Impact of Atmospheric Conditions and Source Identification of Gaseous Polycyclic Aromatic Hydrocarbons (PAHs) during a Smoke Haze Period in Upper Southeast Asia
Abstract
:1. Introduction
2. Materials and Methods
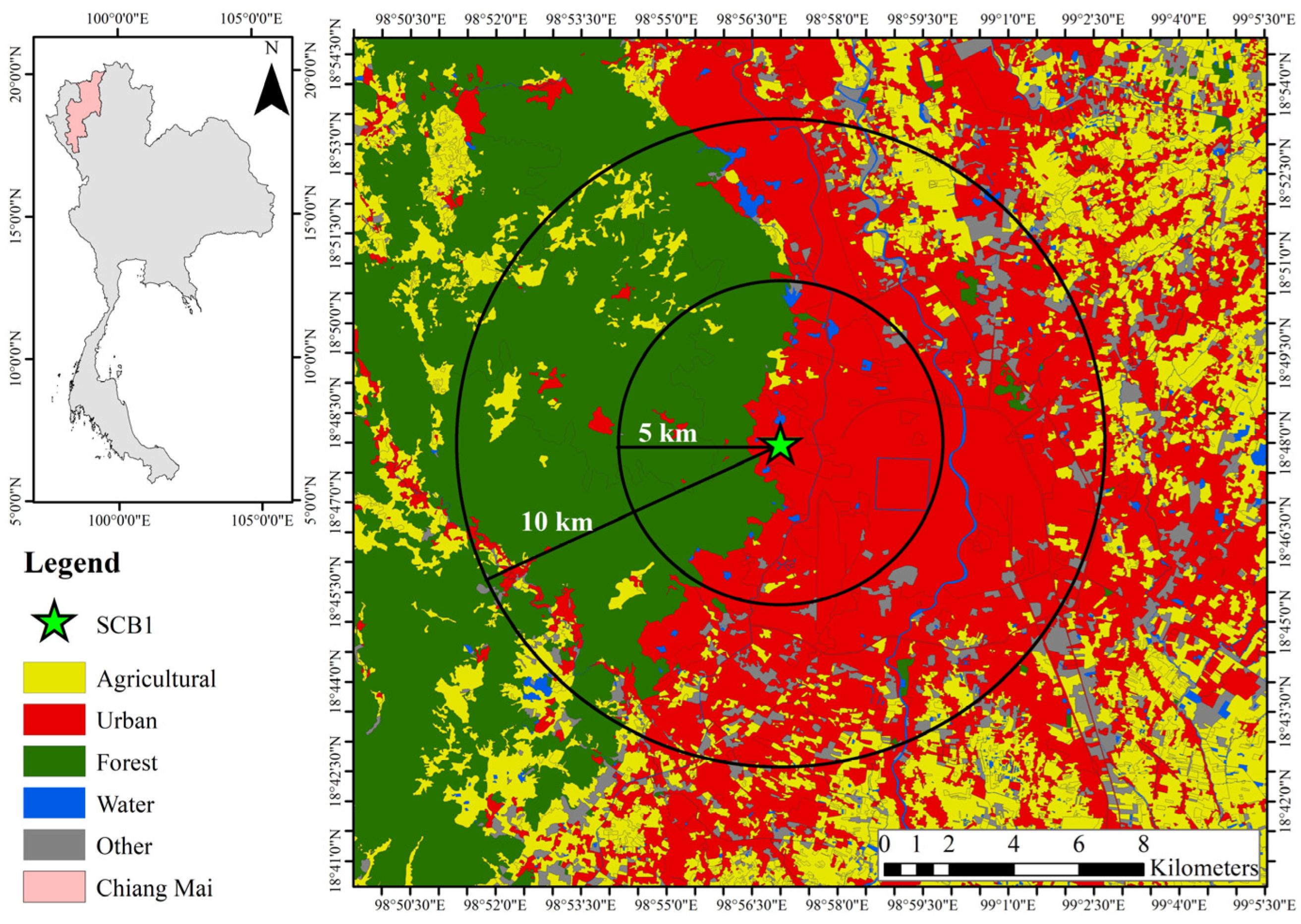
2.1. Sampling Site and Sample Collection
2.2. Chemicals and Standards
2.3. Sample Preparation
2.4. Instrumental Analysis
2.5. Quality Control
2.6. Air Pollution Data
2.7. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Measurement
3.2. Characteristics of Gaseous PAH Concentrations in the Ambient Air
3.3. Comparison of Measurement Results with Other Studies
| City (Country) | Site | Period | Concentration (ng m−3) (Mean (SD)) | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NAP | ACY | ACE | FLU | PHE | ANT | FLA | PYR | ||||
| Chiang Mai, (Thailand) | Urban | Summer | 61.52 (18.53) | 4.15 (3.51) | 6.86 (1.15) | 10.96 (2.66) | 20.44 (2.81) | 2.84 (0.60) | 5.57 (1.50) | 5.80 (1.72) | This study |
| Athens (Greece) | Urban | Summer | - | - | - | 1.28 (0.46) | 6.08 (2.76) | 0.89 (0.22) | 2.79 (0.56) | 1.91 (0.41) | [41] |
| Baltimore & northern Chesapeake Bay (USA) | Urban | Winter - Chesapeake | - | - | - | 2.09 | 3.61 | 0.13 | 0.58 | 0.42 | [42] |
| Summer | |||||||||||
| - Chesapeake | - | - | - | 2.65 | 5.57 | 0.18 | 0.848 | 0.548 | |||
| - Baltimore inner harbor | - | - | - | 4.03 | 12.5 | 0.312 | 3.43 | 2.14 | |||
| Guangzhou (China) | Urban | Summer (July) | [43] | ||||||||
| - Ground level (1.5 m) | - | 2.73 | 0.23 | 3.67 | 35.92 | 4.5 | 34.02 | 32.97 | |||
| - High level (25 m) | 0.18 | 0.05 | 1.1 | 15.3 | 1.31 | 23.36 | 17.02 | ||||
| Spring (April) | |||||||||||
| - High level (25 m) | - | 1.74 | 0.25 | 3.34 | 23.92 | 5.08 | 16.49 | 16.53 | |||
| Taichung (Taiwan) | Industry | Summer to Winter | 409 | 177 | 196 | 129 | 90 | 158 | 80.5 | 79.9 | [44] |
| Urban | 283 | 118 | 137 | 85.8 | 60 | 105 | 53.7 | 53.3 | |||
| Rural | 223 | 126 | 47.4 | 73.3 | 33.2 | 48.3 | 31.3 | 32.9 | |||
| Rome (Italy) | Urban | Winter | 687 (580) | 39 (18) | 57 (20) | 18 (8) | 71 (22) | 5.6 (1.9) | 18 (9) | 7.6 (6.0) | [45] |
| Shimizu (Japan) | Urban | Summer | 174.29 (1.21) | - | 3.54 (1.51) | 5.56 (1.17) | 17.25 (1.33) | 0.32 (1.54) | 1.85 (1.33) | 1.51 (1.14) | [46] |
| Winter | 213.44 (1.17) | - | 2.46 (1.34) | 4.74 (1.10) | 10.10 (1.14) | 0.34 (1.44) | 1.62 (1.16) | 1.19 (1.30) | |||
| Fuji (Japan) | Urban | Summer | 213.01 (1.33) | - | 6.42 (1.35) | 9.84 (1.31) | 26.27 (1.32) | 0.42 (1.55) | 4.57 (1.32) | 3.00 (1.35) | |
| Winter | 345.00 (1.41) | - | 2.87 (1.54) | 5.77 (1.33) | 12.57 (1.47) | 0.93 (1.82) | 3.20 (1.36) | 2.86 (1.37) | |||
| Heraklion (Greece) | Urban | Annual | - | - | - | 5.2 | 19.8 | 3.3 | 4.7 | 6.3 | [47] |
| Guangzhou (South, China) | Urban | Annual | 2.1 (1.9) | 3.9 (3.5) | 0.8 (0.5) | 22.0 (8.8) | 196 (92) | 29.8 (15.4) | 35.4 (19.7) | 21.2 (11.3) | [48] |
| Hanoi (Vietnam) | Urban | Summer | - | - | - | - | 150 (54) | 15 (6.1) | 36 (14) | 65 (30) | [49] |
| Delhi (India) | Urban | Winter | - | 9.8 | 7.6 | 9.9 | 12 | 3.1 | 2.2 | 1.8 | [50] |
| Summer | - | 2.6 | 1.9 | 2.8 | 4.9 | 1.2 | 0.8 | 0.6 | |||
| Monsoon | - | 1.2 | 0.8 | 3.8 | 1.5 | 0.5 | 0.6 | 0.4 | |||
| Akkalkuwa (India) | Rural | Winter | - | - | 13.7 (4.0) | 42.8 (15.8) | 90 (35) | 48.6 (26.2) | 25.6 (12.8) | 19.4 (8.5) | [51] |
| Summer | - | - | 1.3 (0.4) | 3.8 (1.4) | 49.8 (18.5) | 7.7 (3.6) | 3.4 (1.6) | 0.8 (0.3) | |||
| Post-monsoon | - | - | 0.7 (0.2) | 2.3 (0.9) | 35.3 (13.1) | 9.4 (4.3) | 2.4 (1.2) | 0.6 (0.3) | |||
| California (USA) | Prescribed fire firefighter | Training event | 669 (7) | 34 (9) | 6 (4) | 13 (6) | 50 (7) | 4 (6) | 8 (6) | 9 (6) | [38] |
| Wildland firefighter | Willow Fire | 3189 (3) | 72 (4) | 21 (4) | 77 (4) | 210 (3) | 16 (5) | 33 (3) | 22 (5) | ||
3.4. Gas–Particle Partitioning of PAHs
- Kp—gas–particle partitioning coefficient (m3 μg−1);
- Cs—the measured particle-phase concentration (μg μg−1);
- Cg—the measured gas-phase concentration (μg m−3).
