Abstract
The aims of this study were to characterize the exposure of pregnant women living in Portugal to 3-phenoxybenzoic acid (3-PBA) and to evaluate the association of this exposure with maternal outcomes and newborn anthropometric measures. We also aimed to compare exposure in summer with exposure in winter. Pregnant women attending ultrasound scans from April 2018 to April 2019 at a central hospital in Porto, Portugal, were invited to participate. Inclusion criteria were: gestational week between 10 and 13, confirmed fetal vitality, and a signature of informed consent. 3-PBA was measured in spot urine samples by gas chromatography with mass spectrometry (GC-MS). The median 3-PBA concentration was 0.263 (0.167; 0.458) µg/g creatinine (n = 145). 3-PBA excretion was negatively associated with maternal pre-pregnancy body mass index (BMI) (p = 0.049), and it was higher during the summer when compared to winter (p < 0.001). The frequency of fish or yogurt consumption was associated positively with 3-PBA excretion, particularly during the winter (p = 0.002 and p = 0.015, respectively), when environmental exposure is low. Moreover, 3-PBA was associated with levothyroxine use (p = 0.01), a proxy for hypothyroidism, which could be due to a putative 3-PBA—thyroid hormone antagonistic effect. 3-PBA levels were not associated with the anthropometric measures of the newborn. In conclusion, pregnant women living in Portugal are exposed to 3-PBA, particularly during summer, and this exposure may be associated with maternal clinical features.
1. Introduction
Pesticides belong to a large family of compounds used to control insect (insecticides, insect repellents), weeds (herbicides), microbe (fungicides, disinfectants), or mouse and rat (rodenticides) pests [1].
Synthetic pyrethroids (SPs) belong to the class of insecticides and are commonly used. These insecticides derive from natural compounds produced by some species of chrysanthemum flowers, the so-called pyrethrins, and they are used to manage pests both in agriculture and in residences and to reduce the transmission of diseases acquired by insects [2,3].
3-phenoxybenzoic acid (3-PBA) is a general metabolite of several SPs, which is used as a biomarker of SP exposure [4].
In northern Portugal, three studies were carried out to characterize agricultural soil sample contamination with 3-PBA. Those studies showed that 3-PBA was detectable at the range of ng per g of soil, in samples of the north of Portugal [2,5,6]. One other study showed that pyrethroids interfere with the germination and development of plants since their phototoxicity can alter the levels of chlorophyll and carotenoids [4]. The presence of pyrethroids in agricultural soils indicates the relevance of extending monitoring programs for the analysis of these compounds in soils and soil-borne foods [2,5,6].
For example, 75% of fruits and vegetable samples labelled as ‘organic’ (including tomatoes, oranges, grapes, apples, bananas, onions, lettuce, green peppers, carrots, and broccoli) collected from grocery stores in North Carolina, USA, had measurable levels of at least one pyrethroid [7].
The 2018 European Union (EU) report on pesticide residues in food showed that Portugal was one of the countries with the highest maximum residue levels (MRLs) exceeding rates, but none of the residues detected to exceed MRLs were pyrethroids [8].The overall results of this report suggest that the levels of pesticides assessed in the food products analyzed are unlikely to pose a concern for the health of the consumer [8].
Besides food, pyrethroids are also found in pet shampoos, medication used for treating scabies, and topical louse treatments [9].
Exposure to pesticides in the population is widespread, especially via dermal, ingestion, and inhalation routes, and consequently, SPs may enter the food chain, affecting the environment and human health [10].
3-PBA found in the human body may result from absorption of 3-PBA resulting from environmental degradation of several SPs [2], or it may result from the endogenous hydrolysis of SPs by mammalian carboxylesterases (CEs) [11].
Once in the human body, pyrethroid compounds, including 3-PBA, are known to be transported by the placenta, since detectable levels of permethrin were found in cord blood samples collected upon delivery [12].
The developing nervous system is highly susceptible to the neurotoxicity of pesticides, as well as to many types of environmental toxicants. This heightened sensitivity occurs not only during prenatal but also postnatal development, extending into adolescence [13]. Impacts on the developing nervous system can have deleterious effects that last a lifetime, long after exposure has ended, as the toxicant causes alterations of development of the nervous system [14].
Like many other insecticide compounds, pyrethroids are known to be neurotoxic [15]. In fact, intraperitoneal injection of 3-PBA in mice daily for 2 months has shown to induce synuclein aggregation in dopaminergic neurons, which may contribute to dopaminergic neurodegeneration [11]. Given its high lipophilicity, 3-PBA can cross the blood-brain barrier (BBB) and bioaccumulate in the brain, which is rich in lipids [11]. This increases the plausibility of its neurotoxic effects.
Additionally, a recent review provides relevant evidence which confirms that pyrethroids exposure during pregnancy may impact neurodevelopment for example by interference with thyroid hormone (TH) function [15].
Adverse effects on thyroid function warrant caution because THs play an important role in many aspects of human physiology including growth, development, energy metabolism, and reproduction [16,17]. Across vertebrates, particularly during pregnancy and the neonatal period, THs orchestrate metamorphosis, brain development, and metabolism. SPs and their metabolites have structural similarities with THs [18]. These similarities are believed to underlie SPs and 3-PBA interference with nuclear receptors of TH. In fact these compounds have been shown to inhibit TR-mediated gene expression [19]. A study evaluating environmental exposure to pyrethroids and thyroid hormones of pregnant women in Shandong, China, indicated that exposure to pyrethroids was widespread and negatively associated with serum concentrations of free triiodothyronine (FT3) [20]. Nevertheless, Zang et al., in a cohort of women in the first trimester of pregnancy, could not show an association between chemical exposure to pyrethroid pesticides during the early gestation period and maternal thyroid function [21]. There is a need to further explore the effects of pyrethroid exposure on thyroid function in pregnant women.
Some studies have evaluated urinary pyrethroid levels among pregnant women in different regions of the globe. In a randomized trial carried out in Idaho, USA, levels of 3-PBA were measured in 1st trimester pregnant women who received either organic or conventional fruits and vegetables for consumption for 24 weeks. 3-PBA concentrations were significantly higher in urine samples collected from women in the conventional produce group compared to the organic produce group (0.95 vs 0.27 μg/L, p = 0.03) [22]. A study carried out in French pregnant women showed that among 5 pyrethroid metabolites, the urinary concentrations of 3-PBA were the highest with a mean concentration of 0.36 μg/L (0.50 μg/g creatinine) [23]. In China, a study carried out in pregnant women living in a rural area of the Jiangsu Province showed that median urinary 3-PBA concentration was 1.01 g/L (1.55 μg/g creatinine) [24].
A biomonitoring study conducted in the US has indicated that in recent decades, there has been an increase in pyrethroid insecticides home usage and a decrease in the use of organophosphorus pesticides (OP), resulting in detectable amounts of pyrethroid metabolites in population samples [25].
Human fertility rates are known to be decreasing both in developed and developing countries [26]. This reduction has been associated with socioeconomic changes and adverse lifestyle factors [27]. However, pesticide environmental contaminants have attracted international attention and recently came to be considered as possible contributors to human infertility [28].
Environmental exposure to pyrethroids can also adversely impact on pregnancy outcomes and offspring health, including newborn anthropometry, neurodevelopment, and behavioral problems [18]. A study of exposure to pyrethroid sprays during pregnancy has shown associations of this exposure with autism spectrum disorders (ASD) and developmental delay [29]. Cross-sectional studies also implicate pyrethroids in ASD [30] and Attention Deficit Hyperactivity Disorder (ADHD) [31].
Several previous studies of pyrethroid biomarkers and behavior have reported associations between pyrethroid levels and adverse behavioral problems in children. Although detection frequencies of pyrethroid metabolites were low, suggestive evidence that prenatal exposure to 3-PBA may be associated with a variety of behavioral and executive functioning deficits was found [32].
