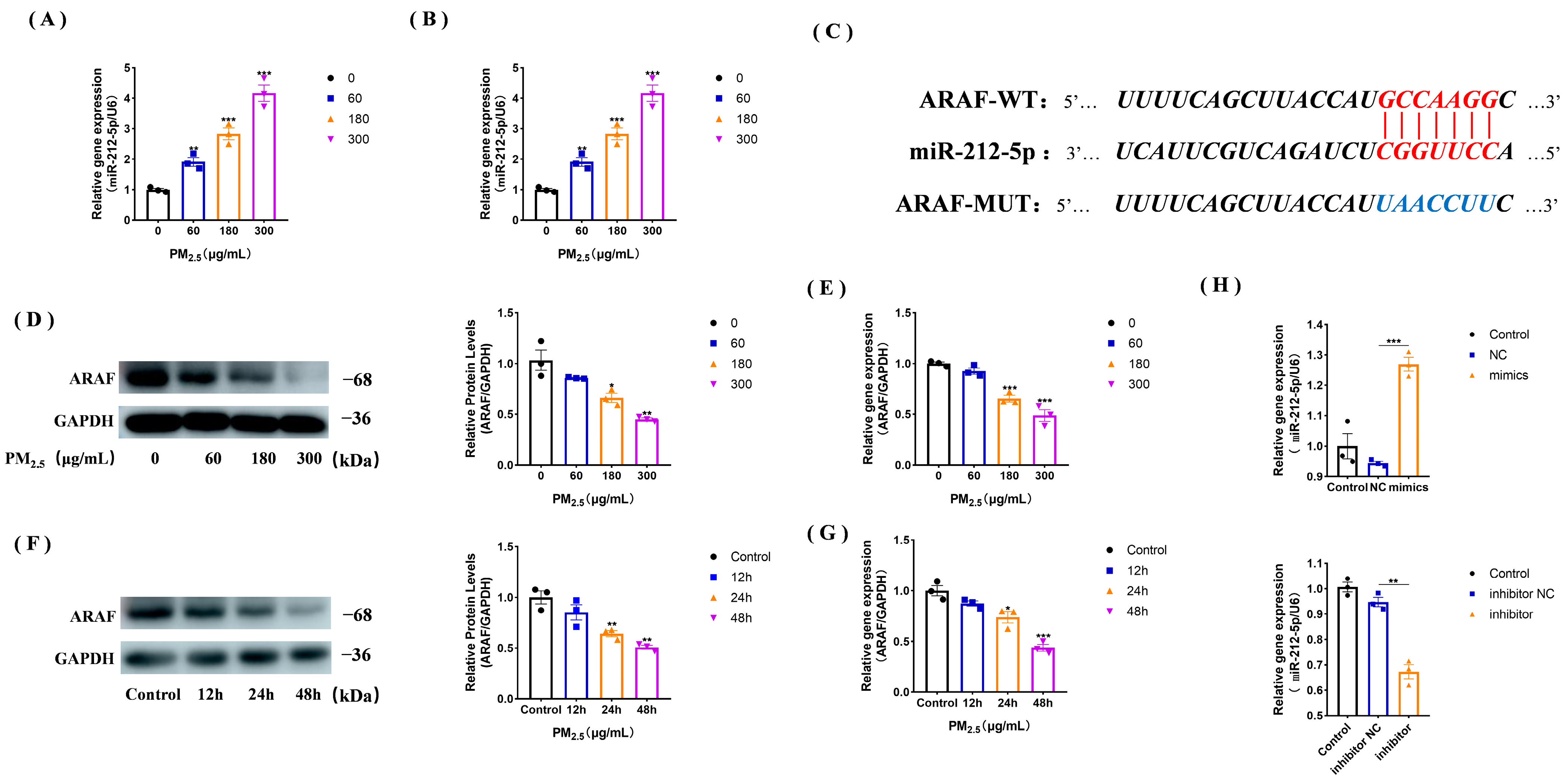
3.3. miR-212-5p Expression Increases under the Cowshed PM2.5 Stimulation
3.4. ARAF Is a Potential Target of miR-212-5p and Its Expression Is Down-Regulated in the Cowshed PM2.5-Stimulated NR8383 Cells
3.5. miR-212-5p Targets ARAF, Promoting Mitochondrial Injury and Apoptosis after Cowshed PM2.5 Stimulation
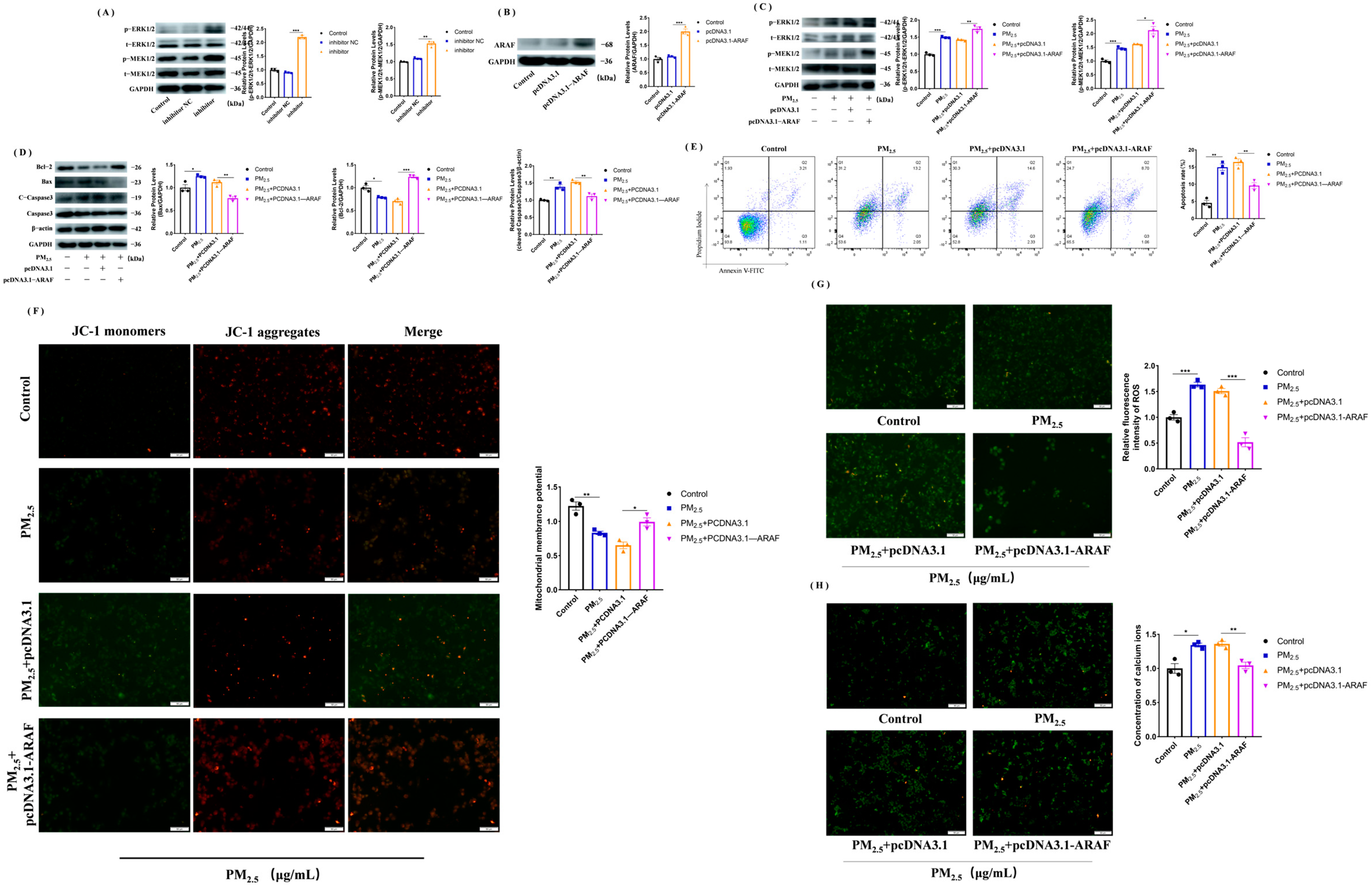
3.6. Activation of MEK/ERK Signaling Pathway after Overexpression of ARAF Inhibits the Cowshed PM2.5-Induced Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.W.; Deng, T.; Huo, T.T.; Dong, F.Q.; Deng, J.J. Mir-140-5p/tlr4 /nf-kappa b signaling pathway: Crucial role in inflammatory response in 16hbe cells induced by dust fall pm2.5. Ecotoxicol. Environ. Saf. 2021, 208, 111414. [Google Scholar] [CrossRef] [PubMed]
- Kangas, T.; Gadeyne, S.; Lefebvre, W.; Vanpoucke, C.; Rodriguez-Loureiro, L. Are air quality perception and pm2.5 exposure differently associated with cardiovascular and respiratory disease mortality in brussels? Findings from a census-based study. Environ. Res. 2023, 219, 115180. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.H.; Zhang, Y.H.; Chen, Z.F.; Yang, C.; Song, Y.Y.; Liao, X.L.; Li, W.Q.; Tsang, S.Y.; Liu, G.G.; Cai, Z.W. Chemical identity and cardiovascular toxicity of hydrophobic organic components in pm2.5. Ecotoxicol. Environ. Saf. 2020, 201, 110827. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.Y.; Li, X.; Zhu, S.Q.; Yin, B.W.; Guo, Z.H.; Cui, Q.Q.; Song, J.S.; Pei, H.T.; Ma, Y.X. Sesamin ameliorates fine particulate matter (pm2.5)-induced lung injury via suppression of apoptosis and autophagy in rats br. J. Agric. Food Chem. 2022, 70, 9489–9498. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Gao, Y.; Hua, C.; Peng, L.; Ci, X. Daphnetin ameliorates pm2.5-induced airway inflammation by inhibiting nlrp3 inflammasome-mediated pyroptosis in cs-exposed mice. Biomed. Pharmacother. 2023, 165, 115047. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Yu, S.-H.; Kuo, H.