3.5. Variations in the Concentration of Gaseous-Phase PAHs
3.6. Effect of Meteorological Conditions
3.7. Effect of Gaseous Pollutants
3.8. Determination of PAH Emission Sources
3.8.1. Pearson Correlation
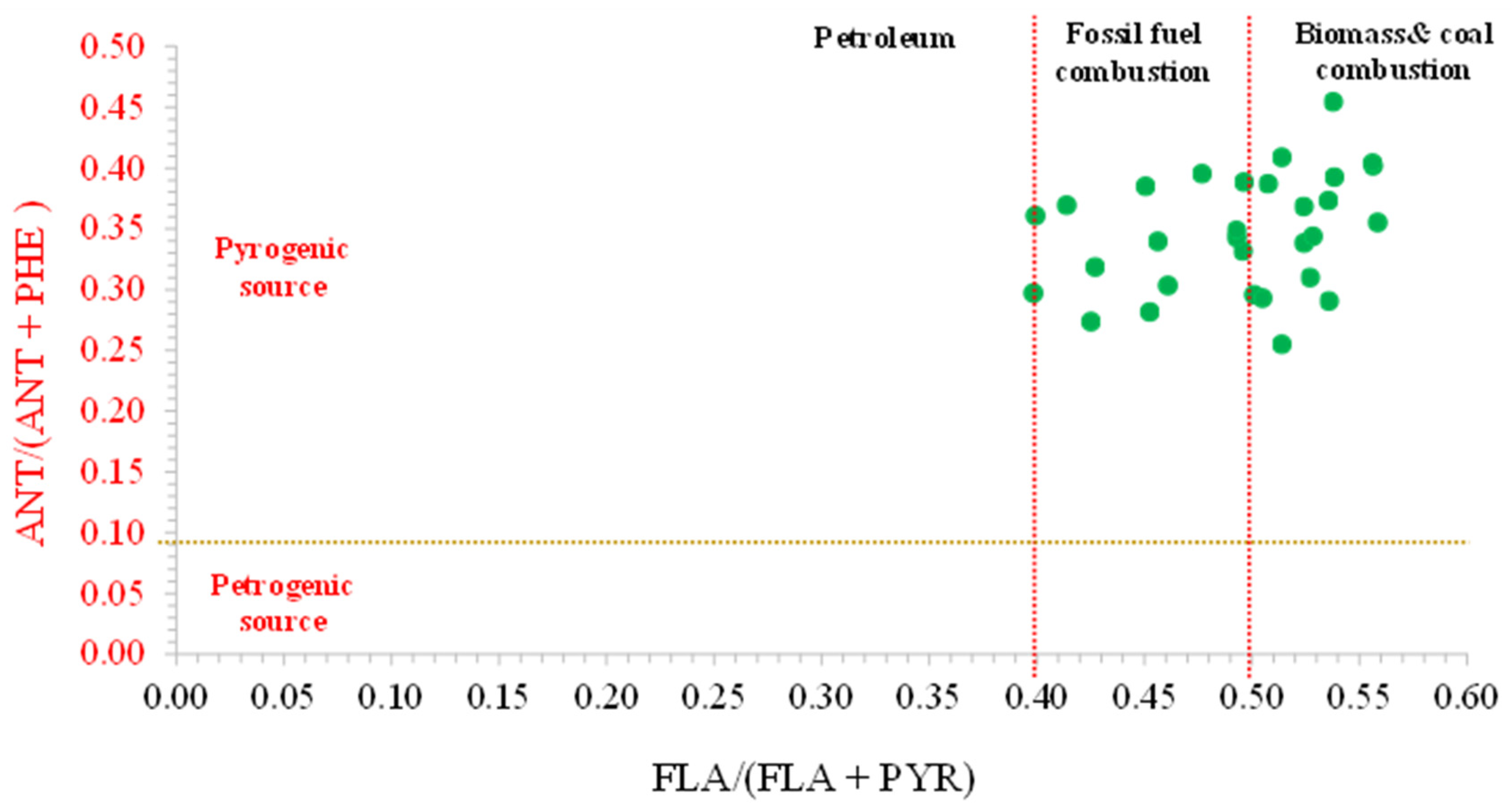
3.8.2. Diagnostic Ratios of PAHs
3.8.3. Principal Component Analysis (PCA)
| Gaseous PAHs | Principal Component | ||
|---|---|---|---|
| PC-1 | PC-2 | PC-3 | |
| NAP | 0.619 | 0.366 | −0.140 |
| ANT | 0.556 | 0.498 | −0.534 |
| PHE | 0.156 | 0.861 | −0.050 |
| FLU | 0.702 | 0.185 | −0.032 |
| ACE | 0.144 | 0.802 | 0.378 |
| ACY | 0.141 | 0.168 | 0.892 |
| FLA | −0.842 | −0.057 | −0.161 |
| PYR | −0.882 | −0.073 | −0.118 |
| Variance (%) | 34.210 | 22.973 | 16.070 |
| Cumulative % | 34.210 | 57.183 | 73.254 |
| Suggested sources | Multiple sources (Biomass burning, coal combustion and vehicle emission) | Biomass burning | |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Tyrpień-Golder, K.; Janoszka, B.; Gierat, B.; Muzyka, R. Mutagenic and carcinogenic polycyclic aromatic hydrocarbons (PAHs) in food-occurrence, human health effects, and assessment methods of exposure. Med. Srod. 2023, 26, 8–15. [Google Scholar] [CrossRef]
- Błaszczyk, E.; Rogula-Kozłowska, W.; Klejnowski, K.; Fulara, I.; Mielżyńska-Švach, D. Polycyclic aromatic hydrocarbons bound to outdoor and indoor airborne particles (PM2.5) and their mutagenicity and carcinogenicity in Silesian kindergartens, Poland. Air Qual. Atmos. Health 2017, 10, 389–400. [Google Scholar] [CrossRef]
- Hussain, K.; Hoque, R.R.; Balachandran, S.; Medhi, S.; Idris, M.G.; Rahman, M.; Hussain, F.L. Monitoring and risk analysis of PAHs in the environment. In Handbook of Environmental Materials Management; Hussain, C., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 1–35. [Google Scholar]
- Idowu, O.; Semple, K.T.; Ramadass, K.; O’Connor, W.; Hansbro, P.; Thavamani, P. Beyond the obvious: Environmental health implications of polar polycyclic aromatic hydrocarbons. Environ. Int. 2019, 123, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Siudek, P. Compositional and seasonal differences of gas and particle phase polycyclic aromatic hydrocarbons (PAHs) over the southern Baltic Sea coast. Sci. Rep. 2022, 12, 21005. [Google Scholar] [CrossRef]
- Chimjarn, S.; Delhomme, O.; Millet, M. Temporal distribution and gas/particle partitioning of polycyclic aromatic hydrocarbons (PAHs) in the atmosphere of Strasbourg, France. Atmosphere 2021, 12, 337. [Google Scholar] [CrossRef]
- Pereira, G.M.; da Silva Caumo, S.E.; Mota do Nascimento, E.Q.; Jomolca Parra, Y.; Vasconcellos, P.D.C. Polycyclic aromatic hydrocarbons in tree barks, gaseous and particulate phase samples collected near an industrial complex in São Paulo (Brazil). Chemosphere 2019, 237, 124499. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhu, L.; Chen, S. Pollution level, phase distribution and health risk of polycyclic aromatic hydrocarbons in indoor air at public places of Hangzhou, China. Environ. Pollut. 2008, 152, 569–575. [Google Scholar] [CrossRef]
- Halek, F.; Nabi Bidhendi, G.R.; Hashtroudi, M.; Kavousi, A. Distribution of polycyclic aromatic hydrocarbons in gas phase in urban atmosphere. Int. J. Environ. Health Res. 2008, 2, 97–102. [Google Scholar]
- Ho, K.F.; Lee, S.C.; Chiu, G.M.Y. Characterization of selected volatile organic compounds, polycyclic aromatic hydrocarbons and carbonyl compounds at a roadside monitoring station. Atmos. Environ. 2002, 36, 57–65. [Google Scholar] [CrossRef]
- Park, S.S.; Kim, Y.J.; Kang, C.H. Atmospheric polycyclic aromatic hydrocarbons in Seoul, Korea. Atmos. Environ. 2002, 36, 2917–2924. [Google Scholar] [CrossRef]
- Kielhorn, J.; Wahnschaffe, U.; Mangelsdorf, I. Environmental Health Criteria 229: Selected Nitro- and Nitro-Oxy-Polycyclic Aromatic Hydrocarbons. Available online: https://www.inchem.org/documents/ehc/ehc/ehc229.htm (accessed on 23 October 2023).
