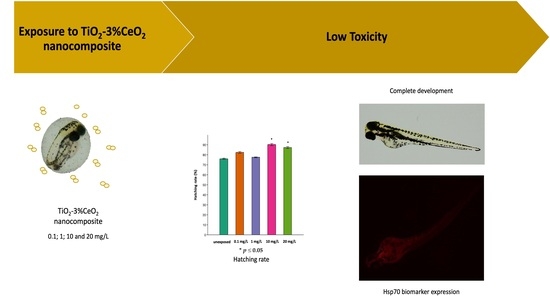
Toxicity of Titanium Dioxide–Cerium Oxide Nanocomposites to Zebrafish Embryos: A Preliminary Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solutions Preparation
2.2. Synthesis and Characterization of Nanocomposites
2.3. Maintenance of Zebrafish and Embryo Collection
2.4. Acute Toxicity Test of TiO2-3%CeO2 on Zebrafish Embryos
2.5. Toxicological Endpoints and DanioScope™ Analysis
2.6. Immunohistochemical Analysis on Larvae
2.7. Statistical Analysis
3. Results
3.1. Toxicological Endpoints on Zebrafish Embryo
3.2. Cardiology and Body Length Measurements Using Danioscope Software
3.3. Immunohistochemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gopalraaj, J.; Manikantan, P.; Arun, M.; Balamuralikrishnan, B.; Anand, A.V. Toxic Effects of Nanoparticles on Fish Embryos. Res. J. Biotechnol. 2021, 16, 12. [Google Scholar] [CrossRef]
- Guinée, J.B.; Heijungs, R.; Vijver, M.G.; Peijnenburg, W.J. Setting the stage for debating the roles of risk assessment and life-cycle assessment of engineered nanomaterials. Nat. Nanotechnol. 2017, 12, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Merinero, M.; Pérez-Aranda, M.; Pérez-Soriano, E.M.; Ortiz, T.; Villamor, E.; Begines, B.; Alcudia, A. Environmental impact of nanoparticles’ application as an emerging technology: A review. Materials 2020, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Baun, A.; Sayre, P.; Steinhäuser, K.G.; Rose, J. Regulatory relevant and reliable methods and data for determining the environmental fate of manufactured nanomaterials. NanoImpact 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Deng, R.; Lin, D.; Zhu, L.; Majumdar, S.; White, J.C.; Gardea-Torresdey, J.L.; Xing, B. Nanoparticle interactions with co-existing contaminants: Joint toxicity, bioaccumulation and risk. Nanotoxicology 2017, 11, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe nanoparticles: Are we there yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef] [PubMed]
- Collin, B.; Auffan, M.; Johnson, A.C.; Kaur, I.; Keller, A.A.; Lazareva, A.; Lead, J.R.; Ma, X.; Merrifield, R.C.; Svendsen, C.; et al. Environmental release, fate and ecotoxicological effects of manufactured ceria nanomaterials. Environ. Sci. Nano 2014, 1, 533–548. [Google Scholar] [CrossRef]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart Res. 2012, 14, 1109. [Google Scholar] [CrossRef]
- Jun, X.I.A.; Zhao, H.Z.; Lu, G.H. Effects of selected metal oxide nanoparticles on multiple biomarkers in Carassius auratus. Biomed. Environ. Sci. 2013, 26, 742–749. [Google Scholar] [CrossRef]
- Gambardella, C.; Mesarič, T.; Milivojević, T.; Sepčić, K.; Gallus, L.; Carbone, S.; Ferrando, S.; Faimali, M. Effects of selected metal oxide nanoparticles on Artemia salina larvae: Evaluation of mortality and behavioural and biochemical responses. Environ. Monit. Assess. 2014, 186, 4249–4259. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, R.; Balsamo, S.A.; D’Urso, L.; Sciré, S.; Brundo, M.V.; Pecoraro, R.; Scalisi, E.M.; Privitera, V.; Impellizzeri, G. CeO2 for water remediation: Comparison of various advanced oxidation processes. Catalysts 2020, 10, 446. [Google Scholar] [CrossRef]
- Correia, A.T.; Rodrigues, S.; Ferreira-Martins, D.; Nunes, A.C.; Ribeiro, M.I.; Antunes, S.C. Multi-biomarker approach to assess the acute effects of cerium dioxide nanoparticles in gills, liver and kidney of Oncorhynchus mykiss. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 238, 108842. [Google Scholar] [CrossRef]
