Microbial Removal of Petroleum Hydrocarbons from Contaminated Soil under Arsenic Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Media
2.3. Enrichment and Isolation of Petroleum Hydrocarbon-Degrading Bacteria
2.4. Petroleum Hydrocarbon Degradation Assay
2.5. Carbon Source Utilization
2.6. Biochemical Test
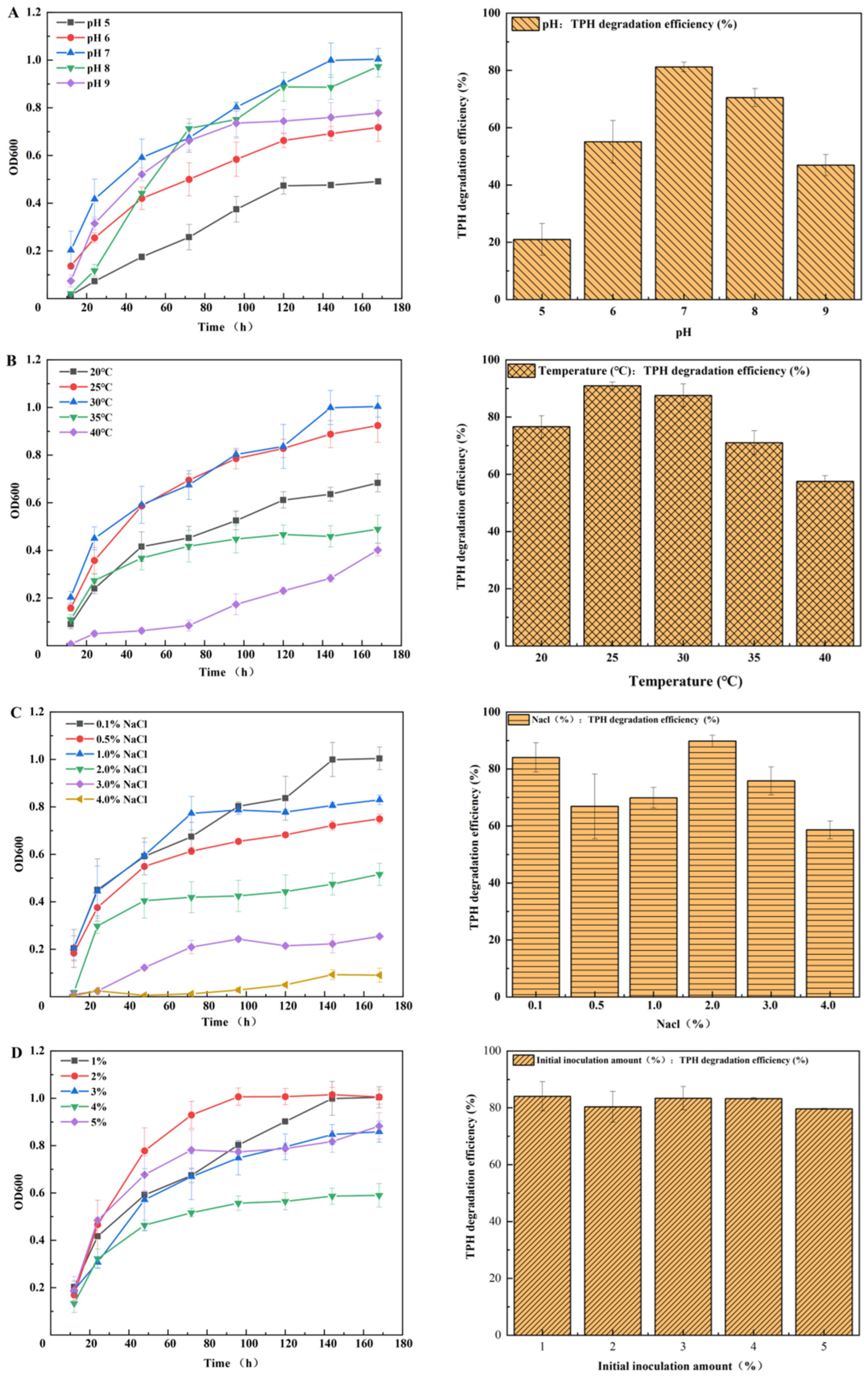
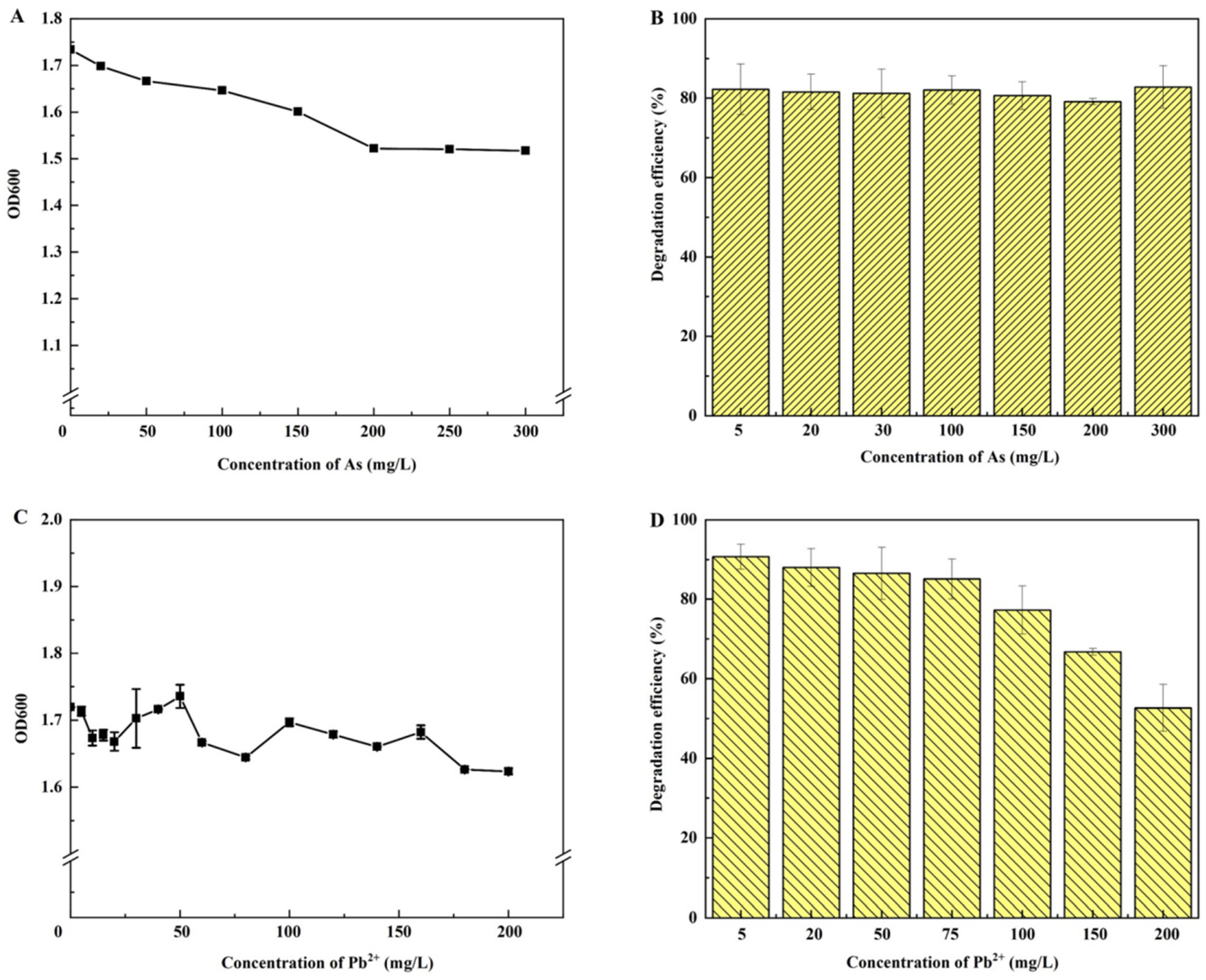
2.7. Effects of Heavy Metal Stress on Bacterial Growth and Petroleum Hydrocarbon Degradation
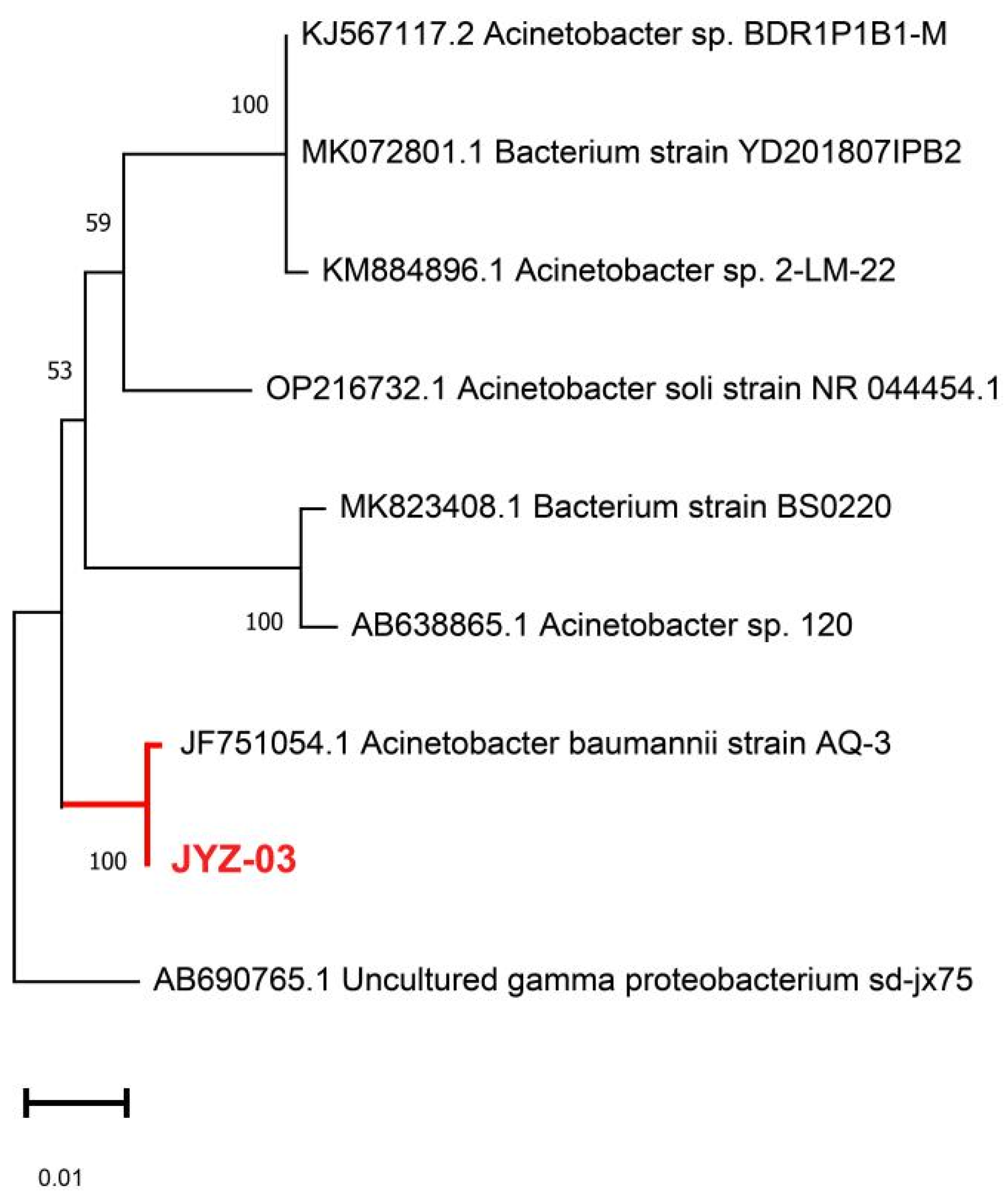
2.8. Identification of the Selected Bacterial Isolates
2.9. Evaluation of the Efficiency of TPH from Soil by JYZ03
3. Results and Discussion
3.1. Metabolic and Taxonomic Characteristics of the Isolates
3.2. Diesel Oil Degradation Assay
3.3. Carbon Source Utilization
3.4. Effects of Environmental Factors on Petroleum Hydrocarbon Removal by JYZ-03
3.5. Effects of Heavy Metal Stress on Petroleum Hydrocarbon Removal by JYZ-03
3.6. Evaluation of the Efficiency of TPH Removal from Soil by JYZ03
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czarny, J.; Staninska-Pięta, J.; Piotrowska-Cyplik, A.; Juzwa, W.; Wolniewicz, A.; Marecik, R.; Ławniczak, Ł.; Chrzanowski, Ł. Acinetobacter sp. as the key player in diesel oil degrading community exposed to PAHs and heavy metals. J. Hazard. Mater. 2020, 383, 121–168. [Google Scholar] [CrossRef]
- Khan, M.A.I.; Biswas, B.; Smith, E.; Naidu, R.; Megharaj, M. Toxicity assessment of fresh and weathered petroleum hydrocarbons in contaminated soil—A review. Chemosphere 2018, 212, 755–767. [Google Scholar] [CrossRef]
- Adeniyi, A.; Afolabi, J.A. Determination of total petroleum hydrocarbons and heavy metals in soils within the vicinity of facilities handling refined petroleum products in Lagos metropolis. Environ. Int. 2002, 28, 79–82. [Google Scholar] [CrossRef]