-C.; Cheng, K.-W.; Hsu, C.-C.; Lin, Y.-P.; Khumsupan, D.; Lin, S.-P.; Angkawijaya, A.E.; Cheng, K.-C. Alleviation of pm2.5-induced alveolar macrophage inflammation using extract of fermented chenopodium formosanum koidz sprouts via regulation of nf-κb pathway. J. Ethnopharmacol. 2024, 318, 116980. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Duan, H.; Zhang, J.; Wang, Q.; Peng, T.; Ye, X.; Cheng, Z.; Li, X. Yap1 protects against pm2.5-induced lung toxicity by suppressing pyroptosis and ferroptosis. Ecotoxicol. Environ. Saf. 2023, 253, 114708. [Google Scholar] [CrossRef]
- Wu, B.; Qin, L.W.; Wang, M.; Zhou, T.; Dong, Y.X.; Chai, T.J. The composition of microbial aerosols, pm2.5, and pm10 in a duck house in shandong province, china. Poult. Sci. 2019, 98, 5913–5924. [Google Scholar] [CrossRef]
- Liu, H.L.; Nie, H.P.; Lai, W.Q.; Shi, Y.; Liu, X.; Li, K.; Tian, L.; Xi, Z.G.; Lin, B.C. Different exposure modes of pm2.5 induces bronchial asthma and fibrosis in male rats through macrophage activation and immune imbalance induced by tipe2 methylation. Ecotoxicol. Environ. Saf. 2022, 247, 114200. [Google Scholar] [CrossRef]
- Hamad, S.H.; Schauer, J.J.; Antkiewicz, D.S.; Shafer, M.M.; Kadhim, A.K.H. Ros production and gene expression in alveolar macrophages exposed to pm2.5 from baghdad, iraq: Seasonal trends and impact of chemical composition. Sci. Total Environ. 2016, 543, 739–745. [Google Scholar] [CrossRef]
- Yang, B.; Guo, J.; Xiao, C.L. Effect of pm2.5 environmental pollution on rat lung. Environ. Sci. Pollut. Res. 2018, 25, 36136–36146. [Google Scholar] [CrossRef]
- Jiang, X.; Han, Y.Q.; Qiu, X.H.; Chai, Q.Q.; Zhang, H.X.Y.; Chen, X.; Cheng, Z.; Wang, Y.W.; Fan, Y.F.; Xue, T.; et al. Organic components of personal pm2.5 exposure associated with inflammation: Evidence from an untargeted exposomic approach. Environ. Sci. Technol. 2021, 55, 10589–10596. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.L.; Li, S.H.; Ma, X.N.; Li, R.Q.; Zhang, H.R.; Li, J.W.; Yan, X.X. Mir-217-5p inhibits smog (pm2.5)-induced inflammation and oxidative stress response of mouse lung tissues and macrophages through targeting stat1. Aging 2022, 14, 6796–6808. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Y.; Cheng, A.; Wang, M.; Liu, M.; Zhu, D.; Chen, S.; Zhao, X.; Yang, Q.; Wu, Y.; et al. Duck circovirus genotype 2 orf3 protein induces apoptosis through the mitochondrial pathway. Poult. Sci. 2023, 102, 102533. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Park, H.J. Perfluorooctanoic acid induces cell death in tm3 cells via the er stress-mitochondrial apoptosis pathway. Reprod. Toxicol. 2023, 118, 108383. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Zhang, J.; Huo, S.M.; Zhang, X.L.; Cui, Y.L.; Li, Y.F. Mitophagy alleviates aif-mediated spleen apoptosis induced by alcl3 through parkin stabilization in mice. Food Chem. Toxicol. 2023, 176, 113762. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.Z.; Zhang, Q.H.; Chang, B.; Xie, P.; Liao, H.; Du, S.X.; Li, X.D. Dioscin induces osteosarcoma cell apoptosis by upregulating ros-mediated p38 mapk signaling. Drug Dev. Res. 2023, 84, 25–35. [Google Scholar] [CrossRef]
- Zhang, L.L.; Chen, L.; Qi, M.; Yu, F.C.; Ni, X.T.; Hong, H.Z.; Xu, H.T.; Xu, S.W. Glyphosate induces autophagy in hepatic l8824 cell line through no-mediated activation of ras/raf/mek/erk signaling pathway and energy metabolism disorders. Fish Shellfish. Immunol. 2023, 137, 108772. [Google Scholar] [CrossRef]
- Song, Y.L.; Bi, Z.F.; Liu, Y.; Qin, F.R.; Wei, Y.Q.; Wei, X.W. Targeting ras-raf-mek-erk signaling pathway in human cancer: Current status in clinical trials. Genes Dis. 2023, 10, 76–88. [Google Scholar] [CrossRef]
- Wu, C.Y.; Zhu, G.Y.; Qiu, F.; Ren, F.L.; Lin, B.B.; Zhang, D.Y.; Yang, Q.Y.; Huang, C.L. Plx8394, a raf inhibitor, inhibits enterovirus 71 replication by blocking raf/mek/erk signaling. Virol. Sin. 2023, 38, 276–284. [Google Scholar] [CrossRef]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the mapk-ras-raf signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Hendrikse, C.S.E.; Theelen, P.M.M.; van