Effects of Cyfluthrin Exposure on Neurobehaviour, Hippocampal Tissue and Synaptic Plasticity in Wistar Rats
Abstract
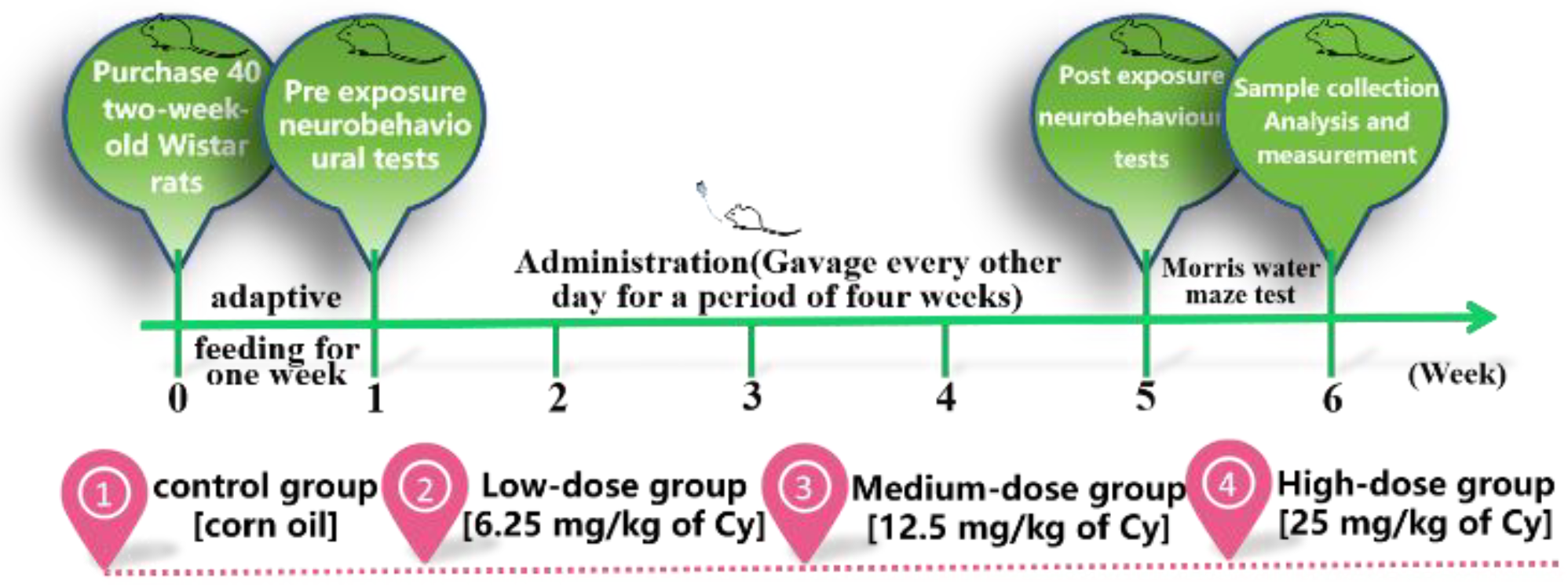
:1. Background
2. Material and Method
2.1. Experimental Reagent and Dose Selection
2.2. Animals
2.3. Tissue Sampling
2.4. Neurobehavioural Tests
2.4.1. Open Field Experiment
2.4.2. Novel Object Recognition Test
2.4.3. Elevated plus Maze Test
2.4.4. Morris Water Maze Test
2.5. HE/Nissl Staining
2.6. Immunohistochemistry
2.7. Transmission Electron Microscopy
2.8. Western Blot
2.9. Q-PCR
2.10. ATP/Glu Test
2.11. Statistical Analysis
3. Results
3.1. General Changes in Rats Exposed to Cy
3.2. Changes in Learning and Memory Ability in Cy-Exposed Rats
3.2.1. Open Field Experiment
3.2.2. Novel Object Recognition Test
3.2.3. Elevated plus Maze Test
3.2.4. Morris Water Maze Test
- (1)
- Positioning navigation phase
- (2)
- Spatial exploration phase
3.3. Hippocampal Neuron Injury in Rats Exposed to Cy
3.3.1. HE Staining
3.3.2. Nissl Staining
3.3.3. Electron Microscopy
3.4. Impairment of Hippocampal Neuron Plasticity Induced by Cy Exposure in Rats
3.5. Changes in the Levels of Inflammatory Factors and A2AR-Related Factors in the Neurons of Rats Exposed to Cy
3.5.1. Expression of Inflammatory Factors in the Hippocampus of Cy-Exposed Rats
3.5.2. Expression of A2AR in the Hippocampus of Cy-Exposed Rats
3.6. Correlation Analysis of A2AR with Neurobehavioural Indices, Inflammatory Factors Levels and Synaptic Plasticity-Related Factor Levels
4. Discussion
4.1. Cy Exposure Can Cause Neurobehavioural Changes in Rats
4.2. Cy Exposure Can Cause Morphological Changes in the Rat Hippocampus
4.3. Cy Exposure Can Cause Abnormal Changes in the Inflammatory Response and Adenosine A2AR Expression in the Rat Hippocampus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Cy | Cyfluthrin |
| A2AR | Adenosine2a receptor |
| ATP | Adenosine tri phosphate |
| Glu | Glutamate |
| Q-PCR | Real-time Quantitative PCR |
| HE | Hematoxylin-eosin |
| ANOVA | Analysis of variance |
References
- Lu, Y.C.; Liang, W.Z.; Kuo, C.C.; Hao, L.J.; Chou, C.T.; Jan, C.R. Action of the insecticide cyfluthrin on Ca signal transduction and cytotoxicity in human osteosarcoma cells. Hum. Exp. Toxicol. 2020, 39, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO; AGP. Manual on the Development and Use of FAO Specifications for Plant Protection Products; FAO: Rome, Italy, 1999. [CrossRef]
- Fatma, H.; Tefide, K. Pesticide residues and health risk appraisal of tomato cultivated in greenhouse from the Mediterranean region of Turkey. Environ. Sci. Pollut. Res. Int. 2021, 28, 22551–22562. [Google Scholar] [CrossRef]