- Krzyszczak, A.; Czech, B. Occurrence and toxicity of polycyclic aromatic hydrocarbons derivatives in environmental matrices. Sci. Total Environ. 2021, 788, 147738. [Google Scholar] [CrossRef] [PubMed]
- Durant, J.L.; Busby, W.F.; Lafleur, A.L.; Penman, B.W.; Crespi, C.L. Human cell mutagenicity of oxygenated, nitrated and unsubstituted polycyclic aromatic hydrocarbons associated with urban aerosols. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1996, 371, 123–157. [Google Scholar] [CrossRef] [PubMed]
- Colby, G.A. Deposition of polycyclic aromatic hydrocarbons (PAHs) into northern Ontario Lake sediments. bioRxiv 2019, 786913. [Google Scholar]
- Ravindra, K.; Bencs, L.; Wauters, E.; De Hoog, J.; Deutsch, F.; Roekens, E.; Bleux, N.; Berghmans, P.; Van Grieken, R. Seasonal and site-specific variation in vapour and aerosol phase PAHs over Flanders (Belgium) and their relation with anthropogenic activities. Atmos. Environ. 2006, 40, 771–785. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Ojeda-Castillo, V.; Hernández-Paniagua, I.Y.; Hernández-Mena, L.; López-López, A.; Díaz-Torres, J.J.; Alonso-Romero, S.; Del Real-Olvera, J. Observed daily profiles of polyaromatic hydrocarbons and quinones in the gas and PM1 phases: Sources and secondary production in a metropolitan area of Mexico. Sustainability 2019, 11, 6345. [Google Scholar] [CrossRef]
- Małiszewska-Kordybach, B. Sources, concentrations, fate and effects of polycyclic aromatic hydrocarbons (PAHs) in the environment. Part A: PAHs in air. Pol. J. Environ. Stud. 1999, 8, 131–136. [Google Scholar]
- Verma, P.K.; Sah, D.; Kumari, K.M.; Lakhani, A. Atmospheric concentrations and gas-particle partitioning of polycyclic aromatic hydrocarbons (PAHs) and nitro-PAHs at Indo-Gangetic sites. Environ. Sci. Process. Impacts 2017, 19, 1051–1060. [Google Scholar] [CrossRef]
- Pollution Control Department (PCD). Meteorological Data. Available online: http://air4thai.pcd.go.th/ (accessed on 12 October 2023).
- Spicer, C.W.; Holdren, M.W.; Smith, D.L.; Miller, S.E.; Smith, R.N.; Hughes, D.P. Aircraft Emissions Characterization: F100 and F110 Engines Report ESL-TR-89-13; Air Force Engineering and Service Center Engineering and Service Laboratory, Environics Division: Bay County, FL, USA, 1990. [Google Scholar]
- Tala, W.; Chantara, S. Use of spent coffee ground biochar as ambient PAHs sorbent and novel extraction method for GC-MS analysis. Environ. Sci. Pollut. Res. 2019, 26, 13025–13040. [Google Scholar] [CrossRef]
- Tala, W.; Chantara, S. Effective solid phase extraction for highly volatile substances and application for analysis of ambient gaseous PAHs. New J. Chem. 2019, 43, 18726–18740. [Google Scholar] [CrossRef]
- Elminir, H.K. Dependence of urban air pollutants on meteorology. Sci. Total Environ. 2015, 350, 225–237. [Google Scholar]
- Schäfer, K.; Elsasser, M.; Arteaga-Salas, J.M.; Gu, J.; Pitz, M.; Schnelle-Kreis, J.; Cyrys, J.; Emeis, S.; Prevot, A.S.H.; Zimmermann, R. Source apportionment and the role of meteorological conditions in the assessment of air pollution exposure due to urban emissions. Atmos. Chem. Phys. Discuss. 2014, 14, 2235–2275. [Google Scholar]
- Xu, W.; Han, T.; Du, W.; Wang, Q.; Chen, C.; Zhao, J.; Zhang, Y.; Li, J.; Fu, P.; Wang, Z.; et al. Effects of aqueous-phase and photochemical processing on secondary organic aerosol formation and evolution in Beijing, China. Environ. Sci. Technol. 2017, 51, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Wiriya, W.; Prapamontol, T.; Chantara, S. PM10-bound polycyclic aromatic hydrocarbons in Chiang Mai (Thailand): Seasonal variations, source identification, health risk assessment and their relationship to air-mass movement. Atmos. Res. 2013, 124, 109–122. [Google Scholar] [CrossRef]
- Amnuaylojaroen, T.; Kaewkanchanawong, P.; Panpeng, P. Distribution and meteorological control of PM2.5 and its effect on visibility in Northern Thailand. Atmosphere 2023, 14, 538. [Google Scholar] [CrossRef]
- Sagar, V.; Verma, G.; Das, R.M. Influence of temperature and relative humidity on PM2.5 concentration over Delhi. MAPAN J. Metrol. Soc. India 2023, 38, 759–769. [Google Scholar]
- Wexler, H. A boundary layer interpretation of the low-level jet. Tellus 1961, 13, 368–378. [Google Scholar] [CrossRef]
- Zhang, N.; Cao, J.; Li, L.; Ho, S.S.H.; Wang, Q.; Zhu, C.; Wang, L. Characteristics and source identification of polycyclic aromatic hydrocarbons and n-Alkanes in PM2.5 in Xiamen. Aerosol Air Qual. Res. 2018, 18, 1673–1683. [Google Scholar] [CrossRef]
- Bamford, H.A.; Bezabeh, D.Z.; Schantz, M.M.; Wise, S.A.; Baker, J.E. Determination and comparison of nitrated-polycyclic aromatic hydrocarbons measured in air and diesel particulate reference materials. Chemosphere 2003, 50, 575–587. [Google Scholar] [CrossRef]
- Reisen, F.; Arey, J. Atmospheric reactions influence seasonal PAH and nitro-PAH concentrations in the Los Angeles Basin. Environ. Sci. Technol. 2005, 39, 64–73. [Google Scholar] [CrossRef]
- Barbas, J.T.; Sigman, M.E.; Dabestani, R. Photochemical of phenanthrene sorbed on silica gel. Environ. Sci. Technol. 1996, 30, 1776–1780. [Google Scholar] [CrossRef]
- Kamens, R.M.; Karam, H.; Guo, J.; Perry, J.M.; Stockburger, L. The behavior of oxygenated polycyclic aromatic hydrocarbons on atmospheric soot particles. Environ. Sci. Technol. 1989, 23, 801–806. [Google Scholar] [CrossRef]