- Osuji, C.; Onojake, C.M. Trace heavy metals associated with crude oil: A case study of ebocha-8 oil-spill-polluted site in Niger Delta, Nigeria. Chem. Biodivers. 2004, 1, 1708–1715. [Google Scholar] [CrossRef]
- Verla, A.; Opara, A.I.; Enyoh, C.E.; Ngozi, V.E.; Natheniel, C.O.; Kingsley, O.U.; Chizoruo, I.F.; Amaka, A.P. Petroleum hydrocarbons and heavy metals risk of consuming fish species from Oguta Lake, Imo State, Nigeria. J. Chem. Health Risks 2021, 1, 1–15. [Google Scholar]
- Ebunu, I.A.; Bamidele, L.B.; Peter, A.O.; Adelana, R.A.; Azikiwe, P.O. Characterization of barite ores from selected locations in Nigeria for drilling fluid formulation. Sci. Afr. 2021, 14, e01057. [Google Scholar]
- Lakmun, C.; Nithiya, A.; Sathiabama, T.T.; Shreeshivadasan, C. Drilling fluids: Presence of hazardous BTEXs and crystalline silica. Environ. Manag. Tech. 2020, 8, 1029–1035. [Google Scholar]
- Giller, K.E.; Witter, E.; McGrath, S.P. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol. Biochem. 1998, 30, 1389–1414. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Balgobind, A.; Pillay, B. Bioavailability of heavy metals in soil: Impact on microbial biodegradation of organic compounds and possible improvement strategies. Int. J. Mol. Sci. 2013, 14, 10197–10228. [Google Scholar] [CrossRef]
- Thavamani, P.; Megharag, M.; Naidu, R. Bioremediation of high molecular weight polyaromatic hydrocarbons co-contaminated with metals in liquid and soil slurries by metal tolerant PAHs degrading bacterial consortium. Biodegradation 2012, 23, 823–835. [Google Scholar] [CrossRef]
- Oriomah, C.; Adelowo, O.O.; Adekanmbi, A.O. Bacteria from spent engine-oil-contaminated soils possess dual tolerance to hydrocarbon and heavy metals, and degrade spent oil in the presence of copper, lead, zinc and combinations thereof. Ann. Microbiol. 2015, 65, 207–215. [Google Scholar] [CrossRef]