- Marsha, K. Dietary Pyrethroid Exposures and Intake Doses for 188 Duplicate-Single Solid Food Items Consumed by North Carolina Adults. Toxics 2020, 8, 6. [Google Scholar] [CrossRef]
- Ni, W.; Gao, H.X.; Wu, B.; Zhao Ji Sun, J.; Song, Y.N.; Shi, Y.P.; Yang, H.F. Gestational Exposure to Cyfluthrin through Endoplasmic Reticulum (ER) Stress-Mediated PERK Signaling Pathway Impairs Placental Development. Toxics 2022, 10, 733. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ma, L.Y.; Xie, Y.X.; Zhu, L.Q.; Ni, W.S.; Wang, R.; Song, Y.N.; Li, X.Y.; Yang, H.F. The role of stimulator of interferon genes-mediated AMPK/mTOR/P70S6K autophagy pathway in cyfluthrin-induced testicular injury. Environ. Toxicol. 2022, 38, 727–742. [Google Scholar] [CrossRef]
- Mousavi, L.; Zadeh-hashem, E.; Imani, M. β-Cyfluthrin-Mediated Cytotoxicity of Cultured Rat Primary Hepatocytes Ameliorated by Cotreatment with Luteolin. Evid. -Based Complement. Altern. Med. Ecam 2022, 2022, 3647988. [Google Scholar] [CrossRef]
- Zhi, X.Y.; Wu, B.; Dong, L.J.; Zhao, J.; Yang, H.F. Analysis on the current status and influencing factors of neurological subhealth among vegetable greenhouse growers in the suburbs of Yinchuan City. J. Ningxia Med. Univ. 2017, 39, 293–298. [Google Scholar]
- Deepika, D.; Kumar, S.; Bravo, N.; Esplugas, R.; Capodiferro, M.; Sharma, R.P.; Schuhmacher, M.; Grimalt, J.O.; Blanco, J.; Kumar, V. Chlorpyrifos, permethrin and cyfluthrin effect on cell survival, permeability, and tight junction in an in-vitro model of the human blood-brain barrier (BBB). Neurotoxicology 2022, 93, 152–162. [Google Scholar] [CrossRef]
- Vanacker, M.; Quindroit, P.; Angeli, K.; Mandin, C.; Glorenne, P.; Brochot, C.; Crépet, C. Aggregate and cumulative chronic risk assessment for pyrethroids in the French adult population. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 143, 111519. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, R.; Luo, S.; Liu, Y.; Jin, Y.; Li, Y.; Li, B.; Chen, Y.; Jia, L.; Yuan, X. Combined effects of chlorpyrifos and cyfluthrin on neurobehavior and neurotransmitter levels in larval zebrafish. J. Appl. Toxicol. JAT 2022, 42, 1662–1670. [Google Scholar] [CrossRef]
- Rodríguez, J.L.; Ares, I.; Martínez, M.; Martínez-Larrañaga, M.R.; Anadón, A.; Martínez, M.A. Bioavailability and nervous tissue distribution of pyrethroid insecticide cyfluthrin in rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 118, 220–226. [Google Scholar] [CrossRef]