- Rundle, A.; Robertson, A.B.; Blay, A.M.; Butler, K.M.; Callaghan, N.I.; Dieni, C.A.; MacCormack, T.J. Cerium oxide nanoparticles exhibit minimal cardiac and cytotoxicity in the freshwater fish Catostomus commersonii. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 181, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.J.; Jameel, Z.N.; Al-Hussaini, I.H. Review on: Titanium dioxide applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Mamboungou, J.; Canedo, A.; Qualhato, G.; Rocha, T.L.; Vieira, L.G. Environmental risk of titanium dioxide nanoparticle and cadmium mixture: Developmental toxicity assessment in zebrafish (Danio rerio). J. Nanopart. Res. 2022, 24, 186. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Ibrahim, I.; Falaras, P. Sensitizer effects on DSSC performance under pan-illumination conditions. J. Photochem. Photobiol. A 2022, 433, 11420. [Google Scholar] [CrossRef]
- Bodaghi, H.; Mostofi, Y.; Oromiehie, A.; Zamani, Z.; Ghanbarzadeh, B.; Costa, C.; Conte, A.; Del Nobile, M.A. Evaluation of the photocatalytic antimicrobial effects of a TiO2 nanocomposite food packaging film by in vitro and in vivo tests. LWT-Food Sci. Technol. 2013, 50, 702–770. [Google Scholar] [CrossRef]
- Emran, M.Y.; Miran, W.; Gomaa, H.; Ibrahim, I.; Belessiotis, G.V.; Abdelwahab, A.A.; Othman, M.B. Biowaste Materials for Advanced Biodegradable Packaging Technology. In Hand Book of Biodegradable Materials; Springer Nature: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Tobajas, M.; Belver, C.; Rodriguez, J.J. Degradation of emerging pollutants in water under solar irradiation using novel TiO2-ZnO/clay nanoarchitectures. Chem. Eng. J. 2017, 309, 596–606. [Google Scholar] [CrossRef]
- Carbajo, J.; Jiménez, M.; Miralles, S.; Malato, S.; Faraldos, M.; Bahamonde, A. Study of application of titania catalysts on solar photocatalysis: Influence of type of pollutants and water matrices. Chem. Eng. J. 2016, 291, 64–73. [Google Scholar]
- Cantarella, M.; Impellizzeri, G.; Privitera, V. Functional nanomaterials for water purification. Riv. Nuovo Cim. 2017, 40, 595–632. [Google Scholar] [CrossRef]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Iaria, C.; Scalisi, E.M.; Brundo, M.V.; Gulino, V.; Spitaleri, L.; Nicotra, G.; Dattilo, S.; et al. Preferential removal of pesticides from water by molecular imprinting on TiO2 photocatalysts. J. Chem. Eng. 2020, 379, 122309. [Google Scholar] [CrossRef]
- Ayub, K.S.; Zaman, W.Q.; Miran, W.; Ali, M.; Abbas, Z.; Mushtaq, U.; Shahzad, A.; Yang, J. Efficient post-plasma catalytic degradation of toluene via series of Co–Cu/TiO2 catalysts. Res. Chem. Intermed. 2022, 48, 4227–4248. [Google Scholar] [CrossRef]
- Dreno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of titanium dioxide nanoparticles in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, P.; Li, X.; Thuyet, D.Q.; Fan, W. Effect of chronic toxicity of the crystalline forms of TiO2 nanoparticles on the physiological parameters of Daphnia magna with a focus on index correlation analysis. Ecotoxicol. Environ. Saf. 2019, 181, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Rocco, L.; Santonastaso, M.; Nigro, M.; Mottola, F.; Costagliola, D.; Bernardeschi, M.; Guidi, P.; Lucchesi, P.; Scarcelli, V.; Corsi, I.; et al. Genomic and chromosomal damage in the marine mussel Mytilus galloprovincialis: Effects of the combined exposure to titanium dioxide nanoparticles and cadmium chloride. Mar. Environ. Res. 2015, 111, 144–148. [Google Scholar] [CrossRef]
- Gornati, R.; Longo, A.; Rossi, F.; Maisano, M.; Sabatino, G.; Mauceri, A.; Bernardini, G.; Fasulo, S. Effects of titanium dioxide nanoparticle exposure in Mytilus galloprovincialis gills and digestive gland. Nanotoxicology 2016, 10, 807–817. [Google Scholar] [CrossRef]
- Pecoraro, R.; Scalisi, E.M.; Messina, G.; Fragalà, G.; Ignoto, S.; Salvaggio, A.; Zimbone, M.; Impellizzeri, G.; Brundo, M.V. Artemia salina: A microcrustacean to assess engineered nanoparticles toxicity. Microsc. Res. Tech. 2021, 84, 531–536. [Google Scholar] [CrossRef]