However, to date, there have been no studies evaluating the levels of exposure of Portuguese pregnant women to this pesticide neither its impact on maternal nor neonatal outcomes. So, the aims of this study were to characterize the exposure of pregnant women living in Portugal to 3-PBA and to evaluate the association of this exposure with maternal outcomes and newborn anthropometric measures. Additionally, considering that the exposure of the global population to 3-PBA is expected to vary with the seasons [24,33,34,35], with the maximum likelihood of exposure in summer [36], we decided to compare samples collected in summer with those collected in winter.
2. Materials and Methods
2.1. Ethical Approval
This study was performed according to the protocol approved by the Ethics Committee of São João University Hospital Center (CHUSJoão)/Faculty of Medicine of the University of Porto. Informed written consent was obtained from all study participants.
2.2. Study Design and Participants
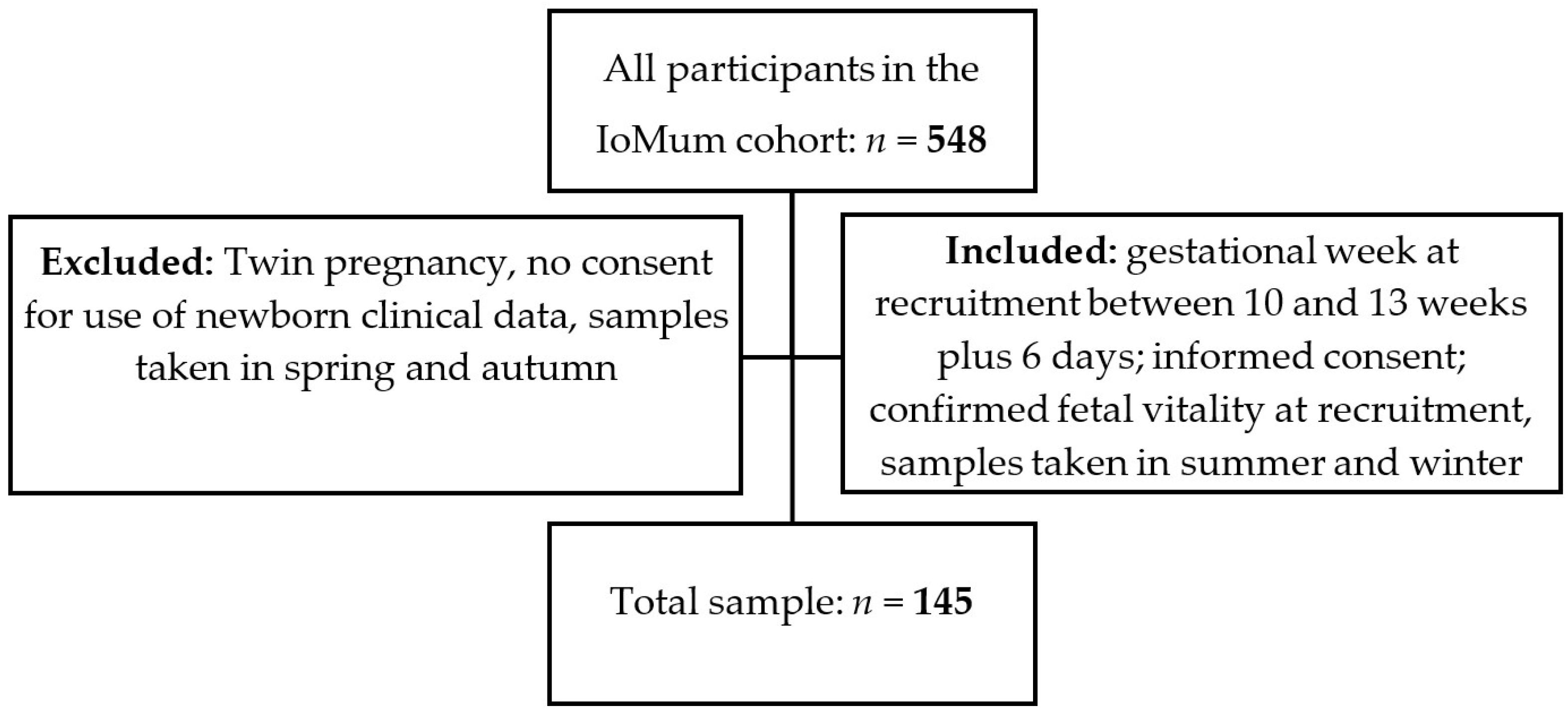
A prospective observational study was carried out from the IoMum cohort (Monitoring iodine status in Portuguese pregnant women and the impact of supplementation—trial registration number NCT04010708) according to the guidelines laid down in the Declaration of Helsinki. Pregnant women attending their first trimester routine ultrasound scan at Centro Hospitalar Universitário de São João (CHUSJ), Porto, between April 2018 and April 2019 were invited to participate as described previously [37,38,39]. All women who had a routine ultrasound scan between 10 and 13 weeks of gestation with confirmed fetal vitality, who signed the informed consent form, and who provided a urine sample at recruitment in summer or in the winter were included in the study. Exclusion criteria were twin pregnancy, declaration of informed consent for use of the data of the newborn not being signed by the mother, and urine sample collection in spring or autumn (Figure 1).
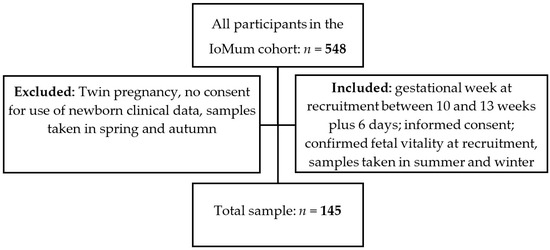
Figure 1.
Recruitment and inclusion flowchart of the study.
Gestational age was determined from the measurement of the fetal crown-rump length.
At the time of enrollment between 10 and 13 weeks plus 6 days (timepoint 1, T1) and after informed consent, information was collected on various demographic and lifestyle factors, including age, area of residence, education, weight, and height of the pregnant, gestational age, smoking habits, and use of medicines. In the lifestyle questionnaire, food frequency information was obtained from a semi-quantitative food intake assessment questionnaire, where we verified the frequency of consumption of cow’s milk, yogurt, cheese, eggs, and fish.
At this time point (T1), a spot urine sample was also collected, and women were invited to a second contact with the IoMum team from 35 weeks until the end of gestation (time point 2, T2) for additional demographic and lifestyle information collection, spot urine collection and a finger prick blood spot. The urine samples were refrigerated upon collection and transported to the laboratory within the following 24 h for aliquot creation and freezing at −80 °C for future analysis.
Information collected at T2 falls outside the scope of this work, and so, it will not be further detailed. Information regarding both maternal and the newborn’s clinical details were obtained from the clinical records, including pregnancy outcomes and complications, mode of delivery, gestational age at delivery, and newborn’s anthropometric and vitality parameters.
2.3. Biochemical Analysis
2.3.1. Chemical Elements Quantification
The analysis of 3-PBA was performed by gas chromatography with mass spectrometry (GC/MS), as described [2] 1 μL of sample was injected onto a Thermo Trace-Ultra gas chromatography, coupled to an ion trap mass detector Thermo Polaris, operated in the electron impact ionization at 70 eV. The ion source and the MS transfer temperature were set at 250 °C. Operating in the splitless mode (0.5 min), the helium was used as carrier gas at a constant flow rate of 1.3 mL min−1. The temperature of the injector was 240 °C. The column was a 30 m ZB-5MSi (0.25 mm i.d., 0.25 μm film thickness Zebron-Phenomenex), and oven temperature was programmed as described [2]. The analysis was developed in the SIM mode, based on the detection of selected ions for 3-PBA (141, 196, and 364).
