Short-Term Exposure Effect of Ambient Fine Particulate Matter, Ozone and Cold Temperature on Emergency Room Visits for Asthma Patients
Abstract
1. Introduction
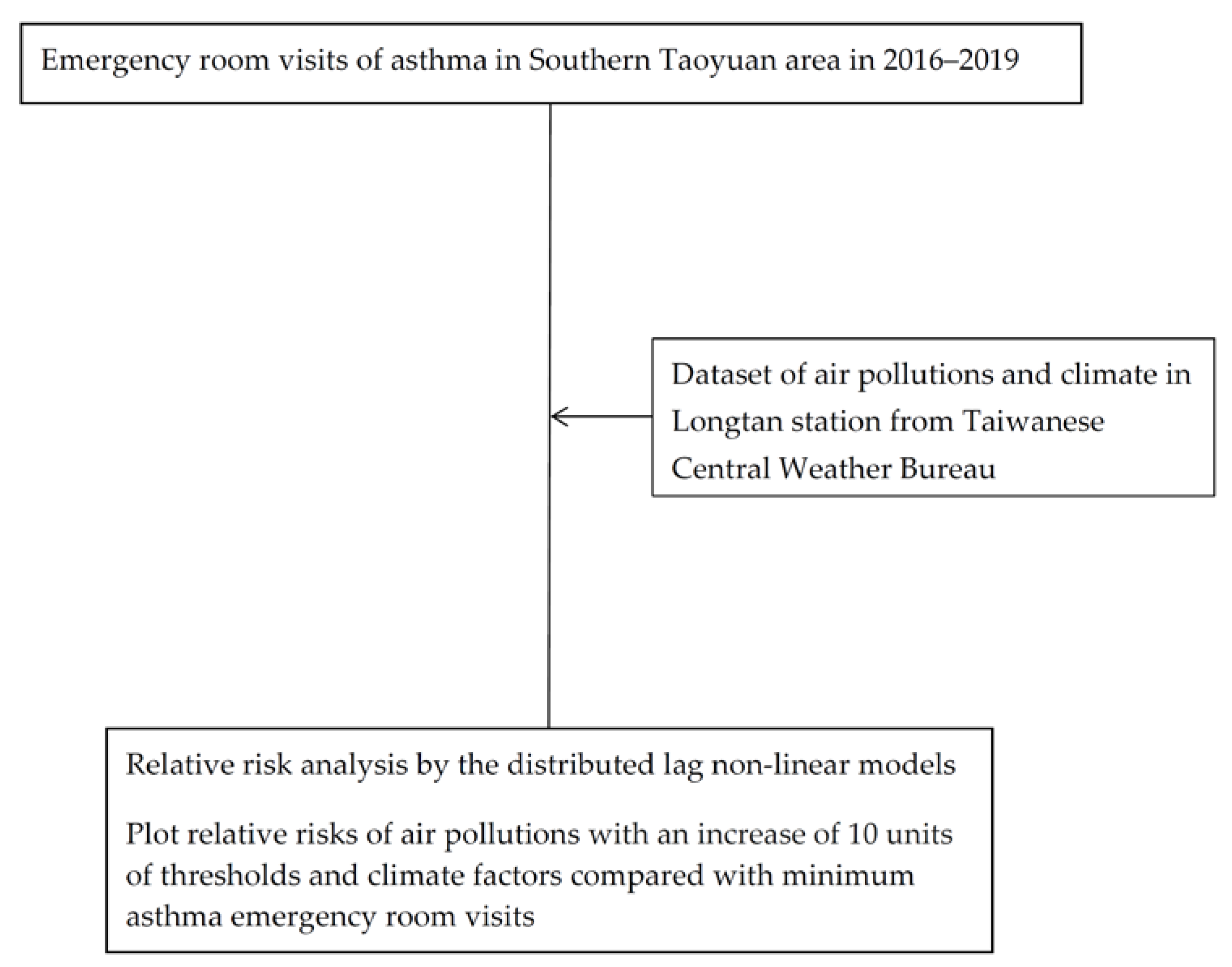
2. Materials and Methods
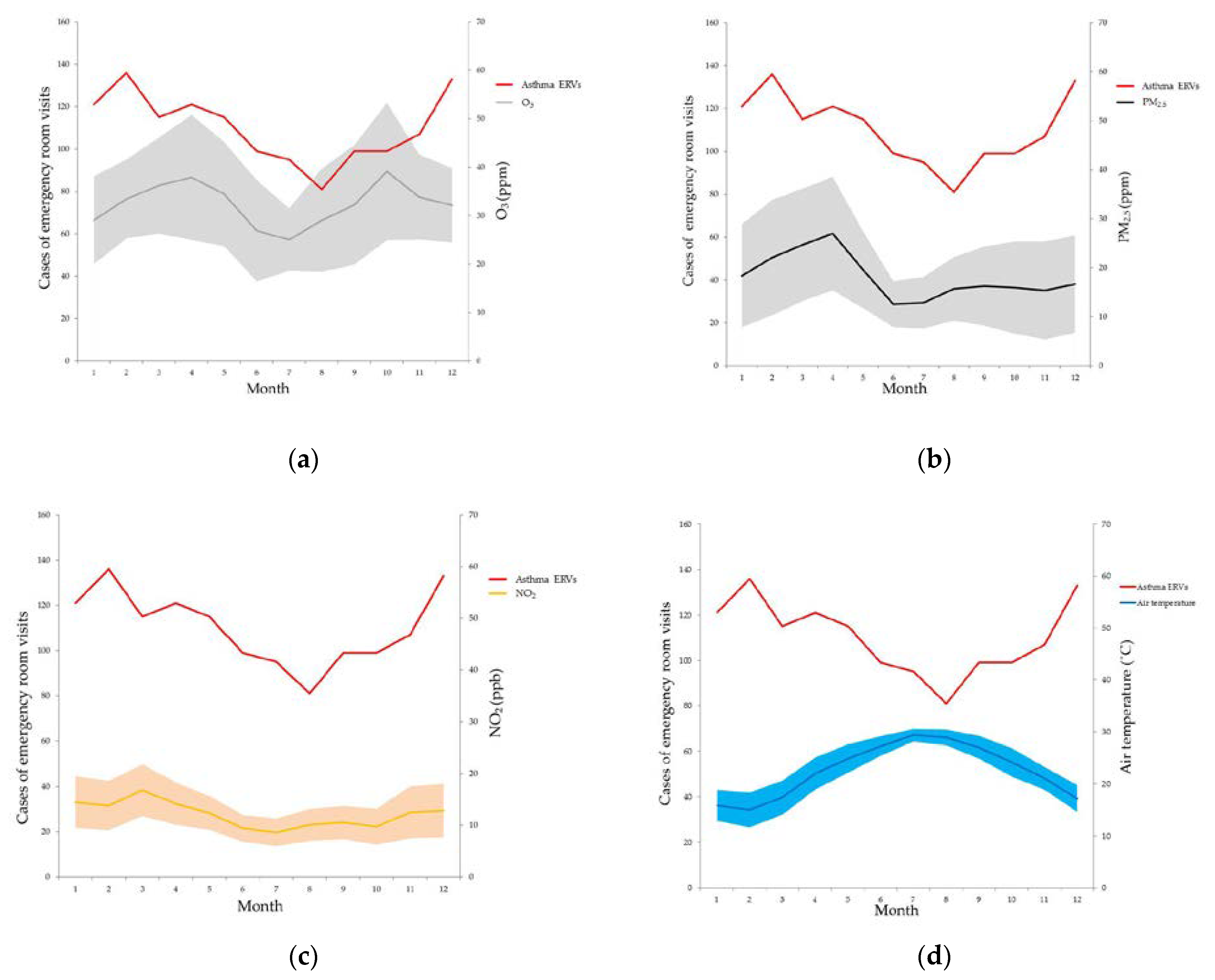