- Pimda, W.; Bunnag, S. Impact of inorganic nutrients and heavy metals present as co-contaminants on biodegradation of petroleum hydrocarbons by Phormidium ambiguum Strain TISTR 8296. Water Air Soil Pollut. 2017, 228, 1–13. [Google Scholar] [CrossRef]
- Ibarrolaza, A.; Coppotelli, B.M.; Delpanno, M.T.; Donati, E.R.; Morelli, I.S. Application of the knowledge-based approach to strain selection for a bioaugmentation process of phenanthrene-and Cr (VI)-contaminated soil. J. Appl. Microbiol. 2011, 111, 26–35. [Google Scholar] [CrossRef]
- Sorkhoh, N.A.; Ali, N.; Alawadhi, H.; Dashti, N.; Al-Mailem, D.; Eliyas, M.; Radwan, S. Phytoremediation of mercury in pristine and crude oil contaminated soils: Contributions of rhizobacteria and their host plants to mercury removal. Ecotoxicol. Environ. Saf. 2010, 73, 1998–2003. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yin, H.; Ye, J.; Peng, H.; Liu, Z.; Dang, Z.; Chang, J. Influence of co-existed benzo[a]pyrene and copper on the cellular characteristics of Stenotrophomonas maltophilia during biodegradation and transformation. Bioresour. Technol. 2014, 158, 181–187. [Google Scholar] [CrossRef]
- Baltrons, O.; Lopez-Mesas, M.; Vilaseca, M.; Gutiérrez-Bouzán, C.; Derf, F.L.; Portet-Koltalo, F.; Palet, C. Influence of a mixture of metals on PAHs biodegradation processes in soils. Sci. Total Environ. 2018, 628–629, 150–158. [Google Scholar] [CrossRef]
- Biswas, B.; Sarkar, B.; Mandal, A.; Naidu, R. Heavy metal-immobilizing organoclay facilitates polycyclic aromatic hydrocarbon biodegradation in mixed-contaminated soil. J. Hazard. Mater. 2015, 298, 129–137. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, 2405–8440. [Google Scholar] [CrossRef]
- Glahn, F.; Schmidt-Heck, W.; Zellmer, S.; Guthke, R.; Wiese, J.; Golka, K.; Hergenröder, R.; Degen, G.H.; Lehmann, T.; Hermes, M.; et al. Cadmium, cobalt and lead cause stress response, cell cycle deregulation and increased steroid as well as xenobiotic metabolism in primary normal human bronchial epithelial cells which is coordinated by at least nine transcription factors. Arch. Toxicol. 2008, 82, 513–524. [Google Scholar] [CrossRef]