- Qin, X.; Li, L.; Nie, X.; Niu, Q. Effects of Chronic Aluminum Lactate Exposure on Neuronal Apoptosis and Hippocampal Synaptic Plasticity in Rats. Biol. Trace Elem. Res. 2020, 197, 571–579. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J.A. A new one-trial test for neurobiological studies of memory in rats. I. Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Belali, R.; Mard, S.A.; Khoshnam, S.E.; Bavarsad, K.; Sarkaki, A.; Farbood, Y. Anandamide Attenuates Neurobehavioral Deficits and EEG Irregularities in the Chronic Sleep Deprivation Rats: The Role of Oxidative Stress and Neuroinflammation. Neurochem. Res. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Knight, P.; Chellian, R.; Wilson, R.; Behnood-Rod, A.; Panunzio, S.; Bruijnzeel, A.W. Sex differences in the elevated plus-maze test and large open field test in adult Wistar rats. Pharmacol. Biochem. Behav. 2021, 204, 173168. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, N.; Mohammad Valipour, S.; Nooritahneh, A.; Motaghinejad, M.; Motevalian, M.; Safari, S.; Gholami, M.; Vatandour, S.; Hekmati, M. Pharmacological Evidences for Curcumin Neuroprotective Effects against Lead-Induced Neurodegeneration: Possible Role of Akt/GSK3 Signaling Pathway. Iran. J. Pharm. Res. IJPR 2020, 19, 494–508. [Google Scholar] [CrossRef]
- Jamal, M.; Ameno, K.; Ameno, S.; Morishita, J.; Wang, W.; Kumihashi, M.; Ikuo, U.; Miki, T.; Ijiri, I. Changes in cholinergic function in the frontal cortex and hippocampus of rat exposed to ethanol and acetaldehyde. Neuroscience 2007, 144, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Zhao, J.; Li, Y.; He, L.X.; Huang, Y.J.; Shu, W.Q.; Cao, J.; Liu, W.B.; Liu, J.Y. Gene expression network regulated by DNA methylation and microRNA during microcystin-leucine arginine induced malignant transformation in human hepatocyte L02 cells. Toxicol. Lett. 2018, 289, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Adedara, I.A.; Owumi, S.E.; Oyelere, A.K.; Farombi, E.O. Neuroprotective role of gallic acid in aflatoxin B -induced behavioral abnormalities in rats. J. Biochem. Mol. Toxic. 2021, 35, e22684. [Google Scholar] [CrossRef]
- Levin, E.D.; Uemura, E.; Bowman, R.E. Neurobehavioral toxicology of halothane in rats. Neurotoxicol Teratol. 1991, 13, 461–470. [Google Scholar] [CrossRef]
- Liao, Z.; Huang, Z.; Li, J.; Li, H.; Miao, L.; Liu, Y.; Zhang, J.; Xu, Y.; Li, Y. Regulation of CRMP2 by Cdk5 and GSK-3β participates in sevoflurane-induced dendritic development abnormalities and cognitive dysfunction in developing rats. Toxicol. Lett. 2021, 341, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.D.S.; de Souza Oliveira Tavares, C.; Araujo, B.H.S.; Mansur, F.; Lopes-Martins, R.Á.B.; Gomes da Silva, S. Improved Spatial Memory And Neuroinflammatory Profile Changes in Aged Rats Submitted to Photobiomodulation Therapy. Cell Mol. Neurobiol. 