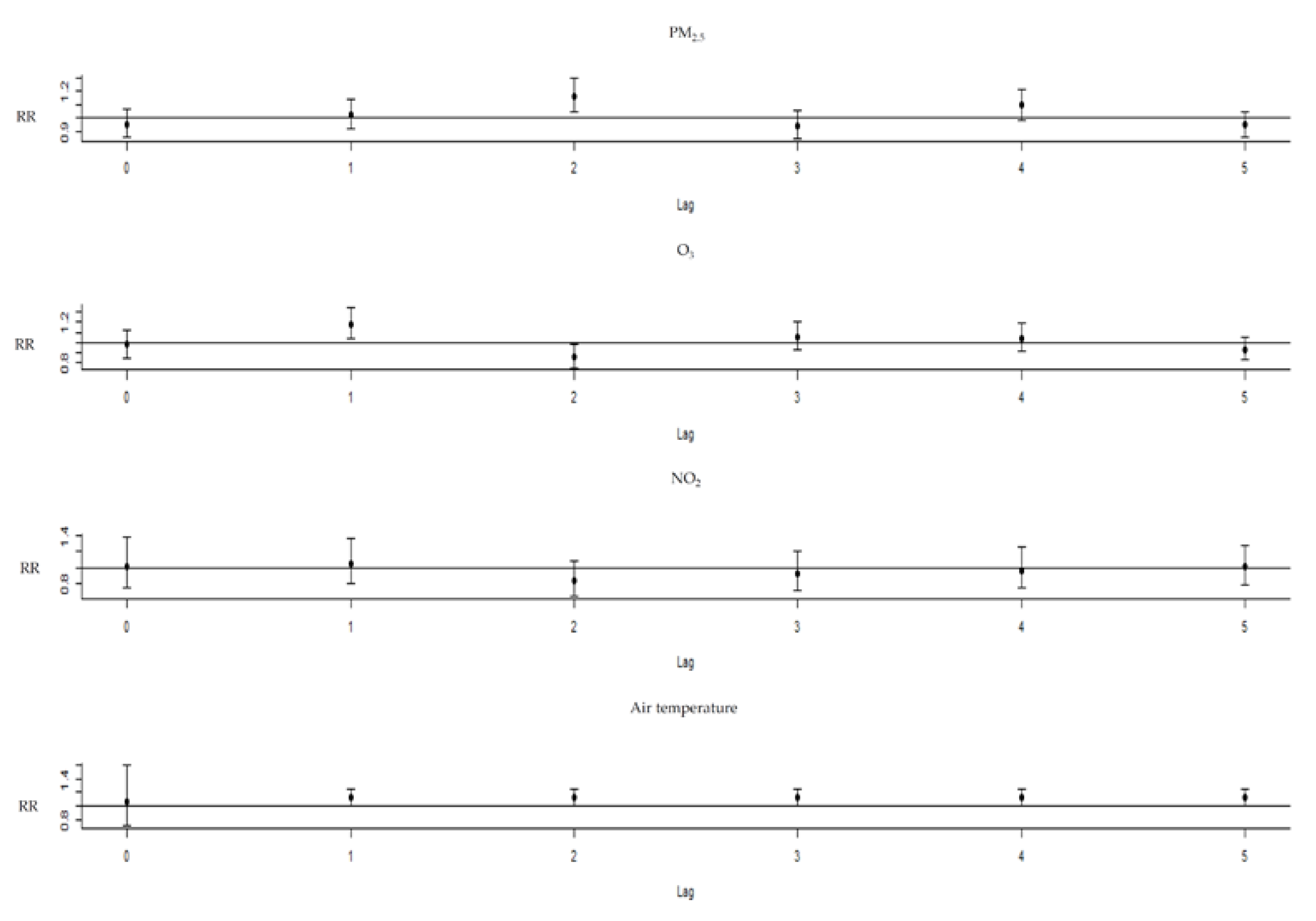
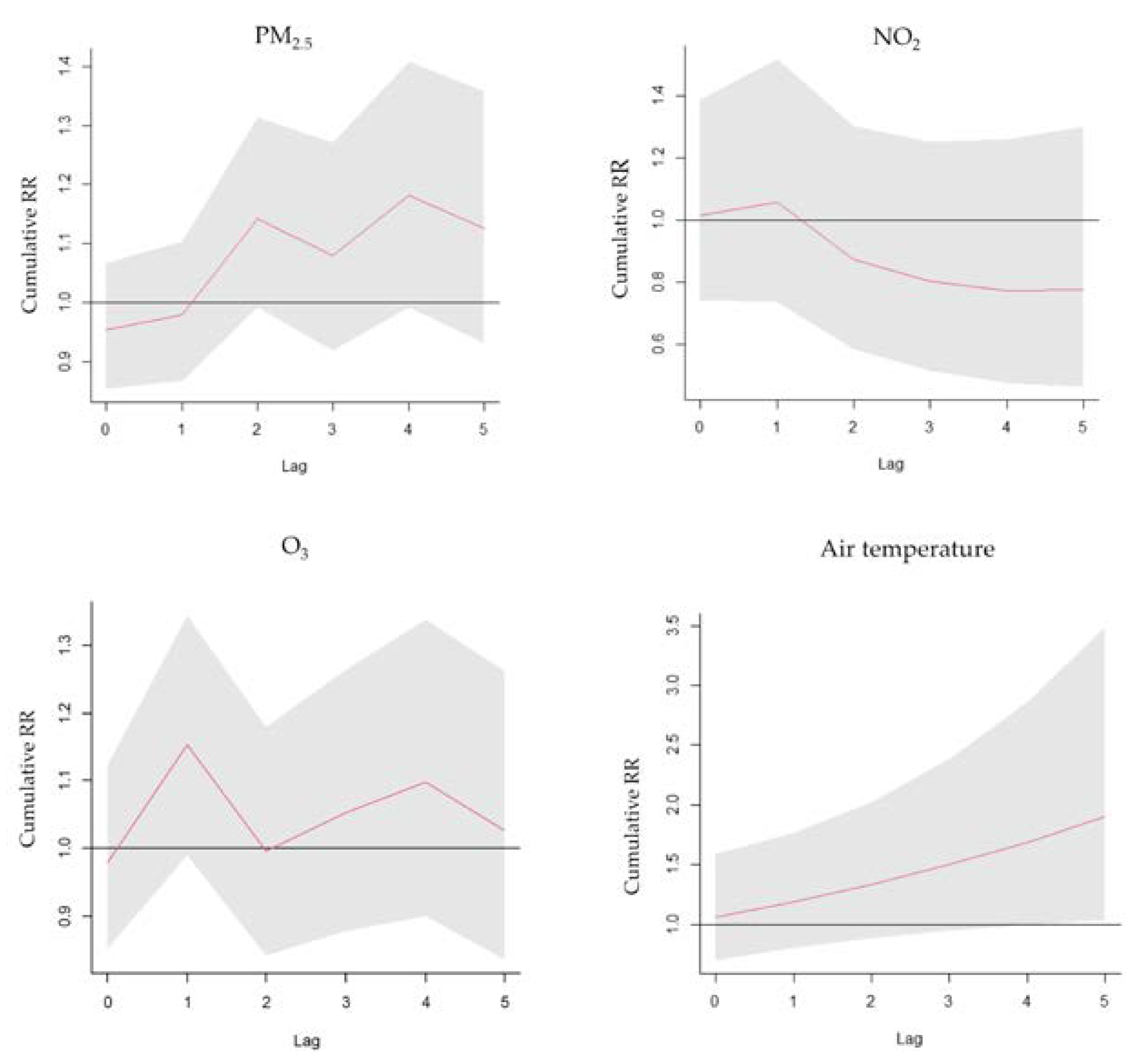
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Asthma Network. Global Asthma Report 2018. 2018. Available online: http://globalasthmareport.org/burden/burden.php (accessed on 11 November 2022).