Sample preparation was performed by solid-phase extraction (SPE). Briefly, the urine samples were thawed at room temperature. Then, a solution of urine in 1.5 mL of deionized water (H2O), 150 μL of sodium hydroxide (NaOH) (Merck), and 100 μL of the internal standard 2-phenoxy benzoic acid (2-PBA) (Sigma Aldrich) (1.5 mL + 1.5 mL + 150 μL + 100 μL, respectively) of were incubated at 37 °C for 15 min. After chemical deconjugation, the samples were transferred to the preconditioned SPE columns (Strata-X) (Phenomenex) with 5 mL methanol (MeOH) (Riedel de Haen) and 5 mL ammonium acetate (Merck). The columns were then immediately washed with 5 mL (MeOH): (H2O) (30/70 V/V). Following a short vacuum pulse to remove excess wash solution, the columns were dried under vacuum for 40 min using the SPE vacuum manifold. Elution was carried out with 5 mL of acidified MeOH (2% formic acid) (Carlo Erba), directly into a glass vial. Subsequently, the eluates were concentrated to 50 μL under a gentle stream of nitrogen.
3-PBA derivatization procedure was necessary prior to GC/MS analysis. The derivatization was performed by addition of 30 μL hexafluoro-2-propanol (HFIP) (Sigma Aldrich Darmstadt, Germany), 20 μL (N, N′-Diisopropylcarbodiimide (DIC) (Sigma Aldrich Darmstadt, Germany) and 400 μL of n-hexane (Merck, Darmstadt, Germany) to the 50 μL of the eluate obtained from the SPE extraction and vortex at room temperature during 10 min. In the final step, liquid-liquid extraction was performed with 1 mL of a 5% aqueous potassium carbonate solution (Panreac, Darmstadt, Germany) (to neutralize the excess derivatizing agent), shaken 5 min in the vortex, and finally, the supernatant was removed and placed in a vial with insert for injecting into GC/MS. The calibration curves and linear ranges of the detector response for 3-PBA were evaluated by analyzing the working standard solutions (15–200 μg L−1, 8 concentrations) in triplicate. In this study, the linearity, selectivity, the limit of detection (LOD) and limit of quantification (LOQ) were evaluated and the determinations that were below the LOD have been replaced by the constant LOD/2, according to Richardson, and Ciampi and Schisterman et al. [40,41,42]. LOD (0.364 μg/L) and LOQ (1.212 μg/L) were calculated as the minimum amount of analyte detectable with a signal-to-noise ratio (S/N) of 3 and 10, respectively; the linearity of the method was established by setting calibration curves using linear regression analysis over the concentration range. Selectivity was verified by comparing the chromatograms of the standards dissolved in n-hexane, the standards extracted from the spiked urine and the matrix blanks.
2.3.2. Creatinine Quantification
Urine-based biomarkers are useful for assessing individuals’ exposure to environmental factors. However, inter-individual variations in urine concentration (which can be assessed by urinary creatinine) can directly affect urinary levels of contaminants. So, urinary creatinine was used to adjust 3-PBA urinary levels to urine concentration [43].
Measurements were performed using an ADVIA 1800 instrument according to the manufacturer’s instructions, based on the enzymatic reaction described by Fossati, Prencipe, and Berti [44].
Briefly, urinary creatinine was quantified by enzymatic conversion (creatininase) to creatine, which was then hydrolyzed by creatinase to produce sarcosine, and this decomposed by the sarcosine oxidase to form glycine, formaldehyde and hydrogen peroxide. The hydrogen peroxide formed produces a blue pigment through the action of peroxidase and by quantitative oxidative condensation with N-(3-sulfopropyl)-3-methoxy-5-methylaniline (HMMPS) and 4-aminoantipyrine. The creatinine concentration was obtained by measuring the absorbance of the blue color at 596/694 nm.
2.4. Maternal Outcomes and Newborn Anthropometric Measures
Maternal outcomes considered for association analyses with levels of 3-PBA were: medication for thyroid disease (as a proxy for hypothyroidism), glycemia in the first trimester, and type of delivery (women who had cesarean delivery, women who had a vaginal delivery).
For the categorization of variables of weight, head circumference and length of the newborn, percentile classification was used [45]. As a result, the newborns were classified into 3 categories regarding weight, head circumference, and length at birth:
- SGA: small for gestational age (below 10th percentile)
- AGA: appropriate for gestational age (between 10th percentile and 90th percentile)
- LGA: large for gestational age (above the 90th percentile)
2.5. Statistical Analysis
Descriptive statistics are presented as absolute and relative frequencies for categorical variables, mean and standard deviation (SD), or median and interquartile range (25th percentile (P25); 75th percentile (P75)) for continuous variables, depending on the symmetry of their distribution.
When testing hypotheses about continuous variables we used non-parametric tests (Mann-Whitney and Kruskal-Wallis tests) considering the hypotheses of non-normality and number of groups. When testing hypotheses on categorical variables, the chi-square test and the Fisher’s exact test were used, as appropriate.
The level of statistical significance was set at 5%, so the differences were considered statistically significant whenever p < 0.05. Statistical analyses were performed using SPSS® v.28.0 (Statistical Package for the Social Sciences: Armonk, NY, USA).
3. Results
3.1. Sociodemographic Data
The sociodemographic characteristics of the study population are presented in Table 1. Most of the population resides in the municipalities corresponding to the coverage areas of CHUSJ (Maia (30%), Valongo (26%), and Porto (18%)), and a minority resides in other municipalities of the North of Portugal, including Matosinhos (n = 5 (4%), Vila Nova de Gaia (n = 4 (3%), and Vila do Conde (n = 3 (2%)). Forty five percent of the population has a low level of education (≤12 years), while around 19% of the population has higher degrees of education equivalent to a Master or PhD degree. The median age of the participants was 31.7 years old, with the youngest and the oldest participants being 19 and 40 years old, respectively. The median pre-pregnancy body mass index (BMI) was 23.6 kg/m2, and 65% of women were within the normal weight range (18.5–24.9 kg/m2). Calculation of BMI was based on the self-reported weights and heights of pregnant women 6 months before the day of recruitment. Regarding the number of pregnancies, 52% of women were primiparous. Only 7% of the pregnancies resulted in preterm births, and in total, this sample gave birth to 68 boys and 73 girls.

Table 1.
General characteristics of the study sample (n = 145).
The mean (SD) weight at birth was 3152 (478) g, and 86% of newborns had adequate weights for their gestational ages. The median (P25; P75) urinary 3-PBA concentration was 0.182 (0.182; 0.372) µg/L, which is above the detection limit (LOD, 0.364 µg/L). To account for the urine concentration, we adjusted 3-PBA urinary excretion for creatinine concentration (median (P25; P72) of 0.263 (0.167; 0.458) µg/g and used this variable in all the remaining analyses.
Table 2 explores the association between urinary concentration of the pyrethroid metabolite 3-PBA with sociodemographic characteristics. The median concentration was the lowest in Porto, although the differences observed were not statistically significant. Maternal education level or smoking habits did not appear to be consistently associated with 3-PBA status. Regarding the BMI categories, a statistically significant difference was observed (p = 0.049), where the lowest medians were found in mothers with obesity.

Table 2.
Urinary levels of 3-PBA (µg/g) by participant characteristics.
