Epiglottis Cartilage, Costal Cartilage, and Intervertebral Disc Cartilage as Alternative Materials in the Postmortem Diagnosis of Methanol Poisoning
Abstract
:1. Introduction
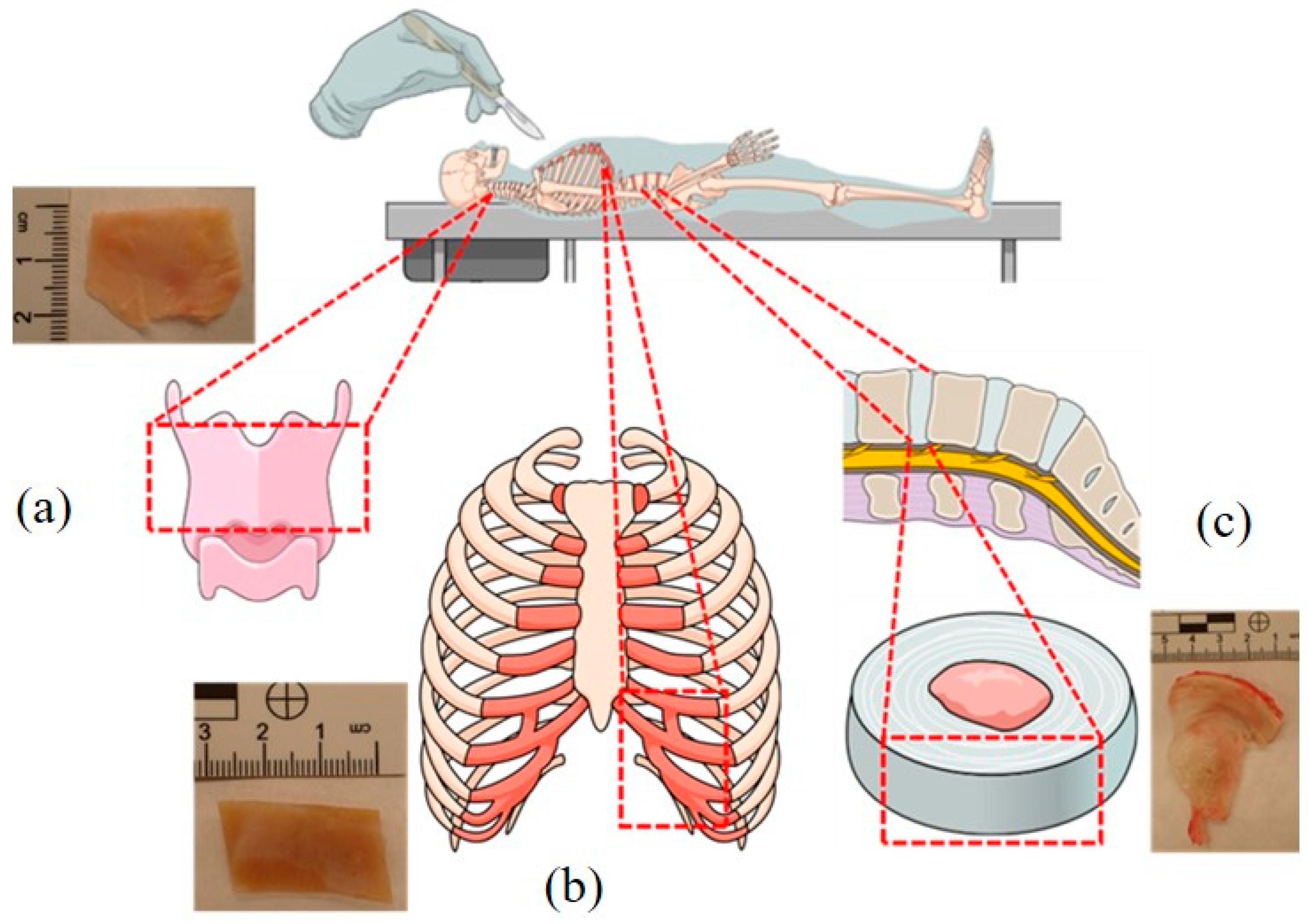
2. Materials and Methods
3. Results
Alcohol Tissue Permeability Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallardo, E.; Queiroz, J.A. The role of alternative specimens in toxicological analysis. Biomed. Chromatogr. 2008, 22, 795–821. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, A.J.; Miller, A.C.; Shaw, L.A.; Champion, J.L.; Neiswonger, M.A. Oral Cavity Fluid as an Investigative Approach for Qualitative and Quantitative Evaluations of Drugs in Postmortem Subjects. J. Anal. Toxicol. 2019, 43, 444–451. [Google Scholar] [CrossRef]
- Truver, M.T.; Palmquist, K.B.; Swortwood, M.J. Oral Fluid and Drug Impairment: Pairing Toxicology with Drug Recognition Expert Observations. J. Anal. Toxicol. 2019, 43, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Kuwayama, K.; Miyaguchi, H.; Kanamori, T.; Tsujikawa, K.; Yamamuro, T.; Segawa, H.; Okada, Y.; Iwata, Y.T. Micro-segmental hair analysis: Detailed procedures and applications in forensic toxicology. Forensic Toxicol. 2022, 40, 215–233. [Google Scholar] [CrossRef]
- Ferreira, C.; Paulino, C.; Quintas, A. Extraction Procedures for Hair Forensic Toxicological Analysis: A Mini-Review. Chem. Res. Toxicol. 2019, 32, 2367–2381. [Google Scholar] [CrossRef]
- Jadoon, S.; Karim, S.; Akram, M.R.; Khan, A.K.; Zia, M.A.; Siddiqi, A.R.; Murtaza, G. Recent Developments in Sweat Analysis and Its Applications. Int. J. Anal. Chem. 2015, 2015, 164974. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ahmed, A.; Sharma, A.; Arya, S. Graphene and Its Derivatives: Synthesis and Application in the Electrochemical Detection of Analytes in Sweat. Biosensors 2022, 12, 910. [Google Scholar] [CrossRef]
- Palmer, K.L.; Krasowski, M.D. Alternate Matrices: Meconium, Cord Tissue, Hair, and Oral Fluid. Methods Mol. Biol. 2019, 1872, 191–197. [Google Scholar] [CrossRef]
- Hernandez, A.; Lacroze, V.; Doudka, N.; Becam, J.; Pourriere-Fabiani, C.; Lacarelle, B.; Solas, C.; Fabresse, N. Determination of Prenatal Substance Exposure Using Meconium and Orbitrap Mass Spectrometry. Toxics 2022, 10, 55. [Google Scholar] [CrossRef]
- Sempio, C.; Wymore, E.; Palmer, C.; Bunik, M.; Henthorn, T.K.; Christians, U.; Klawitter, J. Detection of Cannabinoids by LC–MS-MS and ELISA in Breast Milk. J. Anal. Toxicol. 2021, 45, 686–692. [Google Scholar] [CrossRef]
- Busardò, F.P.; Bertol, E.; Mannocchi, G.; Tittarelli, R.; Pantano, F.; Vaiano, F.; Baglio, G.; Kyriakou, C.; Marinelli, E. Determination of GHB levels in breast milk and correlation with blood concentrations. Forensic Sci. Int. 2016, 265, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Metushi, I.G.; Fitzgerald, R.L.; McIntyre, I.M. Assessment and Comparison of Vitreous Humor as an Alternative Matrix for Forensic Toxicology Screening by GC–MS. J. Anal. Toxicol. 2016, 40, 243–247. [Google Scholar] [CrossRef]