- Salam, L.B.; Obayori, S.O.; Nwaokorie, F.O.; Suleiman, A.; Mustapha, R. Metagenomic insights into effects of spent engine oil perturbation on the microbial community composition and function in a tropical agricultural soil. Environ. Sci. Pollut. Res. 2017, 24, 7139–7159. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Zeng, G.M.; Niu, Q.Y.; Gong, J.-L.; Hu, X.-J.; Lu, L.-H.; Zhou, Y.-Y.; Hu, X.; Chen, M.; Yan, M. Effect of Pb (II) on phenanthrene degradation by new isolated Bacillus sp. P1. RSC Adv. 2015, 5, 55812–55818. [Google Scholar] [CrossRef]
- Kaur, D.; Singh, R.P.; Gupta, S. Screening and characterization of next-generation biofuels producing bacterial strains. Curr. Microbiol. 2022, 79, 85. [Google Scholar]
- Maeng, J.H.; Sakai, Y.; Ishige, T.; Tani, Y.; Kato, N. Diversity of dioxygenases that catalyze the first step of oxidation of long-chain n-alkanes in Acinetobacter sp M-1. FEMS Microbiol. Lett. 1996, 141, 177–182. [Google Scholar] [CrossRef]
- Tani, A.; Tsuchimochi, S.; Nakabeppu, Y.; Nakajo, M. Bone and Tl-201 scintigraphy in a case of hereditary multiple exostoses. Clin. Nucl. Med. 2001, 26, 1028–1031. [Google Scholar] [CrossRef] [PubMed]
- Piubeli, F.; Dos Santos, L.G.; Fernandez, E.N.; Silva, F.H.D.; Durrant, L.R.; Grossman, M.J. The emergence of different functionally equivalent PAH degrading microbial communities from a single soil in liquid PAH enrichment cultures and soil microcosms receiving PAHs with and without bioaugmentation. Pol. J. Microbiol. 2018, 67, 365–375. [Google Scholar] [CrossRef]
- Whang, L.M.; Liu, P.; Ma, C.-C.; Cheng, S.-S. Application of rhamnolipid and surfactin for enhanced diesel biodegradation—Effects of pH and ammonium addition. J. Hazard. Mater. 2009, 164, 1045–1050. [Google Scholar] [CrossRef]