3.2. 3-PBA Exposure by Seasons
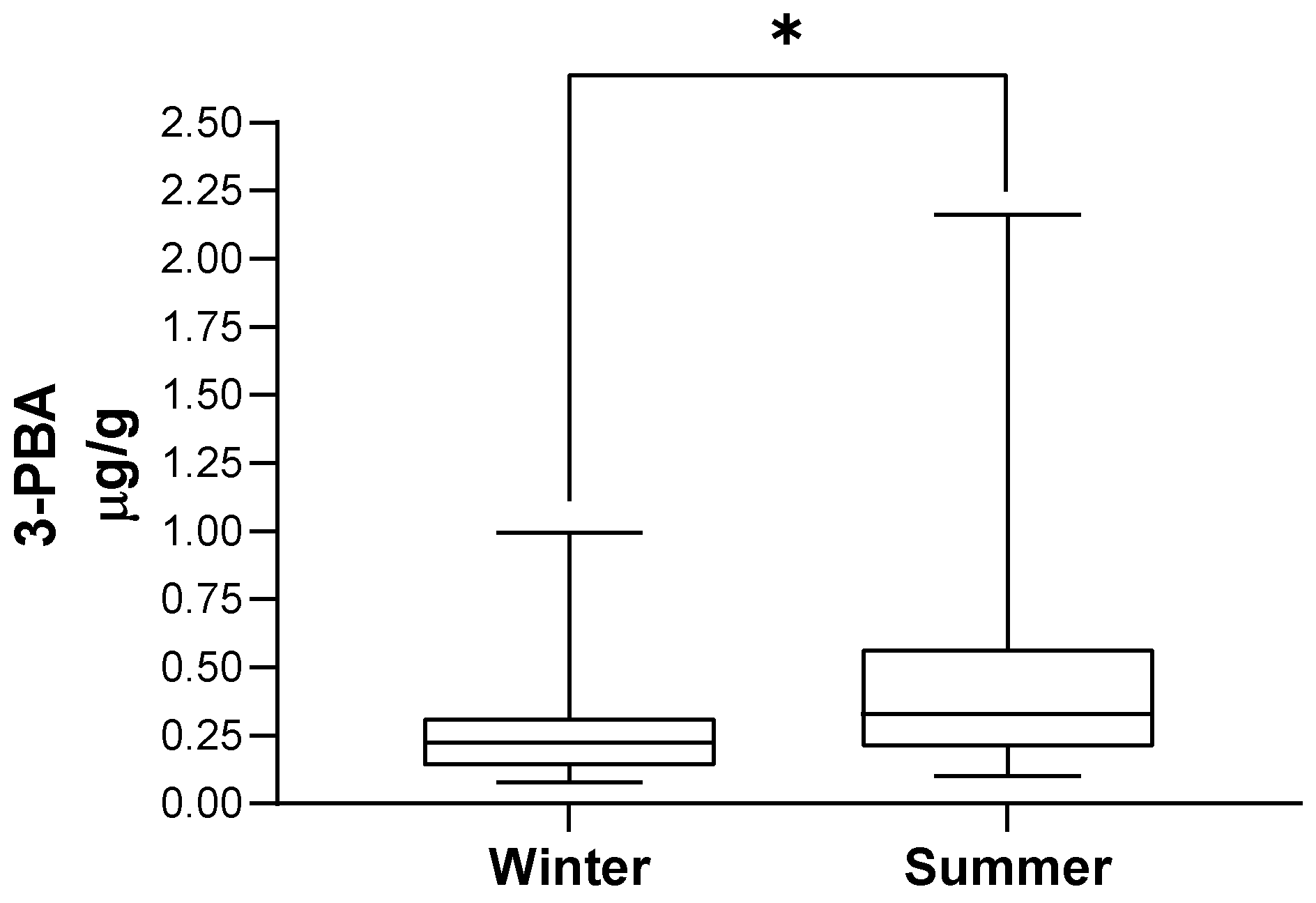
Table 3 presents the distribution of the population by season of urine collection and the corresponding 3-PBA urinary excretion, illustrated in Figure 2. 3-PBA with or without the adjustment for creatinine levels was higher in summer-collected urine (p < 0.001). In addition, we observed that 3-PBA detection rate was much higher in summer when compared with winter samples (53% (n = 39) versus 4% (n = 3); p < 0.001).

Table 3.
Levels of creatinine and 3-PBA with and without adjustment of creatinine in winter and summer samples.
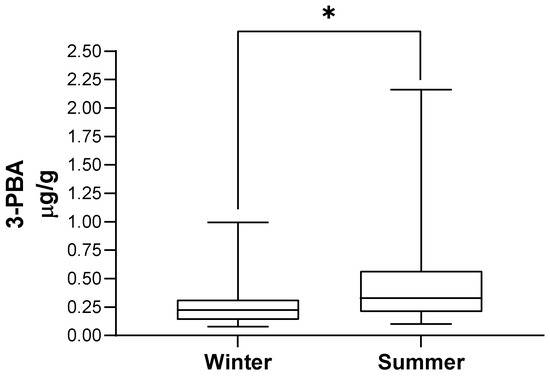
Figure 2.
3-PBA urinary excretion according to season of urine collection. In each box, the central horizontal line marks the median value. * p < 0.001.
3.3. 3-PBA Exposure in Association with Food Intake
Table 4 shows the variation of urinary levels of 3-PBA, separated by seasons (summer and winter) with consumption of fish or yogurt. 3-PBA levels were found to be positively associated with the frequency of consumption of these foods in winter but not in summer. The frequency of consumption of other foods, such as cow’s milk, eggs, and cheese was not associated with 3-PBA levels (data not shown).

Table 4.
3-PBA levels in association with food intake.
3.4. 3-PBA Levels and Maternal Outcomes
The association between 3-PBA levels and maternal and pregnancy clinical characteristics was studied.
Considering the structural similarities found between pyrethroids and THs and the consequent suspicion that they could antagonize thyroid hormone activities [19,21,46], levels of 3-PBA in our sample were analyzed according to medication for thyroid disease (as a proxy for hypothyroidism). We found that women who reported taking levothyroxine had higher median (P25; P75) urinary 3-PBA levels when compared to women who reported not having thyroid disease (0.534 (0.333; 0.976) μg/g, n = 9, and 0.266 (0.168; 0.430) μg/g, n = 124, respectively, p = 0.010).
Additionally, 1st trimester 3-PBA levels had a weak positive correlation with maternal fasting glycemia in the first trimester (r = 0.256; p = 0.011, Spearmen correlation), and women who had cesarean delivery had higher median (P25; P75) first trimester 3-PBA levels when compared with women who had vaginal delivery (0.302 (0.206; 0.528) μg/g versus 0.255 (0.153; 0.410) μg/g, respectively, p = 0.041).
3.5. Neonatal Outcomes
Median 3-PBA concentrations were very similar between male and female offspring (Table 5).

Table 5.
3-PBA levels and newborn sex.
Table 6 shows the variation of urinary 3-PBA levels with neonatal outcomes; the described pyrethroid pesticide metabolite was not associated with anthropometric categories at birth.

Table 6.
Maternal first trimester urinary 3-PBA levels and newborn outcomes.
4. Discussion
Analysis of results for 3-PBA urinary excretion in pregnant women showed concentrations above LOD in 29% of the sample population.
3-PBA urinary excretion tended to associate with residence area, being lower in Porto, when compared to other municipalities. The fact that Porto is a predominantly urban city when compared with Valongo [47], for example, which has a great amount of rural territory, could account for this trend. In line with this rationale, Wielgomas et al. found that the detection of metabolites of SPs in preschool and school children and their parents was more frequent and with higher urinary excretion in rural when compared with urban areas in Poland [48]. In that study, 3-PBA was detected in 77% and 94% of samples from urban and rural areas, respectively, and curiously, 3-PBA urinary levels found in rural and urban areas were very similar to those found in our study (0.272 vs 0.155 µg/g for rural vs urban areas in Poland [48], respectively; 0.278 vs 0.174 µg/g for Porto vs Valongo in Portugal, respectively). Concerning frequency of exposure to 3-PBA, our study revealed a low detection rate (29%) when compared, for example, with reports for Polish, American, French, or Chinese population samples [22,23,24,48]. On the other hand, the 3-PBA detection rate herein described is comparable or higher than those obtained in studies from Germany [49,50,51], Spain [52,53], or France [54,55]. The variation in concentration 3-PBA medians, LOD/LOQ values or rates of detection across studies can be attributable to local exposure characteristics but, importantly, to heterogeneity in urine sampling, sample preparation, quantification methods, and reporting [56]. Altogether, these findings corroborate the claim recently published by Andersen et al. [56] for the need for guidelines to harmonize quantification methods and reporting in human biomonitoring studies.