- Navarro, L.M.; Fernández, N.; Guerra, C.; Guralnick, R.; Kissling, W.D.; Londoño, M.C.; Muller-Karger, F.; Turak, E.; Balvanera, P.; Costello, M.J.; et al. Monitoring biodiversity change through effective global coordination. Curr. Opin. Environ. Sustain. 2017, 29, 158–169. [Google Scholar] [CrossRef]
- Amnuaylojaroen, T.; Inkom, J.; Janta, R.; Surapipith, V. Long range transport of Southeast Asian PM2.5 pollution to Northern Thailand during high biomass burning episodes. Sustainability 2020, 12, 10049. [Google Scholar] [CrossRef]
- Oruc, I. Long-range transport and potential source regions of PM2.5 during the autumn season in Edirne, Türkiye. Front. Life Sci. Relat. Technol. 2022, 3, 95–100. [Google Scholar] [CrossRef]
- Mandalakis, M.; Tsapakis, M.; Tsoga, A.; Stephanou, E.G. Gas–particle concentrations and distribution of aliphatic hydrocarbons, PAHs, PCBs and PCDD/Fs in the atmosphere of Athens (Greece). Atmos. Environ. 2002, 36, 4023–4035. [Google Scholar] [CrossRef]
- Dachs, J.; Glenn, T.R.; Gigliotti, C.L.; Brunciak, P.; Totten, L.A.; Nelson, E.D.; Franz, T.P.; Eisenreich, S.J. Processes driving the short-term variability of polycyclic aromatic hydrocarbons in the Baltimore and northern Chesapeake Bay atmosphere, USA. Atmos. Environ. 2002, 36, 2281–2295. [Google Scholar] [CrossRef]
- Bi, X.; Sheng, G.; Peng, P.A.; Chen, Y.; Zhang, Z.; Fu, J. Distribution of particulate- and vapor-phase n-alkanes and polycyclic aromatic hydrocarbons in urban atmosphere of Guangzhou, China. Atmos. Environ. 2003, 37, 289–298. [Google Scholar] [CrossRef]
- Fang, G.-C.; Wu, Y.-S.; Fu, P.P.-C.; Yang, I.L.; Chen, M.-H. Polycyclic aromatic hydrocarbons in the ambient air of suburban and industrial regions of central Taiwan. Chemosphere 2004, 54, 443–452. [Google Scholar] [CrossRef]
- Possanzini, M.; Di Palo, V.; Gigliucci, P.; Scianò, M.C.T.; Cecinato, A. Determination of phase-distributed PAH in Rome ambient air by denuder/GC-MS method. Atmos. Environ. 2004, 38, 1727–1734. [Google Scholar] [CrossRef]
- Ohura, T.; Sakakibara, H.; Watanabe, I.; Shim, W.J.; Manage, P.M.; Guruge, K.S. Spatial and vertical distributions of sedimentary halogenated polycyclic aromatic hydrocarbons in moderately polluted areas of Asia. Environ. Pollut. 2015, 196, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Tsapakis, M.; Stephanou, E.G. Polycyclic aromatic hydrocarbons in the atmosphere of the eastern mediterranean. Environ. Sci. Technol. 2005, 39, 6584–6590. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xia, X.; Yang, Z.; Wang, R.; Voulvoulis, N. Distribution and sources of polycyclic aromatic hydrocarbons in the middle and lower reaches of the Yellow River, China. Environ. Pollut. 2006, 144, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Kishida, M.; Imamura, K.; Takenaka, N.; Maeda, Y.; Viet, P.H.; Bandow, H. Concentrations of atmospheric polycyclic aromatic hydrocarbons in particulate matter and the gaseous phase at roadside sites in Hanoi, Vietnam. Bull. Environ. Contam. Toxicol. 2008, 81, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Gadi, R.; Mandal, T.K. Levels, sources, and toxic potential of polycyclic aromatic hydrocarbons in urban soil of Delhi, India. Hum. Ecol. Risk. Assess. 2012, 18, 393–411. [Google Scholar] [CrossRef]
- Salve, P.R.; Krupadam, R.J.; Wate, S.R. Distribution of gaseous phase polycyclic aromatic hydrocarbons (PAHs) in rural environment of India. Int. Res. J. Environ. Sci. 2015, 4, 70–74. [Google Scholar]
- Tinsley, F. Chemical Concepts in Pollutant Behavior, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2004. [Google Scholar]
- Pongpiachan, S.; Tipmanee, D.; Deelaman, W.; Muprasit, J.; Feldens, P.; Schwarzer, K. Risk assessment of the presence of polycyclic aromatic hydrocarbons (PAHs) in coastal areas of Thailand affected by the 2004 tsunami. Mar. Pollut. Bull. 2013, 76, 370–378. [Google Scholar] [CrossRef]
- Drotikova, T.; Ali, A.M.; Halse, A.K.; Reinardy, H.C.; Kallenborn, R. Polycyclic aromatic hydrocarbons (PAHs) and oxy- and nitro-PAHs in ambient air of the Arctic town Longyearbyen, Svalbard. Atmos. Chem. Phys. 2020, 20, 9997–10014. [Google Scholar] [CrossRef]
- Elorduy, I.; Elcoroaristizabal, S.; Durana, N.; García, J.A.; Alonso, L. Diurnal variation of particle-bound PAHs in an urban area of Spain using TD-GC/MS: Influence of meteorological parameters and emission sources. Atmos. Environ. 2016, 138, 87–98. [Google Scholar] [CrossRef]
- Fon, T.Y.W.; Noriatsu, O.; Hiroshi, S. Polycyclic aromatic hydrocarbons (PAHs) in the aerosol of Higashi Hiroshima, Japan: Pollution scenario and source identification. Water Air Soil Pollut. 2007, 182, 235–243. [Google Scholar] [CrossRef]
- Li, X.; Kong, S.; Yin, Y.; Li, L.; Yuan, L.; Li, Q.; Xiao, H.; Chen, K. Polycyclic aromatic hydrocarbons (PAHs) in atmospheric PM2.5 around 2013 Asian Youth Games period in Nanjing. Atmos. Res. 2016, 174–175, 85–96. [Google Scholar] [CrossRef]
- Cabrerizo, A.; Dachs, J.; Barceló, D.; Jones, K.C. Climatic and biogeochemical controls on the remobilization and reservoirs of persistent organic pollutants in Antarctica. Environ. Sci. Technol. 2013, 47, 4299–4306. [Google Scholar] [CrossRef] [PubMed]