- Xia, W.; Li, J.; Xia, Y.; Song, Z.; Zhou, J. Optimization of diesel oil biodegradation in seawater using statistical experimental methodology. Water Sci. Technol. 2012, 66, 1301–1309. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Binupriya, A.R.; Baik, S.-H.; Yun, S.-E. Biodegradation of crude oil by individual bacterial strains and a mixed bacterial consortium isolated from hydrocarbon contaminated areas. Clean Soil Air Water 2008, 36, 92–96. [Google Scholar] [CrossRef]
- Verma, S.; Bhargava, R.; Pruthi, V. Oily sludge degradation by bacteria from Ankleshwar, India. Int. Biodeterior. Biodegrad. 2006, 57, 207–213. [Google Scholar] [CrossRef]
- Pointin, X.; Tang, J.C.; Li, D.S.; Zhang, Q.M. Effect of salinity on the bioremediation of petroleum hydrocarbons in a saline-alkaline soil. Lett. Appl. Microbiol. 2012, 55, 210–217. [Google Scholar]
- Gurav, R.; Lyu, H.; Ma, J.; Tang, J.; Liu, Q.; Zhang, H. Degradation of nalkanes and PAHs from the heavy crude oil using salt-tolerant bacterial consortia and analysis of their catabolic genes. Environ. Sci. Pollut. Res. 2017, 24, 11392–11403. [Google Scholar] [CrossRef] [PubMed]
- Mnif, S.; Sayadi, S.; Chamkha, M. Biodegradative potential and characterization of a novel aromatic-degrading bacterium isolated from a geothermal oil field under saline and thermophilic conditions. Int. Biodeterior. Biodegrad. 2014, 86, 258–264. [Google Scholar] [CrossRef]
- Al Farraj, D.A.; Alkufeidy, R.M.; Alkubaisi, N.A.; Alshammari, M.K. Polynuclear aromatic anthracene biodegradation by psychrophilic Sphingomonas sp., cultivated with Tween-80. Chemosphere 2021, 263, 128115. [Google Scholar] [CrossRef] [PubMed]
- Chandran, P.; Das, N. Degradation of diesel oil by immobilized Candida tropicalis and biofilm formed on gravels. Biodegradation 2011, 22, 1181–1189. [Google Scholar] [CrossRef]