Regarding pre-gestational BMI categories, 3-PBA levels were the lowest in mothers with obesity. Being highly lipophilic, 3-PBA conjugates with lipids such as cholesterol, bile acids, and triglycerides, which results in 3-PBA retention in organs particularly rich in lipid content [57] such as adipose tissue. This retention could result in the observed decrease in 3-PBA urinary excretion with increasing BMI (and fat mass).
Yoo M et al. also reported a negative association between 3-PBA levels and BMI for high levels of exposure in Korean adults [58]. Despite this, other studies have shown either a positive or no association between 3-PBA urinary excretion and BMI in pregnant women [59] or in the elder, respectively. We cannot currently explain the disparity of these results when compared to ours.
Importantly, 3-PBA exposure was found to be higher in the summer, when compared to winter-collected urine samples. Several studies have shown that urinary pyrethroid metabolite levels of pregnant women follow trends of seasonal insecticide use related to pest management practice [24,33,34,35,36]. A study carried out in China also observed a trend of seasonal variation, with levels of urinary metabolites in the summer significantly higher than those in the winter. These data indicate the need to assess the potential adverse effects of exposure to pyrethroid pesticides on fetuses and infants, in order to take appropriate measures to protect pregnant women from higher exposure to pesticides in more susceptible seasons [24]. Despite this, we cannot exclude that seasonal variations in 3-PBA levels could also be due to seasonal variations in fruits or vegetables consumption. In fact, a systematic review with metanalysis has shown that fruit consumption across the world tends to be higher in autumn and winter, but vegetable consumption tends to be higher in spring and summer [60]. This may, in all probability, result in differential SPs exposure according to seasons.
In relation to food consumption, 3-PBA levels were associated with a higher consumption of fish and yogurt in winter-collected samples; the lack of association between food consumption and 3-PBA urinary levels in summer-collected samples could be due to a greater exposure to environmental 3-PBA in the summer, which could mask the foodborne exposure.
Although the available evidence regarding associations between dairy or fish consumption and 3-PBA urinary levels is currently weak, 3-PBA-parent pesticides, such as cypermethrin, bifenthrin, and cyhalothrin have been detected both in fish and in dairy samples [61,62] and our data show that these foods could act as vehicles for 3-PBA exposure. In fact, 3-PBA levels were 4.5 times higher in the group of people consuming fish more than 3 times a week and 1.5 times higher in the group of people consuming at least one yogurt a day. Despite the magnitude of the difference and the statistical significance, association of 3-PBA levels with fish consumption should be interpreted with caution, because of the small sample size in the category of fish consumption >3 times/week.
In addition, we cannot explain the reason why yogurt was the only dairy food which consumption associated with 3-PBA levels. Despite this, our data suggest that fish and yogurt may represent a form of pyrethroid bioaccumulation.
With respect to the association of 3-PBA with maternal clinical characteristics, we observed higher levels of 3-PBA in women medicated for hypothyroidism, which corroborates the idea that pyrethroids, whose structure is similar to THs, could associate with thyroid dysfunction, as suggested by others [19,46]. However, in our study, only 9 women are included in the hypothyroidism medicated group, and so, these results should be read with caution.
It is estimated that at least 10% of approved pesticides in the European Union (EU) possess endocrine disruption (ED) properties [63,64]. Experimental studies have reported that many currently used pesticides (or their metabolites) may interfere with the hypothalamic-pituitary-gonadal and hypothalamic-pituitary-thyroid axes [46,65]. Especially among vulnerable human populations, such as developing fetuses and infants, changes in THs beyond the reference range may cause a significant adverse impact on health, including the development of neurodevelopmental problems [16,66]. However, the effects of current human pyrethroid exposure levels on reproductive and thyroid function are poorly understood.
Still, with regard to maternal clinical characteristics, there was a weak but statistically significant correlation between 3-PBA levels and maternal fasting glycemia in the first trimester. This result is in line with a study conducted in China that studied the association between serum levels of pyrethroid insecticides and risk of type 2 diabetes which found that high concentrations of serum pyrethroid insecticides were significantly associated with an increased risk of type 2 diabetes [67]. A US study suggests that exposure to pyrethroids, as estimated by urinary 3-PBA concentrations, was associated with an increased risk of diabetes in the general adult population [68]. Hansen et al. found a severely increased prevalence of prediabetes among Bolivian pesticide sprayers compared with a control group. Within the sprayer group, an association between cumulative exposure to pyrethroids and abnormal glucose regulation was seen [69].
Finally, in our study, we found no associations between pyrethroid pesticide metabolite levels and the anthropometric profile of newborns at birth. Like us, Berkowitz et al., found no significant association between newborn anthropometric measures (birth weight, length, and head circumference) and maternal 3-PBA urinary in early pregnancy [70]. In a prospective birth cohort in rural northern China between September 2010 and 2012, no associations were found between 3-PBA levels and birth length, head circumference, or gestational duration [18]. Contrarily, a study conducted in New York City aimed to investigate the association between delivery outcomes and urinary biomarkers of pyrethroids among healthy pregnant women and found that 3-PBA concentrations were positively associated with head circumference in boys (p = 0.53, 95% CI: 0.03, 1.04) [3]. Also, in a study carried out in northern China, aimed at linking pesticides and other environmental exposures with the health of pregnant women and their children, it was observed that the total levels of pyrethroid metabolites in the mother’s urine maternal urine were positively associated with birth weight and head circumference [71].
One main limitation of this study was the small sample size, which resulted from the selection of samples collected during the summer and winter and exclusion of spring- or autumn-collected samples. In addition, the initial questionnaire did not ask pregnant women about the frequency of consumption of other foods, such as fruits and vegetables, and exposure to additional agents that may contain environmental pollutants.
Another limitation was that we could not analyze occupational exposure to 3-PBA due to lack of detailed information regarding the profession of the participants. In fact, information regarding professional occupations was extracted from clinical registries with no possible association with the likelihood of exposure.
A strong point of our study was that, although the recruitment was carried out at central hospital, we could invite pregnant women undergoing routine prenatal surveillance, not being restricted to pregnant women in hospital consultation, and therefore, we included women with and without pathology.
5. Conclusions
In conclusion, the present study characterized 3-PBA status in a sample of pregnant women living in the Porto metropolitan area. Nonetheless, 3-PBA excretion associated negatively with maternal pre-pregnancy BMI, suggesting 3-PBA adipose tissue retention. Our data also suggest that 3-PBA exposure is higher during the summer, and food such as fish and yogurt may be a source of 3-PBA dietary exposure, particularly when environmental exposure is low.
As to clinical features, our data suggest that 3-PBA may be associated with thyroid dysfunction, which could be due to a thyroid hormone antagonistic effect, as previously described by other authors. This conclusion should be considered cautiously given the small sample size of the hypothyroidism medicated group in our study.
In fact, this data, together with the association herein found between 3-PBA and fasting glycemia, deserve future attention.
Finally, in this work, we did not find an association between 3-PBA maternal urinary excretion in the first trimester and anthropometric measures of the newborn.
This study highlights that pregnant women living in Portugal may be exposed to 3-PBA and that this exposure may associate with maternal clinical features during pregnancy. More studies are needed to confirm data regarding association of 3-PBA exposure and newborn outcomes and to analyze the impact of these exposures on long-term maternal or childhood outcomes.