2022, 42, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.F.; Ross, D.G.; Starr, J.M.; Scollon, E.J.; Wolansky, M.J.; Crofton, K.M.; Devito, M.J. Environmentally relevant pyrethroid mixtures: A study on the correlation of blood and brain concentrations of a mixture of pyrethroid insecticides to motor activity in the rat. Toxicology 2016, 359, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Calipari, E.S. Dopamine Release in the Midbrain Promotes Anxiety. Biol. Psychiat 2020, 88, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Commins, S.; Duffin, J.; Chaves, K.; Leahy, D.; Corcoran, K.; Caffrey, M.; Keenan, L.; Finan, D.; Thornberry, C. NavWell: A simplified virtual-reality platform for spatial navigation and memory experiments. Behav. Res. Methods 2020, 52, 1189–1207. [Google Scholar] [CrossRef] [PubMed]
- Bromley-Brits, K.; Deng, Y.; Song, W. Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J. Vis. Exp. JoVE 2011, 20, 2920. [Google Scholar] [CrossRef] [PubMed]
- Syed, F.; Chandravanshi, L.P.; Khanna, V.K.; Soni, I. Beta-cyfluthrin induced neurobehavioral impairments in adult rats. Chem-Biol. Interact. 2016, 243, 19–28. [Google Scholar] [CrossRef]
- Lu, D.; Yu, L.; Li, M.; Zhai, Q.; Tian, F.; Chen, W. Behavioral disorders caused by nonylphenol and strategies for protection. Chemosphere 2021, 275, 129973. [Google Scholar] [CrossRef] [PubMed]
- Piavchenko, G.; Soldatov, V.; Venediktov, A.; Kartashkina, N.; Novikova, N.; Gorbunova, M.; Boronikhina, T.; Yatskovskiy, A.; Meglinski, I.; Kuznetsov, S. A combined use of silver pretreatment and impregnation with consequent Nissl staining for cortex and striatum architectonics study. Front. Neuroanat. 2022, 16, 940993. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, H.; Liu, W.; Liu, X.; Jiang, X.; Li, X.; Wu, Q.; Xu, Z.; Zhao, Q. Arctium lappa L. roots ameliorates cerebral ischemia through inhibiting neuronal apoptosis and suppressing AMPK/mTOR-mediated autophagy. Phytomed. Int. J. Phytother. Phytopharm. 2021, 85, 153526. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, S.W.; Wen, S.Q.; Zhu, Q.P.; Wang, L.; Zou, H.; Gu, J.H.; Liu, X.Z.; Bian, J.C.; Liu, Z.P. Alpha-Lipoic Acid Attenuates Cadmium- and Lead-Induced Neurotoxicity by Inhibiting Both Endoplasmic-Reticulum Stress and Activation of Fas/FasL and Mitochondrial Apoptotic Pathways in Rat Cerebral Cortex. Neurotox. Res. 2021, 39, 1103–1115. [Google Scholar] [CrossRef]
- Chen, Z.; Yuan, Z.; Yang, S.; Zhu, Y.; Xue, M.; Zhang, J.; Leng, L. Brain Energy Metabolism: Astrocytes in Neurodegenerative Diseases. Cns Neurosci. Ther. 2023, 29, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Gross, I.; Tschigor, T.; Salman, A.L.; Yang, F.; Luo, J.; Vonk, D.; Hipp, M.S.; Neidhardt, J.; Bräuer, A.U. Systematic expression analysis of plasticity-related genes in mouse brain development brings PRG4 into play. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2022, 251, 714–728. [Google Scholar] [CrossRef] [PubMed]