- Greenwood, P.F.; Wibrow, S.; George, S.J.; Tibbett, M. Sequential hydro-carbon biodegradation in a soil from arid coastal Australia treated with oil under laboratory controlled conditions. Org. Geochem. 2008, 39, 1336–1346. [Google Scholar] [CrossRef]
- Han, G.Q.; Wang, B.; Xu, W.H.; Chen, G.Q.; Wang, H.X.; Zhang, H.B.; Zhang, X.J.; Xiong, Z.T. Effects of heavy metal combined pollution soil microbial indicators and soil enzymatic activity. J. Soil Water Conserv. 2010, 24, 238–242. [Google Scholar]
- Luo, Q.; Zhang, J.-G.; Shen, X.-R.; Sui, X.; Fan, Z.-Q. Characterization of a novel diesel oil-degrading Pseudomonas sp. strain F4. Fresenius Environ. Bull. 2013, 22, 689–697. [Google Scholar]
- Kaczynska, G.; Borowik, A.; Wyszkowska, J. Soil dehydrogenases as an indicator of contamination of the environment with petroleum products. Water Air Soil Pollut. 2015, 226, 372. [Google Scholar] [CrossRef]
- Palanisamy, N.; Ramya, J.; Kumar, S.; Vasanthi, N.S.; Chandran, P.; Khan, S. Diesel biodegradation capacities of indigenous bacterial species isolated from diesel contaminated soil. J. Environ. Health Sci. Eng. 2015, 12, 142. [Google Scholar] [CrossRef]


| Soil Property | Value |
|---|---|
| pH | 8.24 |
| Soil organic matter (SOM) (mg/kg) | 78,650 |
| Total N (mg/kg) | 3600 |
| Total P (mg/kg) | 760 |
| As (mg/kg) | 8.6 |
| Total petroleum hydrocarbons (TPH) (mg/kg) | 318.88 |
| N-Alkanes | Initial Concentrations (mg·L−1) | Final Concentrations (mg·L−1) | Degradation Rate (%) |
|---|---|---|---|
| C11 | 11.94 ± 0.80 | 6.12 ± 0.01 | 48.78 ± 0.97 |
| C12 | 113.70 ± 5.87 | 28.82 ± 0.3.6 | 74.65 ± 6.05 |
| C13 | 294.27 ± 18.76 | 36.75 ± 0.82 | 87.51 ± 4.36 |
| C14 | 644.28 ± 43.60 | 120.80 ± 3.03 | 81.25 ± 6.95 |
| C15 | 794.74 ± 54.45 | 128.35 ± 2.78 | 83.85 ± 5.10 |
| C16 | 596.48 ± 40.25 | 93.47 ± 1.78 | 84.33 ± 4.42 |
| C17 | 355.69 ± 25.30 | 52.64 ± 0.89 | 85.20 ± 3.41 |