- Park, H.W.; Tantisira, K.G. Genetic signatures of asthma exacerbation. Allergy Asthma Immunol. Res. 2017, 9, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Adeloye, D.; Salim, H.; Dos Santos, J.P.; Campbell, H.; Sheikh, A.; Rudan, I. Global, regional, and national prevalence of asthma in 2019: A systematic analysis and modelling study. J. Glob. Health 2022, 12, 04052. [Google Scholar] [CrossRef] [PubMed]
- Gushue, C.; Miller, R.; Sheikh, S.; Allen, E.D.; Tobias, J.D.; Hayes, D., Jr.; Tumin, D. Gaps in health insurance coverage and emergency department use among children with asthma. J. Asthma 2019, 56, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.C.; Lin, C.C.; Yang, S.Y.; Chen, H.J.; Li, T.C.; Lin, J.G. Time Trend Analysis of the Prevalence and Incidence of Diagnosed Asthma and Traditional Chinese Medicine Use among Adults in Taiwan from 2000 to 2011: A Population-Based Study. PLoS ONE 2015, 10, e0140318. [Google Scholar] [CrossRef] [PubMed]
- National Health Interview Survey in Taiwan in 2017. 2021. Available online: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=364&pid=13636, (accessed on 11 November 2022).
- Population Health and Welfare Quality Indicators Report in 2017. 2019. Available online: https://www.mohw.gov.tw/cp-3232-18296-1.html (accessed on 11 November 2022).
- World Health Organization. Outdoor air Pollution a Leading Environmental Cause of Cancer Deaths. Available online: https://www.iarc.who.int/wp-content/uploads/2018/07/pr221_E.pdf (accessed on 11 November 2022).
- Vidale, S.; Campana, C. Ambient air pollution and cardiovascular diseases: From bench to bedside. Eur. J. Prev. Cardiol. 2018, 25, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Simkovich, S.M.; Goodman, D.; Roa, C.; Crocker, M.E.; Gianella, G.E.; Kirenga, B.J.; Wise, R.A.; Checkley, W. The health and social implications of household air pollution and respiratory diseases. NPJ Prim. Care Respir. Med. 2019, 29, 12. [Google Scholar] [CrossRef]
- Pope, C.A., III; Coleman, N.; Pond, Z.A.; Burnett, R.T. Fine particulate air pollution and human mortality: 25+ years of cohort studies. Environ. Res. 2020, 183, 108924. [Google Scholar] [CrossRef]
- Cheng, C.G.; Chen, Y.-H.; Yen, S.-Y.; Lin, H.-C.; Lin, H.-C.; Chou, K.-R.; Cheng, C.-A. Air Pollution Exposure and the Relative Risk of Sudden Sensorineural Hearing Loss in Taipei. Int. J. Environ. Res. Public Health 2022, 19, 6144. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Sung, H.-J. Particulate-Matter Related Respiratory Diseases. Tuberc. Respir. Dis. 2020, 83, 116–121. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Hsu, S.-C.; Chang, J.-W.; Lee, C.-L.; Huang, W.-C.; Hsu, Y.-P.; Liu, C.-T.; Jean, S.-S.; Huang, S.-K.; Hsu, C.-W. Differential time-lag effects of ambient PM2.5 and PM2.5-PAHs on asthma emergency department visits. Environ. Sci. Pollut. Res. Int. 2020, 27, 43117–43124. [Google Scholar] [CrossRef] [PubMed]
- Zafirah, Y.; Lin, Y.K.; Andhikaputra, G.; Deng, L.W.; Sung, F.C.; Wang, Y.C. Mortality and morbidity of asthma and chronic obstructive pulmonary disease associated with ambient environment in metropolitans in Taiwan. PLoS ONE 2021, 16, e0253814. [Google Scholar] [CrossRef] [PubMed]
- Schinasi, L.H.; Kenyon, C.C.; Hubbard, R.A.; Zhao, Y.; Maltenfort, M.; Melly, S.J.; Moore, K.; Forrest, C.B.; Roux, A.V.D.; de Roos, A.J. Associations between high ambient temperatures and asthma exacerbation among children in Philadelphia, PA: A time series analysis. Occup. Environ. Med. 2022, 79, 326–332. [Google Scholar] [CrossRef]
- Lam, H.C.Y.; Li, A.M.; Chan, E.Y.Y.; Goggins, W.B. The short-term association between asthma hospitalisationshospitalizations, ambient temperature, other meteorological factors and air pollutants in Hong Kong: A time-series study. Thorax 2016, 71, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Xu, X.; Zhang, Y.; Wang, Q.; Xu, L.; Huo, X. Temperature drop and the risk of asthma:a systematic review and meta-analysis. Environ. Sci. Pollut. Res. 2017, 24, 22535–22546. [Google Scholar] [CrossRef]
- Available online: https://www.longtan.tycg.gov.tw/home.jsp?id=7&parentpath=0,1 (accessed on 11 November 2022).