der Ploeg, P.; Westgeest, H.M.; Boere, I.A.; Thijs, A.M.J.; Ottevanger, P.B.; van de Stolpe, A.; Lambrechts, S.; Bekkers, R.L.M.; et al. The potential of ras/raf/mek/erk (mapk) signaling pathway inhibitors in ovarian cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2023, 171, 83–94. [Google Scholar] [CrossRef]
- An, S.; Yang, Y.; Ward, R.; Liu, Y.; Guo, X.X.; Xu, T.R. A-raf: A new star of the family of raf kinases. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 520–531. [Google Scholar] [CrossRef]
- Yang, S.F.; Liu, G.H. Targeting the ras/raf/mek/erk pathway in hepatocellular carcinoma. Oncol. Lett. 2017, 13, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.; Kolch, W. Taming the hippo—Raf-1 controls apoptosis by suppressing mst2/hippo. Cell Cycle 2005, 4, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-C.; Huang, Y.; Zhang, R.-X.; Han, Z.-J.; Zhou, L.-L.; Sun, N.; Dong, W.-Y.; Zhuang, G.-S. Mir-338–3p inhibits autophagy in a rat model of allergic rhinitis after pm2.5 exposure through akt/mtor signaling by targeting ube2q1. Biochem. Biophys. Res. Commun. 2021, 554, 1–6. [Google Scholar] [CrossRef]
- Zhou, T.Y.; Zhong, Y.J.; Hu, Y.; Sun, C.; Wang, Y.X.; Wang, G.F. Pm2.5 downregulates mir-194-3p and accelerates apoptosis in cigarette-inflamed bronchial epithelium by targeting death-associated protein kinase i. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, M.; Park, M.; Jin, Y.; Lee, S.J.; Lee, H. Specific upregulation of extracellular mir-6238 in particulate matter-induced acute lung injury and its immunomodulation. J. Hazard. Mater. 2023, 445, 130466. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, M.C.; Song, J.; Zeng, Y.Y.; Xia, S.J.; Chen, C.C.; Jin, M.L.; Song, Y.L. The circular rna circtxnrd1 promoted ambient particulate matter-induced inflammation in human bronchial epithelial cells by regulating mir-892a/cox-2 axis. Chemosphere 2022, 286, 131614. [Google Scholar] [CrossRef]