- De la Torre-Iturbe, S.; Vázquez-Roque, R.A.; De la Cruz-López, F.; Flores, G.; Garcés-Ramírez, L. Dendritic and behavioral changes in rats neonatally treated with homocysteine; A proposal as an animal model to study the attention deficit hyperactivity disorder. J. Chem. Neuroanat. 2022, 119, 102057. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Chen, J.; Hu, P.; Jia, M.; Sun, J.H.; Feng, H.Y.; Qiao, F.C.; Zang, Y.Y.; Shi, Y.Y.; Chen, G.; et al. Enhancing GluN2A-type NMDA receptors impairs long-term synaptic plasticity and learning and memory. Mol. Psychiatr. 2022, 27, 3468–3478. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Chiu, S.L.; Zhu, Q.; Huganir, R.L. GRIP1 regulates synaptic plasticity and learning and memory. Proc. Natl. Acad. Sci. USA 2020, 117, 25085–25091. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. Synaptic Plasticity, Dementia and Alzheimer Disease. CNS Neurol. Disord. Drug Targets 2017, 16, 220–233. [Google Scholar] [CrossRef]
- Toledano, A.; Álvarez, M.I.; Toledano-Díaz, A.; Merino, J.J.; Rodríguez, J.J. Brain local and regional neuroglial alterations in Alzheimer’s Disease: Cell types, responses and implications. Curr. Alzheimer Res. 2016, 13, 321–342. [Google Scholar] [CrossRef]
- Xu, T.; Liu, J.; Li, X.R.; Yu, Y.; Luo, X.; Zheng, X.; Cheng, Y.; Yu, P.Q.; Liu, Y. The mTOR/NF-κB Pathway Mediates Neuroinflammation and Synaptic Plasticity in Diabetic Encephalopathy. Mol. Neurobiol. 2021, 58, 3848–3862. [Google Scholar] [CrossRef]
- Deng, D.; Cui, Y.; Gan, S.; Xie, Z.; Cui, S.; Cao, K.; Wang, S.; Shi, G.; Yang, L.; Bai, S.; et al. Sinisan alleviates depression-like behaviors by regulating mitochondrial function and synaptic plasticity in maternal separation rats. Phytomed. Int. J. Phytother. Phytopharm. 2022, 106, 154395. [Google Scholar] [CrossRef]
- Cousin, M.A. Synaptophysin-dependent synaptobrevin-2 trafficking at the presynapse-Mechanism and function. J. Neurochem. 2021, 159, 78–89. [Google Scholar] [CrossRef]
- Yuan, J.; Gao, J.; Su, K.Q.; Feng, X.D. Electroacupuncture improves learning and memory impairment and enhances hippocampal synaptic plasticity through BDNF/TRKB/CREB signaling pathway in cerebral ischemia-reperfusion injury rats. Zhen Ci Yan Jiu = Acupunct. Res. 2023, 48, 843–851. [Google Scholar] [CrossRef]
- Rodríguez, J.L.; Ares, I.; Castellano, V.; Martínez, M.; Martínez-Larrañaga, M.R.; Anadón, A.; Martínez, M.A. Effects of exposure to pyrethroid cyfluthrin on serotonin and dopamine levels in brain regions of male rats. Env. Res. 2016, 146, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, N.; Chen, J.; Zheng, P.; Niu, J.; Tang, S.; Peng, X.; Wu, J.; Yu, J.; Ma, L. Neuropharmacological insight into preventive intervention in posttraumatic epilepsy based on regulating glutamate homeostasis. Cns Neurosci. Ther. 2023, 29, 2430–2444. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, H.; Liang, J.; Huang, J.; Chen, N. Exercise suppresses neuroinflammation for alleviating Alzheimer’s disease. J. Neuroinflamm. 2023, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.M.; Huang, Y.; Wan, P.P.; Lu, Y.H.; Zhou, N.; Li, J.J.; Yu, C.Y.; Chou, J.J.; Zhang, L.; Zhang, C.; et al. Ursolic Acid Protects Neurons in Temporal Lobe Epilepsy and Cognitive Impairment by Repressing Inflammation and Oxidation. Front. Pharmacol. 2022, 13, 877898. [Google Scholar] [CrossRef] [PubMed]
- Sayad-Fathi, S.; Zaminy, A.; Babaei, P.; Yousefbeyk, F.; Azizi, N.; Nasiri, E. The methanolic extract of Cinnamomum zeylanicum bark improves formaldehyde-induced neurotoxicity through reduction of phospho-tau (Thr231), inflammation, and apoptosis. Excli J. 2020, 19, 671–686. [Google Scholar]
- Zeng, Y.; Fang, Q.; Chen, J.; Wang, Y.; Liu, X.; Zhang, X.; Shi, Y.; Zhan, H.; Zhong, X.; Yao, M.; et al. Melatonin Improves Mitochondrial Dysfunction and Attenuates Neuropathic Pain by Regulating SIRT1 in Dorsal Root Ganglions. Neuroscience 2023, 534, 29–40. [Google Scholar] [CrossRef]
- Magalhães, C.A.; Ferreira, C.N.; Loures, C.M.G.; Fraga, V.G.; Chaves, A.C.; Oliveira, A.C.R.; de Souza, L.C.; Resende, E.P.F.; Carmona, K.C.; Guimarães, H.C.; et al. Leptin, hsCRP, TNF-α and IL-6 levels from normal aging to dementia: Relationship with cognitive and functional status. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2018, 56, 150–155. [Google Scholar] [CrossRef]
- Shang, J.; Wang, Q.; Tian, C.; Zhang, R.; Zhao, S.; Zhou, X. Study on effect of beta-cypermethrin on expression of TH and TNF-α in rat brain tissue. Occup. Health 2019, 35, 2187–2190. [Google Scholar]
- Temido-Ferreira, M.; Ferreira, D.G.; Batalha, V.L.; Marques-Morgado, I.; Coelho, J.E.; Pereira, P.; Gomes, R.; Pinto, A.; Carvalho, S.; Canas, P.M.; et al. Age-related shift in LTD is dependent on neuronal adenosine A receptors interplay with mGluR5 and NMDA receptors. Mol. Psychiatr. 2020, 25, 1876–1900. [Google Scholar] [CrossRef]
- Silvia, V.D.S.; Haberl, M.G.; Zhang, P.; Bethge, P.; Lemos, C.; Gon Alves, N.; Gorlewicz, A.; Malezieux, M.; Gon Alves, F.Q.; Grosjean, N.L. Early synaptic deficits in the APP/PS1 mouse model of Alzheimer’s disease involve neuronal adenosine A2A receptors. Nat. Commun. 2016, 7, 11915. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Pedata, F. Modulation of ischemic brain injury and neuroinflammation by adenosine A2A receptors. Curr. Pharm. Des. 2008, 14, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Dong, H.; Zhang, H.; Wang, S.; Hou, L.; Chen, S.; Zhang, J.; Xiong, L. Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Res. 2012, 1459, 81–90. [Google Scholar] [CrossRef] [PubMed]