- Cabrerizo, A.; Dachs, J.; Moeckel, C.; Ojeda, M.-J.; Caballero, G.; Barceló, D.; Jones, K.C. Factors influencing the soil-air partitioning and the strength of soils as a secondary source of polychlorinated biphenyls to the atmosphere. Environ. Sci. Technol. 2011, 45, 4785–4792. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.T.; Jung, K.-S.; Son, J.-M.; Kwon, H.-O.; Choi, S.-D. Seasonal variation, phase distribution, and source identification of atmospheric polycyclic aromatic hydrocarbons at a semi-rural site in Ulsan, South Korea. Environ. Pollut. 2018, 236, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Ailijiang, N.; Zhong, N.; Zhou, X.; Anwar Mamat, A.; Chang, J.; Cao, S.; Hua, Z.; Li, N. Levels, sources, and risk assessment of PAHs residues in soil and plants in urban parks of Northwest China. Sci. Rep. 2022, 12, 21448. [Google Scholar] [CrossRef]
- Keyte, I.J.; Albinet, A.; Harrison, R.M. On-road traffic emissions of polycyclic aromatic hydrocarbons and their oxy- and nitro- derivative compounds measured in road tunnel environments. Sci. Total Environ. 2016, 566–567, 1131–1142. [Google Scholar] [CrossRef]
- Singla, V.; Pachauri, T.; Satsangi, A.; Kumari, K.M.; Lakhani, A. Characterization and mutagenicity assessment of PM2.5 and PM10 PAH at Agra, India. Polycycl. Aromat. Compd. 2012, 32, 199–220. [Google Scholar] [CrossRef]
- Pehnec, G.; Jakovljević, I.L.; Šišović, A.; Bešlić, I.; Vađić, V. Influence of ozone and meteorological parameters on levels of polycyclic aromatic hydrocarbons in the air. Atmos. Environ. 2016, 131, 263–268. [Google Scholar] [CrossRef]
- Golomb, D.; Barry, E.; Fisher, G.; Varanusupakul, P.; Koleda, M.; Rooney, T. Atmospheric deposition of polycyclic aromatic hydrocarbons near New England coastal waters. Atmos. Environ. 2001, 35, 6245–6258. [Google Scholar] [CrossRef]
- Motelay-Massei, A.; Ollivon, D.; Garban, B.; Chevreuil, M. Polycyclic aromatic hydrocarbons in bulk deposition at a suburban site: Assessment by principal component analysis of the influence of meteorological parameters. Atmos. Environ. 2003, 37, 3135–3146. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Liu, M.; Lu, M.; Ge, R.; Li, S.; Liu, X.; Dong, W.; Qadeer, A. Characterization and source identification of PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) in different seasons from Shanghai, China. Sci. Total Environ. 2018, 644, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Hrdina, A.I.H.; Kohale, I.N.; Kaushal, S.; Kelly, J.; Selin, N.E.; Engelward, B.P.; Kroll, J.H. The parallel transformations of polycyclic aromatic hydrocarbons in the body and in the atmosphere. Environ. Health Perspect. 2022, 130, 25004. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Zhang, Y.; Zhao, H.; Tan, F.; Wu, X.; Chen, J. Source apportionment of polycyclic aromatic hydrocarbons (PAHs) in the air of Dalian, China: Correlations with six criteria air pollutants and meteorological conditions. Chemosphere 2019, 216, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.W.F.; Takeda, K.; Sakugawa, H. Exploring the correlation of particulate PAHs, sulfur dioxide, nitrogen dioxide and ozone, a preliminary study. Water Air Soil Pollut. 2008, 194, 5–12. [Google Scholar] [CrossRef]
- Williams, A.G.; Chambers, S.D.; Conen, F.; Reimann, S.; Hill, M.; Griffiths, A.D.; Crawford, J. Radon as a tracer of atmospheric influences on traffic-related air pollution in a small inland city. Tellus B Chem. Phys. Meteorol. 2016, 68, 30967. [Google Scholar] [CrossRef]
- Arey, J.; Zielinska, B.; Atkinson, R.; Aschmann, S.M. Nitroarene products from the gas-phase reactions of volatile polycyclic aromatic hydrocarbons with the OH radical and N2O5. Int. J. Chem. Kinet. 1989, 21, 775–799. [Google Scholar] [CrossRef]
- Arey, J.; Zielinska, B.; Atkinson, R.; Winer, A.M.; Ramdhal, T.; Pitts, J.N., Jr. The formation of nitro-PAH from the gas-phase reactions of fluoranthene and pyrene with the OH radical in the presence of NOx. Atmos. Environ. 1986, 20, 2239–2345. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J. Atmospheric chemistry of gas-phase polycyclic aromatic hydrocarbons: Formation of atmospheric mutagens. Environ. Health Perspect. 1994, 102, 117–126. [Google Scholar]
- Cochran, R.E.; Jeong, H.; Haddadi, S.; Fisseha Derseh, R.; Gowan, A.; Beránek, J.; Kubátová, A. Identification of products formed during the heterogeneous nitration and ozonation of polycyclic aromatic hydrocarbons. Atmos. Environ. 2016, 128, 92–103. [Google Scholar] [CrossRef]
- Sasaki, J.; Aschmann, S.M.; Kwok, E.S.C.; Atkinson, R.; Arey, J. Products of the gas-phase OH and NO3 radical-initiated reactions of naphthalene. Environ. Sci. Technol. 1997, 31, 3173–3179. [Google Scholar] [CrossRef]
- Suzuki, J.; Meguro, S.; Morita, O.; Hirayama, S.; Suzuki, S. Comparison of in vivo binding of aromatic nitro and amino compounds to rat hemoglobin. Biochem. Pharmacol. 1989, 38, 3511–3519. [Google Scholar] [PubMed]
- Vione, D.; Barra, S.; De Gennaro, G.; De Rienzo, M.; Gilardoni, S.; Perrone, M.G.; Pozzoli, L. Polycyclic aromatic hydrocarbons in the atmosphere: Monitoring, sources, sinks and fate. II: Sinks and fate. Ann. Chim. 2004, 94, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Vione, D.; Maurino, V.; Minero, C.; Pelizzetti, E. Aqueous atmospheric chemistry: Formation of 2,4-dinitrophenol upon nitration of 2-nitrophenol and 4-nitrophenol in solution. Environ. Sci. Technol. 2005, 39, 7921–7931. [Google Scholar] [CrossRef] [PubMed]