- Taiwanese Environmental Protection Administration. The Air Pollution Data; Taiwanese Environmental Protection Administration: Taoyuan, Taiwan, 2022. Available online: https://airtw.epa.gov.tw/CHT/Query/His_Data.aspx (accessed on 11 November 2022).
- Gasparrini, A. Distributed Lag Linear and Non-Linear Models in R: The Package dlnm. J Stat Softw. 2011, 43, 1–20. [Google Scholar] [CrossRef]
- Ma, J.-H.; Song, S.-H.; Guo, M.; Zhou, J.; Liu, F.; Peng, L.; Fu, Z.-R. Long-term exposure to PM2.5 lowers influenza virus resistance via down-regulatingby downregulatingating pulmonary macrophage Kdm6a and mediates histones modification in IL-6 and IFN- promoter regions. Biochem. Biophys. Res. Commun. 2017, 493, 1122–1128. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Wang, G.; Ho, K.F.; Han, J.; Dai, W.; Wu, C.; Cao, C.; Liu, L. In- vitro oxidative potential and inflammatory response of ambient PM2.5 in a rural region of Northwest China: Association with chemical compositions and source contribution. Environ. Res. 2022, 205, 112466. [Google Scholar] [CrossRef]
- Li, R.; Kou, X.; Xie, L.; Cheng, F.; Geng, H. Effects of ambient PM2.5 on pathological injury, inflammation, oxidative stress, metabolic enzyme activity, and expression of c-fos and c-jun in lungs of rats. Environ. Sci. Pollut. Res. Int. 2015, 22, 20167–20176. [Google Scholar] [CrossRef]
- Negash, M.; Tsegabrhan, H.; Meles, T.; Tadesse, D.B.; Gidey, G.; Berhane, Y.; Berhanu, K.; Haylemaryam, T. Determinants of Acute Asthma Attack among adult asthmatic patients visiting hospitals of Tigray, Ethiopia, 2019: Case control study. Asthma Res. Pract. 2020, 6, 1. [Google Scholar] [CrossRef]
- Harmon, A.C.; Hebert, V.Y.; Cormier, S.A.; Subramanian, B.; Reed, J.R.; Backes, W.L.; Dugas, T.R. Particulate matter containing environmentally persistent free radicals induces AhR-dependent cytokine and reactive oxygen species production in human bronchial epithelial cells. PLoS ONE 2018, 13, e0205412. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.G.; Morishita, M.; Keeler, G.J.; Harkema, J.R. Divergent effects of urban particulate air pollution on allergic airway responses in experimental asthma: A comparison of field exposure studies. Environ. Health 2012, 11, 45. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, C.; Ji, G.; Liu, H.; Shao, W.; Zhang, C.; Gu, A.; Zhao, P. Effect of exposure to ambient PM2.5 pollution on the risk of respiratory tract diseases: A meta-analysis of cohort studies. J. Biomed. Res. 2017, 31, 130–142. [Google Scholar] [PubMed]