- Javanshir Khoei, A.; Rezaei, K. Toxicity of titanium nano-oxide nanoparticles (TiO2) on the pacific oyster, Crassostrea gigas: Immunity and antioxidant defence. Toxin Rev. 2022, 41, 237–246. [Google Scholar] [CrossRef]
- Ignoto, S.; Pecoraro, R.; Scalisi, E.M.; Contino, M.; Ferruggia, G.; Indelicato, S.; Fiorenza, R.; Balsamo, S.A.; Impellizzeri, G.; Tiralongo, F.; et al. Spermiotoxicity of Nano-TiO2 Compounds in the Sea Urchin Paracentrotus lividus (Lamarck, 1816): Considerations on Water Remediation. J. Mar. Sci. Eng. 2023, 11, 380. [Google Scholar] [CrossRef]
- Mahjoubian, M.; Naeemi, A.S.; Sheykhan, M. Toxicological effects of Ag2O and Ag2CO3 doped TiO2 nanoparticles and pure TiO2 particles on zebrafish (Danio rerio). Chemosphere 2021, 263, 128182. [Google Scholar] [CrossRef] [PubMed]
- Clemente, Z.; Castro, V.L.S.S.; Moura, M.A.M.; Jonsson, C.M.; Fraceto, L.F. Toxicity assessment of TiO2 nanoparticles in zebrafish embryos under different exposure conditions. Aquat. Toxicol. 2014, 147, 129–139. [Google Scholar] [CrossRef]
- Tang, T.; Zhang, Z.; Zhu, X. Toxic effects of TiO2 NPs on zebrafish. Int. J. Environ. Res. Public Health 2019, 16, 523. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Scalisi, E.M.; Pecoraro, R.; Cardaci, V.; Privitera, A.; Truglio, E.; Capparucci, F.; Jarosava, J.; Salvaggio, A.; Caraci, F.; et al. Effects of carnosine on the embryonic development and TiO2 nanoparticles-induced oxidative stress on Zebrafish. Front. Vet. Sci. 2023, 10, 1148766. [Google Scholar] [CrossRef] [PubMed]
- Lee-Estevez, M.; Figueroa, E.; Cosson, J.; Short, S.E.; Valdebenito, I.; Ulloa-Rodríguez, P.; Farias, J.G. Zebrafish as a useful model for immunological research with potential applications in aquaculture. Rev. Aquac. 2018, 10, 213–223. [Google Scholar] [CrossRef]
- Choi, T.Y.; Choi, T.I.; Lee, Y.R.; Choe, S.K.; Kim, C.H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Ward, A.C. Zebrafish as a model to evaluate nanoparticle toxicity. Nanomaterials 2018, 8, 561. [Google Scholar] [CrossRef]
- Metscher, B.D.; Ahlberg, P.E. Zebrafish in context: Uses of a laboratory model in comparative studies. Dev. Biol. 1999, 210, 1–14. [Google Scholar] [CrossRef]
- Meyers, J.R. Zebrafish: Development of a vertebrate model organism. Curr. Protoc. Essent. Lab. 2018, 16, e19. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dynam. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Pecoraro, R.; Salvaggio, A.; Marino, F.; Di Caro, G.; Capparucci, F.; Lombardo, B.M.; Messina, G.; Scalisi, E.M.; Tummino, M.; Loreto, F.; et al. Metallic nano-composite toxicity evaluation by zebrafish embryo toxicity test with identification of specific exposure biomarkers. Curr. Protoc. Toxicol. 2017, 74, 1–14. [Google Scholar] [CrossRef]
- Braunbeck, T.; Kais, B.; Lammer, E.; Otte, J.; Schneider, K.; Stengel, D.; Strecker, R. The fish embryo test (FET): Origin, applications, and future. Environ. Sci. Pollut. Res. 2014, 22, 16247–16261. [Google Scholar] [CrossRef]
- Pereira, A.C.; Gomes, T.; Machado, M.R.F.; Rocha, T.L. The zebrafish embryotoxicity test (ZET) for nanotoxicity assessment: From morphological to molecular approach. Environ. Pollut. 2019, 252, 1841–1853. [Google Scholar] [CrossRef]
- Hou, J.; Liu, H.; Wang, L.; Duan, L.; Li, S.; Wang, X. Molecular toxicity of metal oxide nanoparticles in Danio rerio. Environ. Sci. Technol. 2018, 52, 7996–8004. [Google Scholar] [CrossRef]