- Keyte, I.J.; Harrison, R.M.; Lammel, G. Chemical reactivity and long-range transport potential of polycyclic aromatic hydrocarbons-A review. Chem. Soc. Rev. 2013, 42, 9333–9391. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.; Arey, J.; Zielinska, B.; Pitts, J.N., Jr.; Winer, A.M. Evidence for the transformation of polycyclic organic matter in the atmosphere. Atmos. Environ. 1987, 21, 2261–2264. [Google Scholar] [CrossRef]
- Arey, J.; Zielinska, B.; Atkinson, R.; Winer, A.M. Polycyclic aromatic hydrocarbons and nitroarene concentrations in ambient air during a wintertime high NO2 episode in the Los Angeles Basin. Atmos. Environ. 1987, 21, 1437–1444. [Google Scholar] [CrossRef]
- Helmig, D.; Arey, J.; Atkinson, R.; Harger, W.P.; McElroy, P.A. Products of the OH radical-initiated gas-phase reaction of fluorene in the presence of NOx. Atmos. Environ. Part A Gen. Top. 1992, 26, 1735–1745. [Google Scholar] [CrossRef]
- Lin, Y.; Lee, W.; Li, H.; Chen, C.; Fang, G.; Tsai, P. Impact of using fishing boat fuel with high poly aromatic content on the emission of polycyclic aromatic hydrocarbons from the diesel engine. Atmos. Environ. 2006, 40, 1601–1609. [Google Scholar] [CrossRef]
- Marr, L.C.; Kirchstetter, T.W.; Harley, R.A.; Miguel, A.H.; Hering, S.V.; Hammond, S.K. Characterization of polycyclic aromatic hydrocarbons in motor vehicle fuels and exhaust emissions. Environ. Sci. Technol. 1999, 33, 3091–3099. [Google Scholar] [CrossRef]
- Rajput, N.; Lakhani, A. Measurements of polycyclic aromatic hydrocarbons at an industrial site in India. Environ. Monit. Assess. 2009, 150, 273–284. [Google Scholar] [CrossRef]
- Rajput, P.; Sarin, M.; Sharma, D.; Singh, D. Atmospheric polycyclic aromatic hydrocarbons and isomer ratios as tracers of biomass burning emissions in Northern India. Environ. Sci. Pollut. Res. 2014, 21, 5724–5729. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.O.; Lopes, W.A.; Pereira, P.A.; Vasconcellos, P.D.; Oliveira, F.S.; Carvalho, L.S.; Conceição, L.D.; Andrade, J.B. Quantification and source identification of atmospheric particulate polycyclic aromatic hydrocarbons and their dry deposition fluxes at three sites in Salvador Basin, Brazil, impacted by mobile and stationary sources. J. Braz. Chem. Soc. 2009, 20, 680–692. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namieśnik, J. PAH diagnostic ratios for the identification of pollution emission sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Galarneau, E. Source specificity and atmospheric processing of airborne PAHs: Implications for source apportionment. Atmos. Environ. 2008, 42, 8139–8149. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Guo, X.; Wang, Y. Seasonal variation and source apportionment of organic and inorganic compounds in PM2.5 and PM10 particulates in Beijing, China. J. Environ. Sci. 2013, 25, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tao, S.; Liu, W.; Liu, Y.; Dou, H.; Zhao, J.; Wang, L.; Wang, J.; Tian, Z.; Gao, Y. Atmospheric polycyclic aromatic hydrocarbons in North China: A winter-time study. Environ. Sci. Technol. 2007, 41, 8256–8261. [Google Scholar] [CrossRef]
- Masih, J.; Dyavarchetty, S.; Nair, A.; Taneja, A.; Singhvi, R. Concentration and sources of fine particulate associated polycyclic aromatic hydrocarbons at two locations in the western coast of India. Environ. Technol. Innov. 2019, 13, 179–188. [Google Scholar] [CrossRef]
- Singh, B.P.; Zughaibi, T.A.; Alharthy, S.A.; Al-Asmari, A.I.; Rahman, S. Statistical analysis, source apportionment, and toxicity of particulate- and gaseous-phase PAHs in the urban atmosphere. Front. Public Health 2023, 10, 1070663. [Google Scholar] [CrossRef]
- Zuśka, Z.; Kopcińska, J.; Dacewicz, E.; Skowera, B.; Wojkowski, J.; Ziernicka–Wojtaszek, A. Application of the principal component analysis (PCA) method to assess the impact of meteorological elements on concentrations of particulate matter (PM10): A case study of the mountain valley (the Sącz basin, Poland). Sustainability 2019, 11, 6740. [Google Scholar] [CrossRef]
- Bai, L.; Li, C. Investigation of indoor polycyclic aromatic hydrocarbons (PAHs) in rural Northeast China: Pollution characteristics, source analysis, and health assessment. Buildings 2022, 12, 153. [Google Scholar] [CrossRef]
- Thompson, N.; Adjei, J.K.; Bentum, J.K.; Essumang, D.K.; Duodu, G.O.; Hadzi, G.; Adjei, G.A. Vehicular influence on atmospheric concentrations and source apportionment of polycyclic aromatic hydrocarbons in some major cities in three regions of Ghana using epiphytic lichens. Toxicol. Rep. 2022, 9, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, F.; Yu, Y.; Zhang, J.; Wang, R.; Srinivasulu, M.; Vasenev, V.I. Characterization, source apportionment, and risk assessment of polycyclic aromatic hydrocarbons in urban soil of Nanjing, China. J. Soils Sediments 2017, 17, 1116–1125. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, N.; Yin, S.; Li, X.; Yu, F.; Guo, Y.; Zhang, R. Carbonaceous species in PM2.5 and PM10 in urban area of Zhengzhou in China: Seasonal variations and source apportionment. Atmos. Res. 2017, 191, 1–11. [Google Scholar] [CrossRef]