- Guo, J.H.; Xie, X.X.; Wu, J.Y.; Yang, L.; Ruan, Q.S.; Xu, X.Y.; Wei, D.H.; Wen, Y.Y.; Wang, T.G.; Hu, Y.D.; et al. Association between fine particulate matter and coronary heart disease: A mirna microarray analysis. Environ. Pollut. 2022, 313, 120163. [Google Scholar] [CrossRef]
- Chen, S.; Chen, W.; Li, Z.; Yue, J.; Yung, K.K.L.; Li, R. Regulation of pm2.5 on mitochondrial damage in h9c2 cells through mir-421/sirt3 pathway and protective effect of mir-421 inhibitor and resveratrol. J. Environ. Sci. 2024, 138, 288–300. [Google Scholar] [CrossRef]
- Li, S.H.; Qu, X.; Qin, Z.X.; Gao, J.G.; Li, J.P.; Liu, J.L. Lncfos/mir-212-5p/casp7 axis-regulated mir-212-5p protects the brain against ischemic damage. Mol. Neurobiol. 2023, 60, 2767–2785. [Google Scholar] [CrossRef]
- Guo, Y.J.; Yu, J.J.; Wang, C.X.; Li, K.; Liu, B.; Du, Y.; Xiao, F.; Chen, S.H.; Guo, F.F. Mir-212-5p suppresses lipid accumulation by targeting fas and scd1. J. Mol. Endocrinol. 2017, 59, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Xie, Z.B. Circwhsc1 regulates malignancy and glycolysis by the mir-212-5p/akt3 pathway in triple-negative breast cancer. Exp. Mol. Pathol. 2021, 123, 104704. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.N. Study on Mir-212-5p Targeting Lamc2 and Lama3 to Regulate Cell Apoptosis Induced by pm2.5 in Cowhouse. Jilin Agricultural University, 2023. Available online: https://kns.cnki.net/kcms2/article/abstract?v=JxCH2R2OgonxEmvdodzdIVK9LATq7Mza1UktlqVaU1AvnKYQ2z39D6RQiEvgwtaAZXTFx6Esnf5Sz9Zmj2hFqStlnt_Rx1mR-fda7mM6MOh49Il2zSiPK_ph3f-Xkaa5IeFDTXjizTxLsoZ8l32fMw==&uniplatform=NZKPT&language=CHS (accessed on 1 May 2023).
- Ma, Z.H. Effect of Guanylate Binding Protein 2 on Rat Alveolar Macrophage Injury Induced by pm2.5 in Cowshe. Jilin Agricultural University, 2022. Available online: https://kns.cnki.net/kcms2/article/abstract?v=Eo9-C_M6tLlJ7OAqj-LWX2D4jNPAyLybL6bCSgzvzzdnVIZUtv6jlYYY6_p4S-yu4tJ-B6k4P7spk2keVIGTwrEgz7KtpV-fqYOXzwtlt1d2J7vO0t6NZF1Jo9iuQfrJV7Mf-iKh0dHV96w46skDIA==&uniplatform=NZKPT&language=CHS (accessed on 1 May 2022).