- Bévalot, F.; Cartiser, N.; Bottinelli, C.; Fanton, L.; Guitton, J. Vitreous humor analysis for the detection of xenobiotics in forensic toxicology: A review. Forensic Toxicol. 2016, 34, 12–40. [Google Scholar] [CrossRef]
- Bévalot, F.; Cartiser, N.; Bottinelli, C.; Guitton, J.; Fanton, L. State of the art in bile analysis in forensic toxicology. Forensic Sci. Int. 2016, 259, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Bierly, J.; Labay, L.M. The Utility of Bile in Postmortem Forensic Toxicology. Acad. Forensic Pathol. 2018, 8, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, M.; Wille, S.M.; Fernandez, M.D.M.R.; Di Fazio, V.; Samyn, N.; De Boeck, G.; Bourel, B. Entomotoxicology, experimental set-up and interpretation for forensic toxicologists. Forensic Sci. Int. 2011, 208, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hodecek, J. Revisiting the concept of entomotoxicology. Forensic Sci. Int. Synerg. 2020, 2, 282–286. [Google Scholar] [CrossRef] [PubMed]
- de Campos, E.G.; da Costa, B.R.B.; dos Santos, F.S.; Monedeiro, F.; Alves, M.N.R.; Junior, W.J.R.S.; De Martinis, B.S. Alternative matrices in forensic toxicology: A critical review. Forensic Toxicol. 2022, 40, 1–18. [Google Scholar] [CrossRef]
- Eschweiler, J.; Horn, N.; Rath, B.; Betsch, M.; Baroncini, A.; Tingart, M.; Migliorini, F. The Biomechanics of Cartilage—An Overview. Life 2021, 11, 302. [Google Scholar] [CrossRef]
- Tomsia, M.; Cieśla, J.; Pilch-Kowalczyk, J.; Banaszek, P.; Chełmecka, E. Cartilage Tissue in Forensic Science—State of the Art and Future Research Directions. Processes 2022, 10, 2456. [Google Scholar] [CrossRef]
- Tomsia, M.; Droździok, K.; Javan, G.; Skowronek, R.; Szczepański, M.; Chełmecka, E. Costal cartilage ensures low degradation of DNA needed for genetic identification of human remains retrieved at different decomposition stages and different post-mortem intervals. Postepy Hig. Med. Dosw. 2021, 75, 852–858. [Google Scholar] [CrossRef]
- Becker, J.; Mahlke, N.S.; Ritz-Timme, S.; Boehme, P. The human intervertebral disc as a source of DNA for molecular identi-fication. Forensic Sci. Med. Pathol. 2021, 17, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Tomsia, M.; Droździok, K.; Banaszek, P.; Szczepański, M.; Pałasz, A.; Chełmecka, E. The intervertebral discs’ fibrocartilage as a DNA source for genetic identification in severely charred cadavers. Forensic Sci. Med. Pathol. 2022, 18, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Tomsia, M.; Głaz, M.; Nowicka, J.; Szczepański, M. Sodium nitrite detection in costal cartilage and vitreous humor—Case report of fatal poisoning with sodium nitrite. J. Forensic Leg. Med. 2021, 81, 102186. [Google Scholar] [CrossRef]