- Bugel, S.M.; Tanguay, R.L.; Planchart, A. Zebrafish: A marvel of high through put biology for 21st century toxicology. Curr. Environ. Health. Rep. 2014, 1, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Horzmann, K.A.; Freeman, J.L. Making waves: New developments in toxicology with the zebrafish. Toxicol. Sci. 2018, 163, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Bellardita, M.; Fiorenza, R.; D’Urso, L.; Spitaleri, L.; Gulino, A.; Compagnini, G.; Scirè, S.; Palmisano, L. Exploring the Photothermo-Catalytic Performance of Brookite TiO2-CeO2 Composites. Catalysts 2020, 10, 765. [Google Scholar] [CrossRef]
- Fiorenza, R.; Bellardita, M.S.; Balsamo, A.; Spitaleri, L.; Gulino, A.; Conderelli, M.; D’Urso, L.; Scirè, S.; Palmisano, L. A solar photothermocatalytic approach for the CO2 conversion: Investigation of different synergisms on CoO-CuO/brookiteTiO2-CeO2 catalysts. Chem. Eng. J. 2022, 428, 131249. [Google Scholar] [CrossRef]
- Fiorenza, R.; Bellardita, M.; Balsamo, S.A.; Gulino, A.; Condorelli, M.; Compagnini, G.; Scirè, S.; Palmisano, L. A solar photothermo-catalytic combined process for the VOCs combustion and the subsequent CO2 valorization using noble metal-free catalysts. Catal. Today 2023, 413–415, 113949. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th ed.; University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Fiorenza, R.; Spitaleri, L.; Gulino, A.; Sciré, S. High-performing au-ag bimetallic catalysts supported on macro-mesoporous CeO2 for preferential oxidation of CO in H2-rich gases. Catalysts 2020, 10, 49. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test, OECD Guidelines for the Testing of Chemicals; Section 2; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Sobanska, M.; Scholz, S.; Nyman, A.M.; Cesnaitis, R.; Gutierrez, A.S.; Klüver, N.; De Coen, W. Applicability of the fish embryo acute toxicity (FET) test (OECD 236) in the regulatory context of registration, evaluation, authorisation, and restriction of chemicals (REACH). Environ. Toxicol. Chem. 2018, 37, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.T.; Bartman, T. Analysis of heart valve development in larval zebrafish. Dev. Dyn. 2009, 238, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Stainier, D.Y.; Lee, R.K.; Fishman, M.C. Cardiovascular development in the zebrafish. I. Myocardial fate map and heart tube formation. Development 1993, 119, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Beis, D.; Bartman, T.; Jin, S.W.; Scott, I.C.; D’Amico, L.A.; Ober, E.A.; Verkade, H.; Frantsve, J.; Field, H.A.; Wehman, A.; et al. Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development 2005, 132, 4193–4204. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Browning, L.M.; Nallathamby, P.D.; Desai, T.; Cherukuri, P.K.; Xu, X.H. In vivo quantitative study of sized-dependent transport and toxicity of single silver nanoparticles using zebrafish embryos. Chem Res Toxicol. 2012, 25, 1029–1046. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, Y.L.; Song, C.; Wu, L.L.; Gao, H.W. Interactions of hydroxyapatite with proteins and its toxicological effect to zebrafish embryos development. PLoS ONE 2012, 7, e32818. [Google Scholar] [CrossRef]
- Dziegiel, P. Expression of metallothioneins in tumor cells. Pol. J. Pathol. 2004, 55, 3–12. [Google Scholar] [PubMed]
- Krug, H.F.; Wick, P. Nanotoxicology: An interdisciplinary challenge. Angew. Chem. Int. Ed. 2011, 50, 1260–1278. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 65. [Google Scholar] [CrossRef]
- Kaur, J.; Sohal, I.S.; Singh, H.; Gupta, N.K.; Sehrawat, S.; Puri, S.; Bello, D.; Khatri, M. Toxicity screening and ranking of diverse engineered nanomaterials using established hierarchical testing approaches with a complementary in vivo zebrafish model. Environ. Sci. Nano 2022, 9, 2726–2749. [Google Scholar] [CrossRef]