- Xing, Q.; Sun, M. Characteristics of PM2.5 and PM10 Spatio-Temporal Distribution and Influencing Meteorological Conditions in Beijing. Atmosphere 2022, 13, 1120. [Google Scholar] [CrossRef]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.K.; Zhang, Q.; Qiu, Z.; Chung, K.F. Mechanistic impact of outdoor air pollution on asthma and allergic diseases. J. Thorac. Dis. 2015, 7, 23–33. [Google Scholar] [CrossRef]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef]
- Huang, W.; Wu, J.; Lin, X. Ozone Exposure and Asthma Attack in Children. Front. Pediatr. 2022, 10, 830897. [Google Scholar] [CrossRef]
- Mumby, S.; Chung, K.F.; Adcock, I.M. Transcriptional Effects of Ozone and Impact on Airway Inflammation. Front. Immunol. 2019, 10, 1610. [Google Scholar] [CrossRef]
- Sun, S.; Weinberger, K.R.; Nori-Sarma, A.; Spangler, K.R.; Sun, Y.; Dominici, F.; Wellenius, G.A. Ambient heat and risks of emergency department visits among adults in the United States: Time stratified case crossover study. BMJ 2021, 375, e065653. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lin, Y.-K.; Chuang, C.-Y.; Li, M.-H.; Chou, C.-H.; Liao, C.-H.; Sung, F.-C. Associating Emergency room visits with first and prolonged extreme temperature event in Taiwan: A population-based cohort study. Sci. Total Environ. 2012, 416, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Huang, C.; Hu, W.; Turner, L.R.; Su, H.; Tong, S. Extreme temperatures and emergency department admissions for childhood asthma in Brisbane, Australia. Occup. Environ. Med. 2013, 70, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Tokuda, Y.; Ohde, S.; Ishimatsu, S.; Nakamura, T.; Birrer, R.B. The relationship of short-term air pollution and weather to ED visits for asthma in Japan. Am. J. Emerg. Med. 2009, 27, 153–159. (In Tokyo) [Google Scholar] [CrossRef]
- Lee, S.L.; Wong, W.H.S.; Lau, Y.L. Association between air pollution and asthma admission among children in Hong Kong. Clin. Exp. Allergy 2006, 36, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Survey Results of Taiwanese Smoking Behavior. 2021. Available online: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=1718&pid=9913 (accessed on 11 November 2022).
- Lai, L.-W.; Lin, C.-Y. Influence of the Geographic Channel Effect on PM2.5 Concentrations over the Taipei Basin in Relation to Continental High-Pressure Systems during Winter. Atmosphere 2022, 13, 1539. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Chang, C.-K.; Chang, S.-C.; Chen, P.-S.; Lin, C.; Wang, Y.-C. Temperature, nitrogen dioxide, circulating respiratory viruses and acute upper respiratory infections among children in Taipei, Taiwan: A population-based study. Environ. Res. 2013, 120, 109–118. [Google Scholar] [CrossRef]
- WHO Air Pollution Guideline Values. 2022. Available online: https://apps.who.int/iris/bitstream/handle/10665/345329/9789240034228-eng.pdf (accessed on 11 November 2022).