- Tomsia, M.; Nowicka, J.; Skowronek, R.; Woś, M.; Wójcik, J.; Droździok, K.; Zorychta, M.; Javan, G.T.; Chełmecka, E. A Comparative Study of Ethanol Concentration in Costal Cartilage in Relation to Blood and Urine. Processes 2020, 8, 1637. [Google Scholar] [CrossRef]
- Tomsia, M.; Nowicka, J.; Skowronek, R.; Javan, G.T.; Chełmecka, E. Concentrations of volatile substances in costal cartilage in relation to blood and urine–preliminary studies. Arch. Forensic Med. Criminol. 2021, 71, 38–46. [Google Scholar] [CrossRef]
- Tian, M.; He, H.; Liu, Y.; Li, R.; Zhu, B.; Cao, Z. Fatal methanol poisoning with different clinical and autopsy findings: Case report and literature review. Leg. Med. Tokyo 2022, 54, 101995. [Google Scholar] [CrossRef]
- Liesivuori, J.; Savolainen, H. Methanol and Formic Acid Toxicity: Biochemical Mechanisms. Pharmacol. Toxicol. 1991, 69, 157–163. [Google Scholar] [CrossRef]
- International Programme on Chemical Safety (IPCS). Methanol; Environmental Health Criteria 196; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Tomsia, M.; Głaz, M.; Nowicka, J.; Cieśla, J.; Sosnowski, M.; Chełmecka, E. Fatal Methanol Poisoning Caused by Drinking Industrial Alcohol: Silesia Region, Poland, April–June. Toxics 2022, 10, 800. [Google Scholar] [CrossRef]
- Abolin, C.; McRae, J.D.; Tozer, T.N.; Takki, S. Gas chromatographic head-space assay of formic acid as methyl formate in biologic fluids: Potential application to methanol poisoning. Biochem. Med. 1980, 23, 209–218. [Google Scholar] [CrossRef]
- Kuo, T. The effects of ethanol on methanol intoxication I. A simple headspace gas chromatography for the determination of blood formic acid. Jpn. J. Leg. Med. 1982, 36, 669–675. [Google Scholar]
- Buszewicz, G.; Teresiński, G.; Mądro, R. Distribution of methanol in biological specimens of victims of a fatal group poisoning. Probl. Forensic Sci. 2004, 59, 115–126. [Google Scholar]
- Cieśla, J.; Tomsia, M. Cadaveric Stem Cells: Their Research Potential and Limitations. Front. Genet. 2021, 12, 798161. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-V.T.; Sykes, K.; Kriet, J.D.; Humphrey, C. Cartilage Graft Donor Site Morbidity following Rhinoplasty and Nasal Reconstruction. Craniomaxillofacial Trauma Reconstr. 2017, 11, 278–284. [Google Scholar] [CrossRef]



| Variable | n | Mean | SD | Median | Q1 | Q3 | xmin | xmax |
|---|---|---|---|---|---|---|---|---|
| Age | 17 | 50.8 | 12.1 | 49.0 | 43.0 | 61.0 | 33.0 | 74.0 |
| t1 [days] | 15 | 5.8 | 4.9 | 3.0 | 1.0 | 12.0 | 1.0 | 13.0 # |
| t2 [days] | 17 | 7.2 | 3.4 | 7.0 | 6.0 | 8.0 | 1.0 | 16.0 |
| Methanol [mg/mL or mg/g *] | ||||||||