Author Contributions
J.G.: conceptualization, methodology, investigation, formal analysis, writing—original draft, visualization, funding acquisition; I.B.: investigation, writing—review and editing; C.P.: investigation, writing—review and editing; N.X.M.: investigation; C.M.C.: conceptualization; D.P.: investigation, methodology; M.d.C.P.: resources; C.M.: resources; V.F.D.: resources; C.D.-M.: investigation; C.C.D.: formal analysis; L.F.R.A.: conceptualization, methodology; C.C.: conceptualization, methodology, funding acquisition; J.C.L.: conceptualization, methodology; C.R.: methodology, resources, investigation, methodology, supervision, writing—review and editing; E.K.: conceptualization, methodology, supervision, project administration, funding acquisition, writing—review and editing. V.C.F.: investigation, formal analysis, methodology, supervision, writing—review and editing, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This article was supported by national funds through the FCT Foundation for Science and Technology, I.P., within the scope of the projects RISE - LA/P/0053/2020; CINTESIS, R&D UNIT (reference UIDB/4255/2020) and LAQV (references: UIDB/50006/2020 AND UIDP/50006/2020). Virgínia Cruz Fernandes was funded by FCT/MCTES (Foundation for Science and Technology and Ministry of Science, Technology and Higher Education) and the ESF (European Social Fund) through NORTE 2020 (North Region Operational Program) through a grant of Post-Doc (reference SFRH/BPD/109153/2015). Juliana Guimarães was funded by FCT/MCTES (Foundation for Science and Technology and Ministry of Science, Technology and Higher Education) under CINTESIS by a PhD scholarship (reference UI/BD/152087/2021).
Institutional Review Board Statement
This study was performed according to the protocol approved by the Ethics Committee of São João University Hospital Center (CHUSJoão)/Faculty of Medicine of the University of Porto.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank the kind participation of all pregnant women and the support of the recruitment activities by the health professionals of the Department of Obstetrics, Centro Hospitalar Universitário S. João, Porto, Portugal and also to the professionals of REQUIMTE/LAQV, Instituto Superior de Engenharia, Politécnico do Porto, for their collaboration in carrying out the analyzes of the 3-PBA metabolite. This work received support from PT national funds (FCT/MCTES, Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) through the projects LA/P/0053/2020; UIDB/4255/2020; UIDB/5006/2020 and UIDP/50006/2020. Virgínia Cruz Fernandes thanks FCT/MCTES (Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) and ESF (European Social Fund) through NORTE 2020 (Programa Operacional Região Norte) for his/her Post-Doc grant ref. SFRH/BPD/109153/2015). Juliana Guimarães was funded by FCT/MCTES (Foundation for Science and Technology and Ministry of Science, Technology and Higher Education) under CINTESIS by a PhD scholarship (reference UI/BD/152087/2021).
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no competing interests to declare that are relevant to the content of this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article.
References
- Lewis, R.C.; Cantonwine, D.E.; Anzalota Del Toro, L.V.; Calafat, A.M.; Valentin-Blasini, L.; Davis, M.D.; Baker, S.E.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Urinary biomarkers of exposure to insecticides, herbicides, and one insect repellent among pregnant women in Puerto Rico. Environ. Health 2014, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Braganca, I.; Lemos, P.C.; Delerue-Matos, C.; Domingues, V.F. Pyrethroid pesticide metabolite, 3-PBA, in soils: Method development and application to real agricultural soils. Environ. Sci. Pollut. Res. Int. 2019, 26, 2987–2997. [Google Scholar] [CrossRef]
- Balalian, A.A.; Liu, X.; Herbstman, J.B.; Daniel, S.; Whyatt, R.; Rauh, V.; Calafat, A.M.; Wapner, R.; Factor-Litvak, P. Prenatal exposure to organophosphate and pyrethroid insecticides and the herbicide 2,4-dichlorophenoxyacetic acid and size at birth in urban pregnant women. Environ. Res. 2021, 201, 111539. [Google Scholar] [CrossRef] [PubMed]
- EPA. Evironmental Protection Agency. Pesticide Science and Assessing Pesticide Risks; epa.gov.; EPA: Springfield, IL, USA, 2022.
- Braganca, I.; Lemos, P.C.; Barros, P.; Delerue-Matos, C.; Domingues, V.F. Phytotoxicity of pyrethroid pesticides and its metabolite towards Cucumis sativus. Sci. Total Environ. 2018, 619–620, 685–691. [Google Scholar] [CrossRef]
- Bragança, I.; Mucha, A.P.; Tomasino, M.P.; Santos, F.; Lemos, P.C.; Delerue-Matos, C.; Domingues, V.F. Deltamethrin impact in a cabbage planted soil: Degradation and effect on microbial community structure. Chemosphere 2019, 220, 1179–1186. [Google Scholar] [CrossRef]
- Li, W.; Morgan, M.K.; Graham, S.E.; Starr, J.M. Measurement of pyrethroids and their environmental degradation products in fresh fruits and vegetables using a modification of the quick easy cheap effective rugged safe (QuEChERS) method. Talanta 2016, 151, 42–50. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Medina-Pastor, P.; Triacchini, G. The 2018 European Union report on pesticide residues in food. EFSA J. 2020, 18, e06057. [Google Scholar] [PubMed]
- Saillenfait, A.M.; Ndiaye, D.; Sabaté, J.P. Pyrethroids: Exposure and health effects—An update. Int. J. Hyg. Environ. Health 2015, 218, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Wan, F.; Yu, T.; Hu, J.; Yin, S.; Li, Y.; Kou, L.; Chi, X.; Wu, J.; Sun, Y.; Zhou, Q.; et al. The pyrethroids metabolite 3-phenoxybenzoic acid induces dopaminergic degeneration. Sci. Total Environ. 2022, 838, 156027. [Google Scholar] [CrossRef]
- Personne, S.; Marcelo, P.; Pilard, S.; Baltora-Rosset, S.; Corona, A.; Robidel, F.; Lecomte, A.; Brochot, C.; Bach, V.; Zeman, F. Determination of maternal and foetal distribution of cis- and trans-permethrin isomers and their metabolites in pregnant rats by liquid chromatography tandem mass spectrometry (LC-MS/MS). Anal. Bioanal. Chem. 2019, 411, 8043–8052. [Google Scholar] [CrossRef]
- Connors, S.L.; Levitt, P.; Matthews, S.G.; Slotkin, T.A.; Johnston, M.V.; Kinney, H.C.; Johnson, W.G.; Dailey, R.M.; Zimmerman, A.W. Fetal mechanisms in neurodevelopmental disorders. Pediatr. Neurol. 2008, 38, 163–176. [Google Scholar] [CrossRef]
- Abreu-Villaça, Y.; Levin, E.D. Developmental neurotoxicity of succeeding generations of insecticides. Environ. Int. 2017, 99, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.R.; David, A.; Freire, C.; Fernández, M.F.; D’Cruz, S.C.; Reina-Pérez, I.; Fini, J.B.; Blaha, L. Pyrethroids and developmental neurotoxicity - A critical review of epidemiological studies and supporting mechanistic evidence. Environ. Res. 2022, 214 Pt 2, 113935. [Google Scholar] [CrossRef]
- Hwang, M.; Lee, Y.; Choi, K.; Park, C. Urinary 3-phenoxybenzoic acid levels and the association with thyroid hormones in adults: Korean National Environmental Health Survey 2012-2014. Sci. Total Environ. 2019, 696, 133920. [Google Scholar] [CrossRef]
- Casals-Casas, C.; Desvergne, B. Endocrine disruptors: From endocrine to metabolic disruption. Annu. Rev. Physiol. 2011, 73, 135–162. [Google Scholar] [CrossRef]