| C18 | 360.10 ± 12.02 | 57.51 ± 0.61 | 84.03 ± 5.09 |
| C19 | 346.46 ± 22.48 | 48.95 ± 0.86 | 85.87 ± 3.95 |
| C20 | 235.60 ± 18.70 | 37.84 ± 1.44 | 83.94 ± 7.68 |
| C21 | 202.03 ± 14.08 | 21.64 ± 0.54 | 89.29 ± 3.85 |
| C22 | 114.92 ± 9.14 | 12.43 ± 0.33 | 89.18 ± 3.59 |
| C23 | 47.27 ± 3.43 | 10.07 ± 0.29 | 78.70 ± 8.59 |
| C24 | 26.43 ± 2.30 | 1.68 ± 0.21 | 93.65 ± 8.98 |
| C25 | 13.14 ± 0.80 | 0.00 ± 0.00 | 100.00 ± 0.00 |
| Average | 4157.00 ± 246.00 | 663.04 ± 12.57 | 84.05 ± 5.11 |
| Carbon Sources | Degradation Rate (%) |
|---|---|
| C26 | 17.56 ± 0.04 |
| C28 | 18.56 ± 1.77 |
| C30 | 9.94 ± 2.72 |
| C32 | 25.88 ± 3.44 |
| C34 | 20.24 ± 1.32 |
| C36 | 11.16 ± 1.42 |
| C38 | 3.11 ± 1.90 |
| Pristane | 63.18 ± 9.06 |
| Phytane | 70.52 ± 8.58 |
| Naphthalene | 36.62 ± 2.78 |
| Phenanthrene | 26.71 ± 4.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Q.; Yu, J.; Fang, K.; Dong, P.; Li, Z.; Zhang, W.; Liu, M.; Xiang, L.; Cai, J. Microbial Removal of Petroleum Hydrocarbons from Contaminated Soil under Arsenic Stress. Toxics 2023, 11, 143. https://doi.org/10.3390/toxics11020143
Su Q, Yu J, Fang K, Dong P, Li Z, Zhang W, Liu M, Xiang L, Cai J. Microbial Removal of Petroleum Hydrocarbons from Contaminated Soil under Arsenic Stress. Toxics. 2023; 11(2):143. https://doi.org/10.3390/toxics11020143
Chicago/Turabian StyleSu, Qu, Jiang Yu, Kaiqin Fang, Panyue Dong, Zheyong Li, Wuzhu Zhang, Manxia Liu, Luojing Xiang, and Junxiong Cai. 2023. "Microbial Removal of Petroleum Hydrocarbons from Contaminated Soil under Arsenic Stress" Toxics 11, no. 2: 143. https://doi.org/10.3390/toxics11020143
APA StyleSu, Q., Yu, J., Fang, K., Dong, P., Li, Z., Zhang, W., Liu, M., Xiang, L., & Cai, J. (2023). Microbial Removal of Petroleum Hydrocarbons from Contaminated Soil under Arsenic Stress. Toxics, 11(2), 143. https://doi.org/10.3390/toxics11020143