- Ravindra, K.; Wauters, E.; Van Grieken, R. Variation in particulate PAHs levels and their relation with the transboundary movement of the air masses. Sci. Total Environ. 2008, 396, 100–110. [Google Scholar] [CrossRef]
- Deka, J.; Sarma, K.P.; Hoque, R.R. Source contributions of polycyclic aromatic hydrocarbons in soils around oilfield in the Brahmaputra Valley. Ecotoxicol. Environ. Saf. 2016, 133, 281–289. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Li, Y.-X.; Zhang, J.-Q.; Zhang, T.; Liu, G.-H.; Huang, M.-Z.; Li, X.; Ruan, J.-J.; Kannan, K.; Qiu, R.-L. Associations between polycyclic aromatic hydrocarbon (PAH) exposure and oxidative stress in people living near e-waste recycling facilities in China. Environ. Int. 2016, 94, 161–169. [Google Scholar] [CrossRef]
- Khalili, N.R.; Scheff, P.A.; Holsen, T.M. PAH source fingerprints for coke ovens, diesel and gasoline engines, highway tunnels, and wood combustion emissions. Atmos. Environ. 1995, 29, 533–542. [Google Scholar] [CrossRef]
- Simayi, M.; Yahefu, P.; Han, M. Spatiotemporal variation, source analysis and health risk assessment of particle-bound PAHs in Urumqi, China. Aerosol Air Qual. Res. 2018, 18, 2728–2740. [Google Scholar] [CrossRef]
- Soleimani, M.; Ebrahimi, Z.; Mirghaffari, N.; Moradi, H.; Amini, N.; Poulsen, K.G.; Christensen, J.H. Seasonal trend and source identification of polycyclic aromatic hydrocarbons associated with fine particulate matters (PM2.5) in Isfahan city, Iran, using diagnostic ratio and PMF model. Environ. Sci. Pollut. Res. 2022, 29, 26449–26464. [Google Scholar] [CrossRef]
- Ortiz, J.E.; Sánchez-Palencia, Y.; Gallego, J.L.R.; Borrego, A.G.; Baragaño, D.; Torres, T. Deposition of atmospheric polycyclic aromatic hydrocarbons in rural areas: Current data and historical record from an ombrotrophic peatland. Int. J. Coal Geol. 2023, 268, 104199. [Google Scholar] [CrossRef]
- Jiao, H.; Bian, G.; Chen, X.; Wang, S.; Zhuang, X.; Bai, Z. Distribution, sources, and potential risk of polycyclic aromatic hydrocarbons in soils from an industrial district in Shanxi, China. Environ. Sci. Pollut. Res. 2017, 24, 12243–12260. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lohmann, R.; Yu, N.; Zhang, C.; Gao, Y.; Zhao, J.; Ma, L. Source apportionment of gaseous and particulate PAHs from traffic emission using tunnel measurements in Shanghai, China. Atmos. Environ. 2015, 107, 129–136. [Google Scholar] [CrossRef]
- Chen, C.; Xia, Z.; Wu, M.; Zhang, Q.; Wang, T.; Wang, L.; Yang, H. Concentrations, source identification, and lung cancer risk associated with springtime PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) in Nanjing, China. Arch. Environ. Contam. Toxicol. 2017, 73, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, H.; Zhang, L.; Zhang, Z.; Xing, X.; Qi, S. Fine particle-bound polycyclic aromatic hydrocarbons (PAHs) at an urban site of Wuhan, central China: Characteristics, potential sources and cancer risks apportionment. Environ. Pollut. 2019, 246, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.-C.; Wu, Y.-S.; Chen, J.-C.; Chang, C.-N.; Ho, T.-T. Characteristic of polycyclic aromatic hydrocarbon concentrations and source identification for fine and coarse particulates at Taichung Harbor near Taiwan Strait during 2004–2005. Sci. Total Environ. 2006, 366, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Malik, R.N.; Martellini, T.; Cincinelli, A. PAH exposure biomarkers are associated with clinico-chemical changes in the brick kiln workers in Pakistan. Sci. Total Environ. 2014, 490, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Pulster, E.L.; Johnson, G.; Hollander, D.; McCluskey, J.; Harbison, R. Levels and sources of atmospheric polycyclic aromatic hydrocarbons surrounding an oil refinery in Curaçao. J. Environ. Prot. 2019, 10, 431–453. [Google Scholar] [CrossRef]
- He, X.; Pang, Y.; Song, X.; Chen, B.; Feng, Z.; Ma, Y. Distribution, sources and ecological risk assessment of PAHs in surface sediments from Guan River estuary, China. Mar. Pollut. Bull. 2014, 80, 52–58. [Google Scholar] [CrossRef]

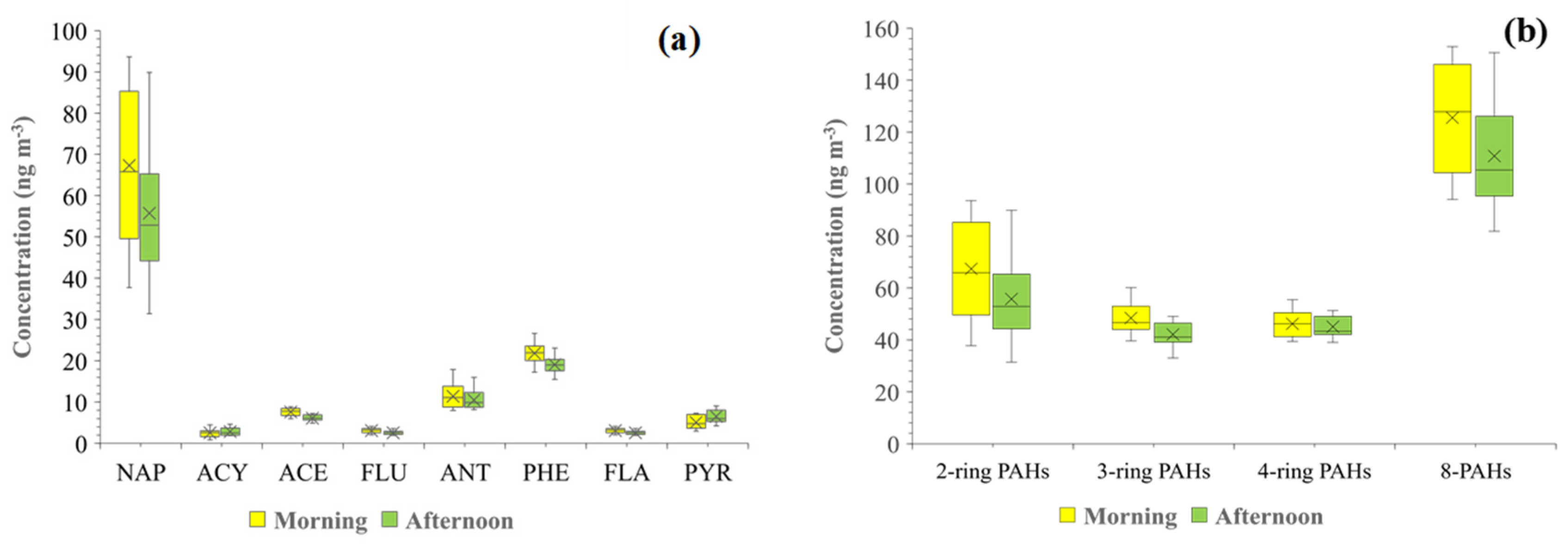
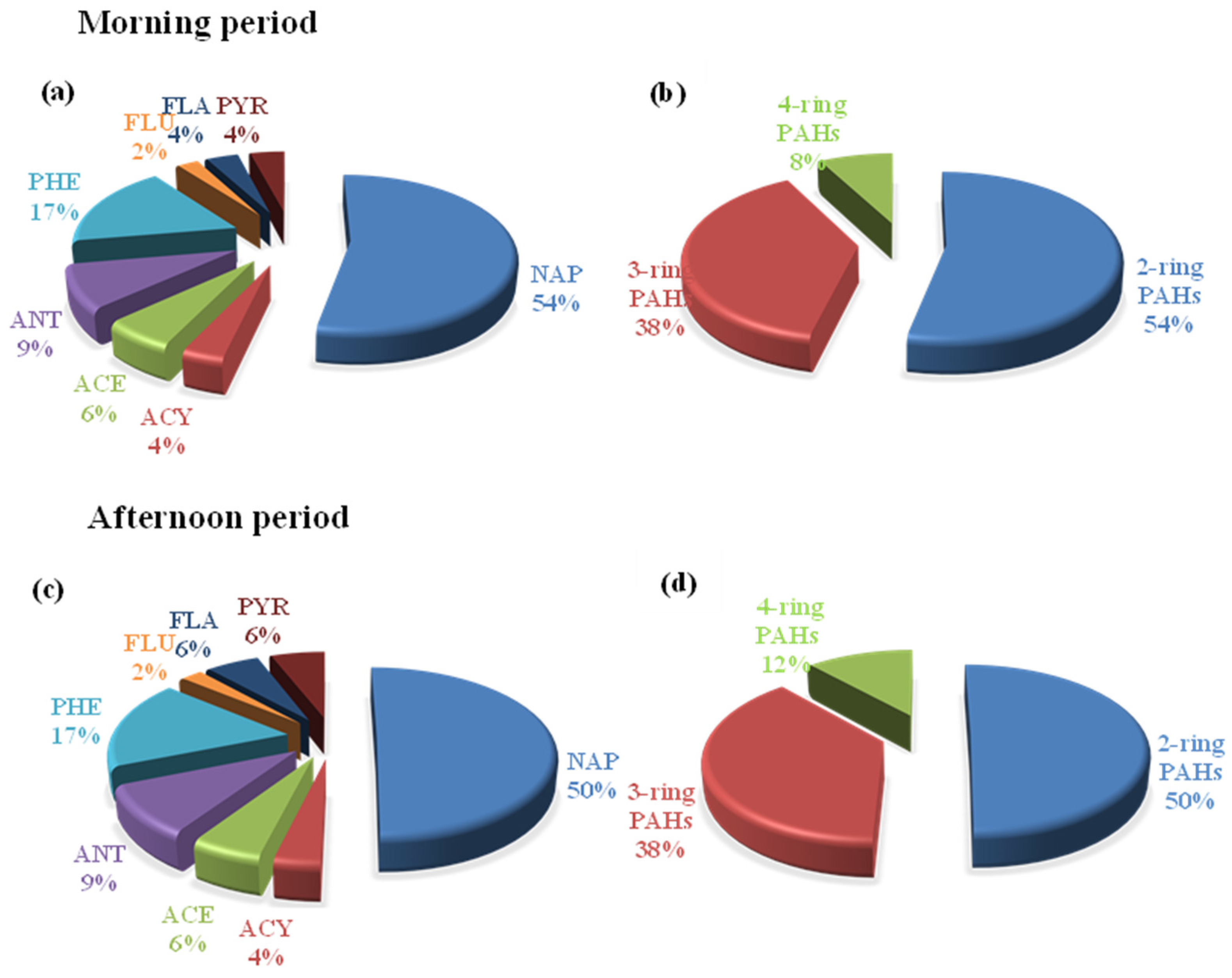
| Compound | Number of Ring | Concentration (ng m−3) | |||
|---|---|---|---|---|---|
| Individual Compound | Ring Compound | ||||
| Min–Max | Average ± SD | Min–Max | Average ± SD | ||
| NAP | 2 | 31–94 | 62 ± 19 | 31–94 | 62 ± 19 |
| ACY | 3 | 0.88–14 | 4.1 ± 3.5 | 33–60 | 45 ± 6.3 |
| ACE | 4.9–8.9 | 6.9 ± 1.1 | |||
| FLU | 7.9–18 | 11 ± 2.7 | |||
| PHE | 15–27 | 20 ± 2.8 | |||
| ANT | 2.0–4.1 | 2.8 ± 0.6 | |||
| FLA | 4 | 3.5–9.2 | 5.6 ± 1.5 | 6.5–18 | 11 ± 3.0 |
| PYR | 3.0–9.0 | 5.8 ± 1.7 | |||
| Compound | a Kp (m3 μg−1) | b Cg (μg m−3) | Cs (μg μg−1) | Distribution Ratio of Ms/Mg |
|---|---|---|---|---|
| NAP | - | 6.15 × 10−3 | - | - |
| ACY | 1.96 × 10−5 | 4.15 × 10−3 | 8.13 × 10−8 | 1.96 × 10−5 |
| ACE | 2.76 × 10−5 | 6.86 × 10−3 | 1.89 × 10−7 | 2.76 × 10−5 |
| Flu | 6.16 × 10−5 | 1.10 × 10−3 | 6.78 × 10−8 | 6.16 × 10−5 |
| HE | 3.13 × 10−4 | 2.04 × 10−3 | 6.39 × 10−7 | 3.13 × 10−4 |
| ANT | 3.39 × 10−4 | 2.84 × 10−3 | 9.63 × 10−7 | 3.39 × 10−4 |
| FLA | 3.25 × 10−3 | 5.57 × 10−3 | 1.81 × 10−5 | 3.25 × 10−3 |
| PYR | 3.01 × 10−2 | 5.80 × 10−3 | 1.75 × 10−4 | 3.01 × 10−2 |