- Zhang, J.; Yoshinaga, J.; Hisada, A.; Shiraishi, H.; Shimodaira, K.; Okai, T.; Koyama, M.; Watanabe, N.; Suzuki, E.; Shirakawa, M.; et al. Prenatal pyrethroid insecticide exposure and thyroid hormone levels and birth sizes of neonates. Sci. Total Environ. 2014, 488–489, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Shen, O.; Sun, H.; Fei, J.; Lu, C.; Song, L.; Xia, Y.; Wang, S.; Wang, X. Assessing hormone receptor activities of pyrethroid insecticides and their metabolites in reporter gene assays. Toxicol. Sci. 2010, 116, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, Z.; Qin, K.; Zhang, Y.; Pan, R.; Wang, Y.; Shi, R.; Gao, Y.; Tian, Y. Environmental pyrethroid exposure and thyroid hormones of pregnant women in Shandong, China. Chemosphere 2019, 234, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hisada, A.; Yoshinaga, J.; Shiraishi, H.; Shimodaira, K.; Okai, T.; Noda, Y.; Shirakawa, M.; Kato, N. Exposure to pyrethroids insecticides and serum levels of thyroid-related measures in pregnant women. Environ. Res. 2013, 127, 16–21. [Google Scholar] [CrossRef]
- Curl, C.L.; Porter, J.; Penwell, I.; Phinney, R.; Ospina, M.; Calafat, A.M. Effect of a 24-week randomized trial of an organic produce intervention on pyrethroid and organophosphate pesticide exposure among pregnant women. Environ. Int. 2019, 132, 104957. [Google Scholar] [CrossRef] [PubMed]
- Dereumeaux, C.; Saoudi, A.; Goria, S.; Wagner, V.; De Crouy-Chanel, P.; Pecheux, M.; Berat, B.; Zaros, C.; Guldner, L. Urinary levels of pyrethroid pesticides and determinants in pregnant French women from the Elfe cohort. Environ. Int. 2018, 119, 89–99. [Google Scholar] [CrossRef]
- Qi, X.; Zheng, M.; Wu, C.; Wang, G.; Feng, C.; Zhou, Z. Urinary pyrethroid metabolites among pregnant women in an agricultural area of the Province of Jiangsu, China. Int. J. Hyg. Environ. Health 2012, 215, 487–495. [Google Scholar] [CrossRef]
- Baker, S.E.; Barr, D.B.; Driskell, W.J.; Beeson, M.D.; Needham, L.L. Quantification of selected pesticide metabolites in human urine using isotope dilution high-performance liquid chromatography/tandem mass spectrometry. J. Expo. Anal. Environ. Epidemiol. 2000, 10, 789–798. [Google Scholar] [CrossRef]
- Skakkebaek, N.E.; Jørgensen, N.; Main, K.M.; Rajpert-De Meyts, E.; Leffers, H.; Andersson, A.M.; Juul, A.; Carlsen, E.; Mortensen, G.K.; Jensen, T.K.; et al. Is human fecundity declining? Int. J. Androl. 2006, 29, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Snijder, C.A.; te Velde, E.; Roeleveld, N.; Burdorf, A. Occupational exposure to chemical substances and time to pregnancy: A systematic review. Hum. Reprod. Update 2012, 18, 284–300. [Google Scholar] [CrossRef] [PubMed]
- Mehrpour, O.; Karrari, P.; Zamani, N.; Tsatsakis, A.M.; Abdollahi, M. Occupational exposure to pesticides and consequences on male semen and fertility: A review. Toxicol. Lett. 2014, 230, 146–156. [Google Scholar] [CrossRef]
- Shelton, J.F.; Geraghty, E.M.; Tancredi, D.J.; Delwiche, L.D.; Schmidt, R.J.; Ritz, B.; Hansen, R.L.; Hertz-Picciotto, I. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: The CHARGE study. Environ. Health Perspect. 2014, 122, 1103–1109. [Google Scholar] [CrossRef]
- Domingues, V.F.; Nasuti, C.; Piangerelli, M.; Correia-Sa, L.; Ghezzo, A.; Marini, M.; Abruzzo, P.M.; Visconti, P.; Giustozzi, M.; Rossi, G.; et al. Pyrethroid Pesticide Metabolite in Urine and Microelements in Hair of Children Affected by Autism Spectrum Disorders: A Preliminary Investigation. Int. J. Environ. Res. Public Health 2016, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Wagner-Schuman, M.; Richardson, J.R.; Auinger, P.; Braun, J.M.; Lanphear, B.P.; Epstein, J.N.; Yolton, K.; Froehlich, T.E. Association of pyrethroid pesticide exposure with attention-deficit/hyperactivity disorder in a nationally representative sample of U.S. children. Environ. Health 2015, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Furlong, M.A.; Barr, D.B.; Wolff, M.S.; Engel, S.M. Prenatal exposure to pyrethroid pesticides and childhood behavior and executive functioning. Neurotoxicology 2017, 62, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Šulc, L.; Janoš, T.; Figueiredo, D.; Ottenbros, I.; Šenk, P.; Mikeš, O.; Huss, A.; Čupr, P. Pesticide exposure among Czech adults and children from the CELSPAC-SPECIMEn cohort: Urinary biomarker levels and associated health risks. Environ. Res. 2022, 214, 114002. [Google Scholar] [CrossRef] [PubMed]
- Osaka, A.; Ueyama, J.; Kondo, T.; Nomura, H.; Sugiura, Y.; Saito, I.; Nakane, K.; Takaishi, A.; Ogi, H.; Wakusawa, S.; et al. Exposure characterization of three major insecticide lines in urine of young children in Japan-neonicotinoids, organophosphates, and pyrethroids. Environ. Res. 2016, 147, 89–96. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Li, Y.; Jagals, P.; Ware, R.S.; Wang, X.; He, C.; Mueller, J.F.; Sly, P.D. Development of a questionnaire-based insecticide exposure assessment method and comparison with urinary insecticide biomarkers in young Australian children. Environ. Res. 2019, 178, 108613. [Google Scholar] [CrossRef]
- Wang, D.; Kamijima, M.; Imai, R.; Suzuki, T.; Kameda, Y.; Asai, K.; Okamura, A.; Naito, H.; Ueyama, J.; Saito, I.; et al. Biological monitoring of pyrethroid exposure of pest control workers in Japan. J. Occup. Health 2007, 49, 509–514. [Google Scholar] [CrossRef]
- Matta Coelho, C.; Guimarães, J.; Bracchi, I.; Xavier Moreira, N.; Pinheiro, C.; Ferreira, P.; Pestana, D.; Barreiros Mota, I.; Cortez, A.; Prucha, C.; et al. Noncompliance to iodine supplementation recommendation is a risk factor for iodine insufficiency in Portuguese pregnant women: Results from the IoMum cohort. J. Endocrinol. Investig. 2022, 45, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Xavier Moreira, N.; Ferreira, P.; Matta Coelho, C.; Guimarães, J.; Pereira, G.; Cortez, A.; Bracchi, I.; Pestana, D.; Barreiros Mota, I.; et al. Iodine knowledge is associated with iodine status in Portuguese pregnant women: Results from the IoMum cohort study. Br. J. Nutr. 2021, 126, 1331–1339. [Google Scholar] [CrossRef]
- Ferreira, P.; Pinheiro, C.; Matta Coelho, C.; Guimarães, J.; Pereira, G.; Xavier Moreira, N.; Cortez, A.; Bracchi, I.; Pestana, D.; Barreiros Mota, I.; et al. The association of milk and dairy consumption with iodine status in pregnant women in Oporto region. Br. J. Nutr. 2021, 126, 1314–1322. [Google Scholar] [CrossRef]
- Richardson, D.B.; Ciampi, A. Effects of exposure measurement error when an exposure variable is constrained by a lower limit. Am. J. Epidemiol. 2003, 157, 355–363. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Vexler, A.; Whitcomb, B.W.; Liu, A. The limitations due to exposure detection limits for regression models. Am. J. Epidemiol. 2006, 163, 374–383. [Google Scholar] [CrossRef]
- Govarts, E.G.L.; Rambaud, L.; Vogel, N.; Montazeri, P.; Berglund, M.; Santonen, T. Deliverable Report; Statistical Analysis Plan for the Co-Funded Studies of WP8; WP10—Data Management and Analysis; Deadline: March 2020 Upload by Coordinator: 10 December 2020; European Human Biomonitoring Initiative (HBM4EU) no.733032; 2020; Available online: https://www.hbm4eu.eu/work-packages/deliverable-10-12-update-statistical-analysis-plan-for-the-co-funded-studies-of-wp8/ (accessed on 18 December 2022).