- Pecoraro, R.; Scalisi, E.M.; Iaria, C.; Capparucci, F.; Rizza, M.T.; Ignoto, S.; Salvaggio, A.; Fiorenza, R.; Impellizzeri, G.; Brundo, M.V. Toxicological assessment of CeO2 nanoparticles on early development of zebrafish. Toxicol. Res. 2021, 10, 570–578. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, Z.; Zhai, Y.; Zhang, F.; Vijver, M.G.; Peijnenburg, W.J. Effects of humic substances on the aqueous stability of cerium dioxide nanoparticles and their toxicity to aquatic organisms. Sci. Total Environ. 2021, 781, 146583. [Google Scholar] [CrossRef] [PubMed]
- Hermsen, S.A.B.; van den Brandhof, E.J.; van der Ven, L.T.M.; Piersma, A.H. Relative embryotoxicity of two classes of chemicals in a modified zebrafish embryotoxicity test and comparison with their in vivo potencies. Toxicol. Vitr. 2011, 25, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Samaee, S.M.; Rabbani, S.; Jovanović, B.; Mohajeri-Tehrani, M.R.; Haghpanah, V. Efficacy of the hatching event in assessing the embryo toxicity of the nano-sized TiO2 particles in zebrafish: A comparison between two different classes of hatching-derived variables. Ecotoxicol. Environ. Saf. 2015, 116, 121–128. [Google Scholar] [CrossRef]
- Scalisi, E.M.; Pecoraro, R.; Salvaggio, A.; Capparucci, F.; Fortuna, C.G.; Zimbone, M.; Impellizzeri, G.; Brundo, M.V. Titanium Dioxide Nanoparticles: Effects on Development and Male Reproductive System. Nanomaterials 2023, 13, 1783. [Google Scholar] [CrossRef]
- Baker, K.; Warren, K.S.; Yellen, G.; Fishman, M.C. Defective “pacemaker” current (I h) in a zebrafish mutant with a slow heart rate. Proc. Natl. Acad. Sci. USA 1997, 94, 4554–4559. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhao, Y.; Xia, T.; Meng, H.; Ji, Z.; Liu, R.; George, S.; Xiong, S.; Wang, X.; Zhang, H.; et al. High content screening in zebrafish speeds up hazard ranking of transition metal oxide nanoparticles. ACS Nano 2011, 5, 7284–7295. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.J.; Verdon, R.; Gillies, S.; Brown, D.M.; Fernandes, T.F. Adoption of in vitro systems and zebrafish embryos as systems and zebrafish embryos as alternative models for reducing rodent use in assessments of immunological and oxidative stress responses to nanoparticles. Crit. Rev. Toxicol. 2018, 48, 252–271. [Google Scholar] [CrossRef]
- Olasagasti, M.; Gatti, A.M.; Capitani, F.; Barranco, A.; Pardo, M.A.; Escuredo, K.; Rainieri, S. Toxic effects of colloidal nanosilver in zebrafish embryos. J. Appl. Toxicol. 2014, 34, 562–575. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecoraro, R.; Scalisi, E.M.; Indelicato, S.; Contino, M.; Coco, G.; Stancanelli, I.; Capparucci, F.; Fiorenza, R.; Brundo, M.V. Toxicity of Titanium Dioxide–Cerium Oxide Nanocomposites to Zebrafish Embryos: A Preliminary Evaluation. Toxics 2023, 11, 994. https://doi.org/10.3390/toxics11120994
Pecoraro R, Scalisi EM, Indelicato S, Contino M, Coco G, Stancanelli I, Capparucci F, Fiorenza R, Brundo MV. Toxicity of Titanium Dioxide–Cerium Oxide Nanocomposites to Zebrafish Embryos: A Preliminary Evaluation. Toxics. 2023; 11(12):994. https://doi.org/10.3390/toxics11120994
Chicago/Turabian StylePecoraro, Roberta, Elena Maria Scalisi, Stefania Indelicato, Martina Contino, Giuliana Coco, Ilenia Stancanelli, Fabiano Capparucci, Roberto Fiorenza, and Maria Violetta Brundo. 2023. "Toxicity of Titanium Dioxide–Cerium Oxide Nanocomposites to Zebrafish Embryos: A Preliminary Evaluation" Toxics 11, no. 12: 994. https://doi.org/10.3390/toxics11120994
APA StylePecoraro, R., Scalisi, E. M., Indelicato, S., Contino, M., Coco, G., Stancanelli, I., Capparucci, F., Fiorenza, R., & Brundo, M. V. (2023). Toxicity of Titanium Dioxide–Cerium Oxide Nanocomposites to Zebrafish Embryos: A Preliminary Evaluation. Toxics, 11(12), 994. https://doi.org/10.3390/toxics11120994