- Zhang, X.Q.; Ma, Z.H. Characteristics of bioaerosols in animal barns: Size distribution, bacterial community, environmental factors, antibiotic resistance genes, and health risks. Jilin Agricultural University: Changchun, China, 2023; submitted. [Google Scholar]
- Ma, Z.H.; Du, X.H. Overexpression of rgs2 ameliorates cowshed pm2.5-induced alveolar macrophage damage by suppressing the gq/11-ca2+ pathway. Jilin Agricultural University: Changchun, China, 2023; submitted. [Google Scholar]
- Pan, S.S.; Qiu, Y.T.; Li, M.; Yang, Z.Q.; Liang, D.P. Recent developments in the determination of pm2.5 chemical composition. Bull. Environ. Contam. Toxicol. 2022, 108, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Roman, K.; Roman, M. Spatial variation in particulate emission resulting from animal farming in poland. Agriculture 2021, 11, 168. [Google Scholar] [CrossRef]
- Liang, S.; Ning, R.H.; Zhang, J.Y.; Liu, J.Y.; Zhang, J.; Shen, H.Q.; Chen, R.; Duan, J.C.; Sun, Z.W. Mir-939-5p suppresses pm2.5-induced endothelial injury via targeting hif-1 alpha in haecs. Nanotoxicology 2021, 15, 706–720. [Google Scholar] [CrossRef]
- Yao, H.M.; Zhao, X.X.; Wang, L.L.; Ren, Y. Atorvastatin ameliorated pm2.5-induced atherosclerosis in rats. Arch. Environ. Occup. Health 2023, 78, 267–272. [Google Scholar] [CrossRef]
- Ma, W.L.; Xu, L.; Sun, X.Y.; Qi, Y.; Chen, S.; Li, D.C.; Jin, Y.; Chen, N.N.; Zhu, X.X.; Luo, J.; et al. Using a human bronchial epithelial cell-based malignant transformation model to explore the function of hsa-mir-200 family in the progress of pm2.5-induced lung cancer development. Environ. Pollut. 2023, 319, 120981. [Google Scholar] [CrossRef]
- Khan, P.S.; Rajesh, P.; Rajendra, P.; Chaskar, M.G.; Rohidas, A.; Jaiprakash, S. Recent advances in b-raf inhibitors as anticancer agents. Bioorg. Chem. 2022, 120, 105597. [Google Scholar] [CrossRef]
- Xu, D.; Lin, W.P.; Gao, J.; Jiang, Y.; Li, L.B.; Gao, F. Pm2.5 exposure and health risk assessment using remote sensing data and gis. Int. J. Environ. Res. Public Health 2022, 19, 6154. [Google Scholar] [CrossRef]
- Chong, Y.L.; Zhu, C.R.; Hu, W.; Jiang, C.R.; Liang, W.B.; Zhu, Z.J. Mir-212-5p suppresses glioma development via targeting sumo2. Biochem. Genet. 2023, 61, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Zhang, N.N. Mir-212-5p inhibits nasopharyngeal carcinoma progression by targeting mettl3. Open Med. 2022, 17, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.X.; Zhang, Z.J.; Wang, Y.M.; Yang, M.L.; Fan, J.Q.; Wang, Q.J.; Deng, L.; Chen, D.; Cai, Y.F.; Li, Q.H.; et al. Down-regulated linc00115 inhibits prostate cancer cell proliferation and invasion via targeting mir-212-5p/fzd5/wnt/beta-catenin axis. J. Cell. Mol. Med. 2021, 25, 10627–10637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Zhang, G.M.; Yang, J.; Lu, R.; Hu, H.J. Dleu2 modulates proliferation, migration and invasion of platelet-derived growth factor-bb (pdgf-bb)-induced vascular smooth muscle cells (vsmcs) via mir-212-5p/ywhaz axis. Cell Cycle 2022, 21, 2013–2026. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Li, X.H.; Ouyang, C.; Ke, H.Y.; Yu, X.L.; Tan, J.F.; Chen, J.H.; Wang, C.P.; Zhang, L.P.; Tang, Y.F.; et al. Salidroside prevents pm2.5-induced beas-2b cell apoptosis via sirt1-dependent regulation of ros and mitochondrial function. Ecotoxicol. Environ. Saf. 2022, 231, 113170. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, R.; OuYang, H.J.; Wang, Y.; Wu, J.; Li, M.Y.; Hu, Y.; Yao, Y.Y.; Liu, Y.H.; Ji, Y.L. Cadmium induced mouse spermatogonia apoptosis via mitochondrial calcium overload mediated by ip3r-mcu signal pathway. Toxicology 2023, 486, 153448. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Meng, X.Z.; Meng, M.L.; Shi, M.; Sun, W.P.; Li, X.J.; Zhang, X.; Liu, R.H.; Fu, Y.; Song, L.Y. Oxidative stress activates the trpm2-ca2+-nlrp3 axis to promote pm2.5-induced lung injury of mice. Biomed. Pharmacother. 2020, 130, 110481. [Google Scholar] [CrossRef]
- Flores-Romero, H.; Dadsena, S.; Garcia-Saez, A.J. Mitochondrial pores at the crossroad between cell death and inflammatory signaling. Mol. Cell 2023, 83, 843–856. [Google Scholar] [CrossRef]
- Wang, C.; Shang, H.; Zhang, S.; Wang, X.; Liu, D.; Shen, M.; Li, N.; Jiang, Y.; Wei, K.; Zhu, R. Hexavalent chromium disrupts the skin barrier by targeting ros-mediated mitochondrial pathway apoptosis in keratinocytes. Chem. Biol. Interact. 2023, 379, 110523. [Google Scholar] [CrossRef]

| Gene | Primer Sequence (5′-3′) |
|---|---|
| ARAF | Forward: CAGTGAGGTACAGCTGTTGAAG |
| Reverse: GCCACCTTGAGCACTTTCA | |
| GAPDH | Forward: CCTGCACCACCAACTGCTTA |
| Reverse: CATCACGCCACAGCTTCCA | |
| U6 | Forward: CTCGCTTCGGCAGCACA |
| Reverse: AACGCTTCACGAATTTGCGT | |
| miR-212-5p | Forward: CGCGACCTTGGCTCTAGACTG |
| Reverse: AGTGCAGGGTCCGAGGTATT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, K.; Sun, Y.; Jia, Y.; Duan, X.; Ma, Z.; Zhang, X.; Wang, L.; Zhu, Y.; Gao, Y.; Basang, W. MicroRNA miR-212-5p Regulates the MEK/ERK Signaling Pathway by Targeting A-Raf proto-oncogene serine/threonine-protein kinase (ARAF) to Regulate Cowshed PM2.5-Induced NR8383 Apoptosis. Toxics 2023, 11, 981. https://doi.org/10.3390/toxics11120981
Sun K, Sun Y, Jia Y, Duan X, Ma Z, Zhang X, Wang L, Zhu Y, Gao Y, Basang W. MicroRNA miR-212-5p Regulates the MEK/ERK Signaling Pathway by Targeting A-Raf proto-oncogene serine/threonine-protein kinase (ARAF) to Regulate Cowshed PM2.5-Induced NR8383 Apoptosis. Toxics. 2023; 11(12):981. https://doi.org/10.3390/toxics11120981
Chicago/Turabian StyleSun, Ke, Yize Sun, Yunna Jia, Xinran Duan, Zhenhua Ma, Xiqing Zhang, Lixia Wang, Yanbin Zhu, Yunhang Gao, and Wangdui Basang. 2023. "MicroRNA miR-212-5p Regulates the MEK/ERK Signaling Pathway by Targeting A-Raf proto-oncogene serine/threonine-protein kinase (ARAF) to Regulate Cowshed PM2.5-Induced NR8383 Apoptosis" Toxics 11, no. 12: 981. https://doi.org/10.3390/toxics11120981
APA StyleSun, K., Sun, Y., Jia, Y., Duan, X., Ma, Z., Zhang, X., Wang, L., Zhu, Y., Gao, Y., & Basang, W. (2023). MicroRNA miR-212-5p Regulates the MEK/ERK Signaling Pathway by Targeting A-Raf proto-oncogene serine/threonine-protein kinase (ARAF) to Regulate Cowshed PM2.5-Induced NR8383 Apoptosis. Toxics, 11(12), 981. https://doi.org/10.3390/toxics11120981