- O’Brien, K.M.; Upson, K.; Buckley, J.P. Lipid and Creatinine Adjustment to Evaluate Health Effects of Environmental Exposures. Curr. Environ. Health Rep. 2017, 4, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Fossati, P.; Prencipe, L.; Berti, G. Enzymic creatinine assay: A new colorimetric method based on hydrogen peroxide measurement. Clin. Chem. 1983, 29, 1494–1496. [Google Scholar] [CrossRef]
- Alexander, G.R.; Kogan, M.D.; Himes, J.H. 1994-1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern. Child Health J. 1999, 3, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Leemans, M.; Couderq, S.; Demeneix, B.; Fini, J.B. Pesticides With Potential Thyroid Hormone-Disrupting Effects: A Review of Recent Data. Front. Endocrinol. 2019, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Report, D. Classification of Parishes on the Mainland into Rural and Non-Rural; Rural Development Program (PRODER); Portugal, 2013; p. 81. Available online: https://enrd.ec.europa.eu/country/portugal_en (accessed on 18 December 2022).
- Wielgomas, B.; Piskunowicz, M. Biomonitoring of pyrethroid exposure among rural and urban populations in northern Poland. Chemosphere 2013, 93, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- Leng, G.; Ranft, U.; Sugiri, D.; Hadnagy, W.; Berger-Preiss, E.; Idel, H. Pyrethroids used indoors--biological monitoring of exposure to pyrethroids following an indoor pest control operation. Int. J. Hyg. Environ. Health 2003, 206, 85–92. [Google Scholar] [CrossRef]
- Hardt, J.; Angerer, J. Biological monitoring of workers after the application of insecticidal pyrethroids. Int. Arch. Occup. Environ. Health 2003, 76, 492–498. [Google Scholar] [CrossRef]
- Berger-Preiss, E.; Levsen, K.; Leng, G.; Idel, H.; Sugiri, D.; Ranft, U. Indoor pyrethroid exposure in homes with woollen textile floor coverings. Int. J. Hyg. Environ. Health 2002, 205, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.; Suárez, B.; Vela-Soria, F.; Castiello, F.; Reina-Pérez, I.; Andersen, H.R.; Olea, N.; Fernández, M.F. Urinary metabolites of non-persistent pesticides and serum hormones in Spanish adolescent males. Environ. Res. 2021, 197, 111016. [Google Scholar] [CrossRef]
- Roca, M.; Miralles-Marco, A.; Ferré, J.; Pérez, R.; Yusà, V. Biomonitoring exposure assessment to contemporary pesticides in a school children population of Spain. Environ. Res. 2014, 131, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Viel, J.F.; Warembourg, C.; Le Maner-Idrissi, G.; Lacroix, A.; Limon, G.; Rouget, F.; Monfort, C.; Durand, G.; Cordier, S.; Chevrier, C. Pyrethroid insecticide exposure and cognitive developmental disabilities in children: The PELAGIE mother-child cohort. Environ. Int. 2015, 82, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Viel, J.F.; Rouget, F.; Warembourg, C.; Monfort, C.; Limon, G.; Cordier, S.; Chevrier, C. Behavioural disorders in 6-year-old children and pyrethroid insecticide exposure: The PELAGIE mother-child cohort. Occup. Environ. Med. 2017, 74, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.R.; Rambaud, L.; Riou, M.; Buekers, J.; Remy, S.; Berman, T.; Govarts, E. Exposure Levels of Pyrethroids, Chlorpyrifos and Glyphosate in EU-An Overview of Human Biomonitoring Studies Published since 2000. Toxics 2022, 10, 789. [Google Scholar] [CrossRef]
- Eljarrat, E. Pyrethroid Insecticides. In The Handbook of Environmental Chemistry; HEC: Paris, France, 2020; Volume 92. [Google Scholar]
- Yoo, M.; Lim, Y.H.; Kim, T.; Lee, D.; Hong, Y.C. Association between urinary 3-phenoxybenzoic acid and body mass index in Korean adults: 1(st) Korean National Environmental Health Survey. Ann. Occup. Environ. Med. 2016, 28, 2. [Google Scholar] [CrossRef] [PubMed]
- Dalsager, L.; Christensen, L.E.; Kongsholm, M.G.; Kyhl, H.B.; Nielsen, F.; Schoeters, G.; Jensen, T.K.; Andersen, H.R. Associations of maternal exposure to organophosphate and pyrethroid insecticides and the herbicide 2,4-D with birth outcomes and anogenital distance at 3 months in the Odense Child Cohort. Reprod. Toxicol. 2018, 76, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Stelmach-Mardas, M.; Kleiser, C.; Uzhova, I.; Peñalvo, J.L.; La Torre, G.; Palys, W.; Lojko, D.; Nimptsch, K.; Suwalska, A.; Linseisen, J.; et al. Seasonality of food groups and total energy intake: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2016, 70, 700–708. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Bilal, R.M.; Gewida, A.G.A.; Dhama, K.; Abdel-Latif, H.M.R.; Amer, M.S.; Rivero-Perez, N.; Zaragoza-Bastida, A.; Binnaser, Y.S.; et al. An Overview on the Potential Hazards of Pyrethroid Insecticides in Fish, with Special Emphasis on Cypermethrin Toxicity. Animals 2021, 11, 1880. [Google Scholar] [CrossRef] [PubMed]
- Riederer, A.M.; Hunter, R.E., Jr.; Hayden, S.W.; Ryan, P.B. Pyrethroid and organophosphorus pesticides in composite diet samples from Atlanta, USA adults. Environ. Sci. Technol. 2010, 44, 483–490. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, R.; Plant, J.A.; Bell, J.N.; Voulvoulis, N. Endocrine disrupting pesticides: Implications for risk assessment. Environ. Int. 2008, 34, 168–183. [Google Scholar] [CrossRef]
- Lyssimachou, A.; Muilerman, H. Impact Assessment of the Criteria for Endocrine Disrupting Pesticides; Pesticide Action Network/PAN Europe: Brussels, Belgium, 2015–2016; p. 8. [Google Scholar]
- Orton, F.; Rosivatz, E.; Scholze, M.; Kortenkamp, A. Widely used pesticides with previously unknown endocrine activity revealed as in vitro antiandrogens. Environ. Health Perspect. 2011, 119, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Berbel, P.; Mestre, J.L.; Santamaría, A.; Palazón, I.; Franco, A.; Graells, M.; González-Torga, A.; de Escobar, G.M. Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: The importance of early iodine supplementation. Thyroid 2009, 19, 511–519. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, S.; Cheng, X.; An, J.; Zhang, X.; Li, P.; Li, W.; Wang, X.; Yuan, Y.; Zheng, H.; et al. Association between serum pyrethroid insecticide levels and incident type 2 diabetes risk: A nested case-control study in Dongfeng-Tongji cohort. Eur. J. Epidemiol. 2022, 37, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, S.K.; Choi, Y.H. Environmental pyrethroid exposure and diabetes in U.S. adults. Environ. Res. 2019, 172, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.R.; Jørs, E.; Lander, F.; Condarco, G.; Schlünssen, V. Is cumulated pyrethroid exposure associated with prediabetes? A cross-sectional study. J. Agromed. 2014, 19, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, G.S.; Wetmur, J.G.; Birman-Deych, E.; Obel, J.; Lapinski, R.H.; Godbold, J.H.; Holzman, I.R.; Wolff, M.S. In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environ. Health Perspect. 2004, 112, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Cui, C.; Chen, L.; Gao, Y.; Zhou, Y.; Shi, R.; Tian, Y. Prenatal exposure to pyrethroid insecticides and birth outcomes in Rural Northern China. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 264–270. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).