| Wind Speed | Net Radiation | Temperature | Pressure | Relative Humidity | |
|---|---|---|---|---|---|
| NAP | 0.002 | −0.339 | −0.502 ** | 0.414 * | 0.503 ** |
| ACY | −0.202 | −0.059 | 0.024 | 0.180 | −0.065 |
| ACE | −0.106 | −0.679 ** | −0.630 ** | 0.492 ** | 0.680 ** |
| ANT | 0.090 | −0.186 | −0.559 ** | 0.491 ** | 0.572 ** |
| PHE | −0.147 | −0.489 ** | −0.523 ** | 0.427 * | 0.594 ** |
| FLU | −0.325 | −0.291 | −0.449 * | 0.596 ** | 0.329 |
| FLA | 0.101 | 0.416 * | 0.608 ** | −0.676 ** | −0.493 ** |
| PYR | −0.022 | 0.331 | 0.659 ** | −0.814 ** | −0.530 ** |
| 2-ring PAHs | 0.002 | −0.339 | −0.502 ** | 0.414 * | 0.503 ** |
| 3-ring PAHs | −0.19 | −0.479 ** | −0.610 ** | 0.641 ** | 0.622 ** |
| 4-ring PAHs | 0.037 | 0.394 * | 0.675 ** | −0.797 ** | −0.545 ** |
| 8-PAHs | −0.047 | −0.369 * | −0.506 ** | 0.424 * | 0.528 ** |
| NO2 | NO | NOX | CO | SO2 | O3 | PM2.5 | PM10 | |
|---|---|---|---|---|---|---|---|---|
| NAP | −0.272 | −0.165 | −0.232 | 0.072 | 0.225 | −0.385 * | 0.215 | −0.315 |
| ACY | 0.125 | 0.291 | 0.221 | 0.213 | 0.063 | −0.062 | −0.098 | −0.237 |
| ACE | −0.162 | −0.119 | −0.156 | 0.367 * | −0.075 | −0.568 ** | −0.121 | −0.311 |
| ANT | −0.541 ** | −0.366 * | −0.501 ** | 0.079 | −0.064 | −0.289 | −0.263 | −0.418 * |
| PHE | −0.231 | −0.203 | −0.246 | 0.236 | −0.070 | −0.434 * | −0.158 | −0.420 * |
| FLU | −0.026 | 0.194 | 0.079 | 0.168 | 0.130 | −0.437 * | −0.076 | −0.115 |
| FLA | 0.262 | 0.027 | 0.188 | −0.396 * | −0.024 | 0.559 ** | 0.278 | 0.26 |
| PYR | 0.559 ** | 0.189 | 0.445 * | −0.195 | 0.105 | 0.440 * | 0.34 | 0.526 ** |
| 2-ring PAHs | −0.272 | −0.165 | −0.232 | 0.072 | 0.225 | −0.385 * | 0.215 | −0.315 |
| 3-ring PAHs | −0.292 | −0.086 | −0.218 | 0.338 | −0.024 | −0.493 ** | −0.259 | −0.569 ** |
| 4-ring PAHs | 0.447 * | 0.121 | 0.346 | −0.307 | 0.047 | 0.526 ** | 0.332 | 0.427 * |
| 8-PAHs | −0.252 | −0.147 | −0.211 | 0.116 | 0.189 | −0.394 * | 0.157 | −0.366 |
| Parent PAHs (1° PAHs) | PAH Derivatives (2° PAHs) | |||
|---|---|---|---|---|
| Name | Detected Concentration a (ng m−3) | Name | Obtained Yield (ng m−3) b from 1° PAHs Reacted with | |
| OH• | NO3• | |||
| NAP | 61.52 | 1-Nitronaphthalene | 0.1846 | 10.4584 |
| 2-Nitronaphthalene | 0.1846 | 4.3064 | ||
| ACY | 6.86 | 5-nitroacenaphthene | 0.0137 | 0.1029 |
| 4-nitroacenaphthene | 0.0137 | 2.7440 | ||
| 3-nitroacenaphthene | 0.0137 | 0.1372 | ||
| ACE | 4.15 | 4-Nitroacenaphthylene | 0.0830 | - |
| FLU | 10.96 | 3-nitrofluorene | 0.1534 | - |
| 1-nitrofluorene | 10.9600 | - | ||
| 4-nitrofluorene | 0.0329 | - | ||
| 2-nitrofluorene | 0.0110 | - | ||
| PHE | 20.44 | 2 nitroisomers (Not 9-nitrophenanthrene) | - | - |
| 4 nitroisomers (Including 9-nitrophenanthrene) | - | - | ||
| ANT | 2.87 | 1-Nitroanthracene | - | - |
| 2-Nitroanthracene | - | - | ||
| FLA | 5.57 | 2-Nitrofluoranthene | 0.0057 | - |
| 7-Nitrofluoranthene | 0.0057 | - | ||
| 8-Nitrofluoranthene | 0.1671 | 1.3368 | ||
| PYR | 5.8 | 2-nitropyrene | 0.0557 | - |
| 4-nitropyrene | 0.0167 | - | ||
| NAP | ACY | ACE | ANT | PHE | FLU | FLA | PYR | 2-Ring PAHs | 3-Ring PAHs | 4-Ring PAHs | 8- PAHs | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NAP | 1.000 | |||||||||||
| ACY | 0.351 | 1.000 | ||||||||||
| ACE | 0.247 | 0.082 | 1.000 | |||||||||
| ANT | 0.626 ** | 0.005 | 0.290 | 1.000 | ||||||||
| PHE | 0.287 | −0.225 | 0.565 ** | 0.432 * | 1.000 | |||||||
| FLU | 0.457 * | 0.080 | 0.202 | 0.405 * | 0.297 | 1.000 | ||||||
| FLA | −0.355 | −0.230 | −0.287 | −0.347 | −0.260 | −0.470 ** | 1.000 | |||||
| PYR | −0.424 * | −0.246 | −0.261 | −0.485 ** | −0.238 | −0.467 ** | 0.768 ** | 1.000 | ||||
| 2-ring PAHs | 1.000 ** | 0.351 | 0.247 | 0.626 ** | 0.287 | 0.457 * | −0.355 | −0.424 * | 1.000 | |||
| 3-ring PAHs | 0.673 ** | 0.480 ** | 0.619 ** | 0.705 ** | 0.632 ** | 0.478 ** | −0.485 ** | −0.537 ** | 0.673 ** | 1.000 | ||
| 4-ring PAHs | −0.417 * | −0.253 | −0.290 | −0.447 * | −0.264 | −0.498 ** | 0.932 ** | 0.948 ** | −0.417 * | −0.545 ** | 1.000 | |
| 8-PAHs | 0.980 ** | 0.399 * | 0.346 | 0.670 ** | 0.387 * | 0.455 * | −0.311 | −0.381 * | 0.980 ** | 0.780 ** | −0.370 * | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tala, W.; Kraisitnitikul, P.; Chantara, S. Impact of Atmospheric Conditions and Source Identification of Gaseous Polycyclic Aromatic Hydrocarbons (PAHs) during a Smoke Haze Period in Upper Southeast Asia. Toxics 2023, 11, 990. https://doi.org/10.3390/toxics11120990
Tala W, Kraisitnitikul P, Chantara S. Impact of Atmospheric Conditions and Source Identification of Gaseous Polycyclic Aromatic Hydrocarbons (PAHs) during a Smoke Haze Period in Upper Southeast Asia. Toxics. 2023; 11(12):990. https://doi.org/10.3390/toxics11120990
Chicago/Turabian StyleTala, Wittaya, Pavidarin Kraisitnitikul, and Somporn Chantara. 2023. "Impact of Atmospheric Conditions and Source Identification of Gaseous Polycyclic Aromatic Hydrocarbons (PAHs) during a Smoke Haze Period in Upper Southeast Asia" Toxics 11, no. 12: 990. https://doi.org/10.3390/toxics11120990
APA StyleTala, W., Kraisitnitikul, P., & Chantara, S. (2023). Impact of Atmospheric Conditions and Source Identification of Gaseous Polycyclic Aromatic Hydrocarbons (PAHs) during a Smoke Haze Period in Upper Southeast Asia. Toxics, 11(12), 990. https://doi.org/10.3390/toxics11120990