| Blood | 17 | 2.53 | 1.89 | 2.69 | 0.00 | 4.28 | <0.1 | 5.14 |
| Urine | 16 | 3.42 | 2.58 | 3.38 | 1.10 | 5.63 | <0.1 | 8.20 |
| Costal cartilage * | 17 | 0.66 | 0.53 | 0.67 | 0.00 | 1.12 | <0.1 | 1.48 |
| Epiglottis * | 17 | 0.95 | 0.91 | 0.71 | 0.00 | 1.62 | <0.1 | 3.16 |
| Annulus fibrosis of intervertebral discs * | 17 | 1.21 | 1.09 | 1.03 | <0.1 | 2.04 | <0.1 | 3.00 |
| Formic acid [mg/mL or mg/g *] | ||||||||
| Blood | 17 | 0.76 | 0.54 | 0.95 | 0.03 | 1.16 | <0.01 | 1.49 |
| Urine | 15 | 2.81 | 2.69 | 2.95 | 0.04 | 4.62 | <0.01 | 8.36 |
| Costal cartilage * | 17 | 0.14 | 0.17 | 0.07 | 0.01 | 0.20 | <0.01 | 0.67 |
| Epiglottis * | 12 | 0.26 | 0.40 | 0.09 | 0.01 | 0.41 | <0.01 | 1.38 |
| Annulus fibrosis of intervertebral discs * | 17 | 0.60 | 1.03 | 0.13 | 0.04 | 0.56 | 0.01 | 4.14 |
| Concentration in the Blood | Concentration in Other Fluids or Tissues | β | SE (β) | r | p |
|---|---|---|---|---|---|
| Methanol | Urine | 1.3043 | 0.1342 | 0.9290 | <0.001 |
| Costal cartilage | 0.2440 | 0.0355 | 0.8714 | <0.001 | |
| Epiglottis | 0.3989 | 0.0713 | 0.8224 | <0.001 | |
| Annulus fibrosis of intervertebral discs | 0.5137 | 0.0672 | 0.8920 | <0.001 | |
| Formic acid | Urine | 3.9346 | 0.8651 | 0.7836 | <0.001 |
| Costal cartilage * | 0.4381 | 0.3123 | 0.3405 | 0.181 | |
| Epiglottis * | 0.8091 | 0.3271 | 0.5382 | <0.05 | |
| Annulus fibrosis of intervertebral discs * | 0.5762 | 0.3829 | 0.4696 | 0.171 |
| Concentration Ratio | Methanol | Ethanol | p | |
|---|---|---|---|---|
| Blood/urine | 0.73 (0.72; 0.84) | 0.79 (0.67; 1.00) | 0.694 | |
| UCC | UCC | GCC | ||
| Blood/cartilage | 3.69 (3.02; 4.92) | 4.39 (3.10; 5.65) | 2.53 (2.18; 3.35) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomsia, M.; Chełmecka, E.; Głaz, M.; Nowicka, J. Epiglottis Cartilage, Costal Cartilage, and Intervertebral Disc Cartilage as Alternative Materials in the Postmortem Diagnosis of Methanol Poisoning. Toxics 2023, 11, 152. https://doi.org/10.3390/toxics11020152
Tomsia M, Chełmecka E, Głaz M, Nowicka J. Epiglottis Cartilage, Costal Cartilage, and Intervertebral Disc Cartilage as Alternative Materials in the Postmortem Diagnosis of Methanol Poisoning. Toxics. 2023; 11(2):152. https://doi.org/10.3390/toxics11020152
Chicago/Turabian StyleTomsia, Marcin, Elżbieta Chełmecka, Małgorzata Głaz, and Joanna Nowicka. 2023. "Epiglottis Cartilage, Costal Cartilage, and Intervertebral Disc Cartilage as Alternative Materials in the Postmortem Diagnosis of Methanol Poisoning" Toxics 11, no. 2: 152. https://doi.org/10.3390/toxics11020152
APA StyleTomsia, M., Chełmecka, E., Głaz, M., & Nowicka, J. (2023). Epiglottis Cartilage, Costal Cartilage, and Intervertebral Disc Cartilage as Alternative Materials in the Postmortem Diagnosis of Methanol Poisoning. Toxics, 11(2), 152. https://doi.org/10.3390/toxics11020152