| Antibody Name | Company |
|---|---|
| Rabbit Anti-A2AR (ab3461) | Abcam (Cambridge, UK) |
| Rabbit Anti-PSD-95 (ab76115) | Abcam (Cambridge, UK) |
| Rabbit Anti-SYP (ab32127) | Abcam (Cambridge, UK) |
| Rabbit Anti-IL-6 | Affinity (Boston, MA, USA) |
| Rabbit Anti-TNF-α | Affinity (Boston, MA, USA) |
| Rabbit Anti-GAPDH | Bioss (Beijing, China) |
| Forward Primer | Reverse Primer | Species | |
|---|---|---|---|
| A2AR | GAAAGACGGGAACTCCACGAAGAC | GGCAGTAACACGAACGCAAAGAAG | Rat |
| PSD-95 | TCCAGTCTGTGCGAGAGGTAGC | GGACGGATGAAGATGGCGATGG | Rat |
| SYP | GCTGTGTTTGCCTTCCTCTACTC | TGATAATGTTCTCTGGGTCCGTG | Rat |
| IL-6 | ACTTCCAGCCAGTTGCCTTCTTG | TGGTCTGTTGTGGGTGGTATCCTC | Rat |
| TNF-α | AAAGGACACCATGAGCACGGAAAG | CGCCACGAGCAGGAATGAGAAG | Rat |
| Experimental Category | Indicators | Pearson Correlation Coefficient r | p |
|---|---|---|---|
| Open field test | |||
| Time spent in the central zone | 0.7154 | <0.001 | |
| Grooming time | −0.6354 | 0.001 | |
| Number of rearings | −0.4381 | 0.032 | |
| Number of grid crossings | −0.5682 | 0.004 | |
| Novel object recognition test | |||
| Discrimination index | −0.6896 | <0.001 | |
| Elevated plus maze test | |||
| Time spent in the open arms | −0.375 | 0.071 | |
| Number of open-arm entries | −0.4633 | 0.023 | |
| Water maze test | |||
| Average escape latency | 0.7000 | <0.001 | |
| Time spent in the target quadrant | −0.6044 | 0.002 | |
| Number of platform crossings | −0.6364 | 0.001 | |
| Inflammation-related factors | |||
| IL-6 | 0.8612 | <0.001 | |
| TNF | 0.8433 | 0.001 | |
| Synaptic plasticity-related factors | |||
| PSD-95 | −0.6377 | 0.026 | |
| SYP | −0.7935 | 0.002 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Zhao, J.; Li, X.; Sun, J.; Yang, H. Effects of Cyfluthrin Exposure on Neurobehaviour, Hippocampal Tissue and Synaptic Plasticity in Wistar Rats. Toxics 2023, 11, 999. https://doi.org/10.3390/toxics11120999
Xie Y, Zhao J, Li X, Sun J, Yang H. Effects of Cyfluthrin Exposure on Neurobehaviour, Hippocampal Tissue and Synaptic Plasticity in Wistar Rats. Toxics. 2023; 11(12):999. https://doi.org/10.3390/toxics11120999
Chicago/Turabian StyleXie, Yongxin, Ji Zhao, Xiaoyu Li, Jian Sun, and Huifang Yang. 2023. "Effects of Cyfluthrin Exposure on Neurobehaviour, Hippocampal Tissue and Synaptic Plasticity in Wistar Rats" Toxics 11, no. 12: 999. https://doi.org/10.3390/toxics11120999
APA StyleXie, Y., Zhao, J., Li, X., Sun, J., & Yang, H. (2023). Effects of Cyfluthrin Exposure on Neurobehaviour, Hippocampal Tissue and Synaptic Plasticity in Wistar Rats. Toxics, 11(12), 999. https://doi.org/10.3390/toxics11120999