| Asthma Emergency Room Visits (1321) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 43.43 ± 29.44 | |||||||||||
| Gender | Males | Females | ||||||||||
| 679 | 642 | |||||||||||
| Age group (years old) | Children (<18) | Younger (18–44) | Middle-aged (45–64) | Older (>65) | ||||||||
| 423 (32.02%) | 228 (17.26%) | 272 (20.59%) | 378 (30.7%) | |||||||||
| Outcomes | Discharged | Hospitalization | Discharged against medical advice | Mortality | ||||||||
| 999 (75.62%) | 296 (22.41%) | 19 (1.44%) | 1 (0.08%) | |||||||||
| Median | 0.25 | 0.5 | 0.75 | Minimum | Maximum | |||||||
| Pm2.5 (ppm) | 16 | 11 | 18.14 | 23 | 3 | 80 | ||||||
| PM10 (ppm) | 36 | 27 | 38.02 | 47 | 5 | 117 | ||||||
| O3 (ppm) | 31.5 | 24.3 | 32.44 | 39.02 | 5.3 | 77.4 | ||||||
| NO2 (ppb) | 11.26 | 8.75 | 12.11 | 14.89 | 1.89 | 30.46 | ||||||
| CO (ppm) | 0.34 | 0.27 | 0.3552 | 0.43 | 0.08 | 0.86 | ||||||
| SO2 (ppb) | 2.4 | 2 | 2.438 | 2.8 | 0.3 | 7.4 | ||||||
| Air temperature (°C) | 23.03 | 18.17 | 22.56 | 27.61 | 3.41 | 32.02 | ||||||
| Relative humidity (%) | 76.72 | 70.72 | 77.47 | 84.54 | 43.93 | 99.7 | ||||||
| PM2.5 | PM10 | O3 | NO2 | SO2 | CO | AT | RH | |
|---|---|---|---|---|---|---|---|---|
| PM2.5 | 1.000 | 0.884 | 0.255 | 0.518 | 0.461 | 0.645 | −0.105 | −0.184 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| PM10 | 0.884 | 1 | 0.24 | 0.483 | 0.378 | 0.575 | −0.113 | −0.242 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| O3 | 0.255 | 0.24 | 1.000 | −0.091 | 0.096 | 0.059 | −0.25 | −0.25 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | 0.025 | <0.001 | <0.001 | |
| NO2 | 0.518 | 0.483 | −0.091 | 1 | 0.415 | 0.846 | −0.316 | 0.195 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| SO2 | 0.461 | 0.378 | 0.096 | 0.415 | 1 | 0.348 | 0.06 | −0.106 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.022 | <0.001 | |
| CO | 0.645 | 0.575 | 0.059 | 0.846 | 0.348 | 1 | −0.432 | 0.23 |
| p | <0.001 | <0.001 | 0.025 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Air temperature | −0.105 | −0.113 | −0.25 | −0.316 | 0.06 | −0.432 | 1 | −0.39 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | 0.022 | <0.001 | <0.001 | |
| Relative humidity | −0.184 | −0.242 | −0.25 | 0.195 | −0.106 | 0.23 | −0.39 | 1 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-G.; Yen, S.-Y.; Hsiao, C.-C.; Lin, Y.-Y.; Chang, Y.-H.; Chen, Y.-H.; Cheng, C.-A. Short-Term Exposure Effect of Ambient Fine Particulate Matter, Ozone and Cold Temperature on Emergency Room Visits for Asthma Patients. Toxics 2023, 11, 94. https://doi.org/10.3390/toxics11020094
Cheng C-G, Yen S-Y, Hsiao C-C, Lin Y-Y, Chang Y-H, Chen Y-H, Cheng C-A. Short-Term Exposure Effect of Ambient Fine Particulate Matter, Ozone and Cold Temperature on Emergency Room Visits for Asthma Patients. Toxics. 2023; 11(2):94. https://doi.org/10.3390/toxics11020094
Chicago/Turabian StyleCheng, Chun-Gu, Shang-Yih Yen, Chih-Chun Hsiao, Yen-Yue Lin, Yin-Han Chang, Yu-Hsuan Chen, and Chun-An Cheng. 2023. "Short-Term Exposure Effect of Ambient Fine Particulate Matter, Ozone and Cold Temperature on Emergency Room Visits for Asthma Patients" Toxics 11, no. 2: 94. https://doi.org/10.3390/toxics11020094
APA StyleCheng, C.-G., Yen, S.-Y., Hsiao, C.-C., Lin, Y.-Y., Chang, Y.-H., Chen, Y.-H., & Cheng, C.-A. (2023). Short-Term Exposure Effect of Ambient Fine Particulate Matter, Ozone and Cold Temperature on Emergency Room Visits for Asthma Patients. Toxics, 11(2), 94. https://doi.org/10.3390/toxics11020094






