Simultaneous Determination of Nine Phthalates in Vegetable Oil by Atmospheric Pressure Gas Chromatography with Tandem Mass Spectrometry (APGC-MS/MS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Sample Preparation
2.3. Phthalates Analysis by APGC-MS/MS
3. Results and Discussion
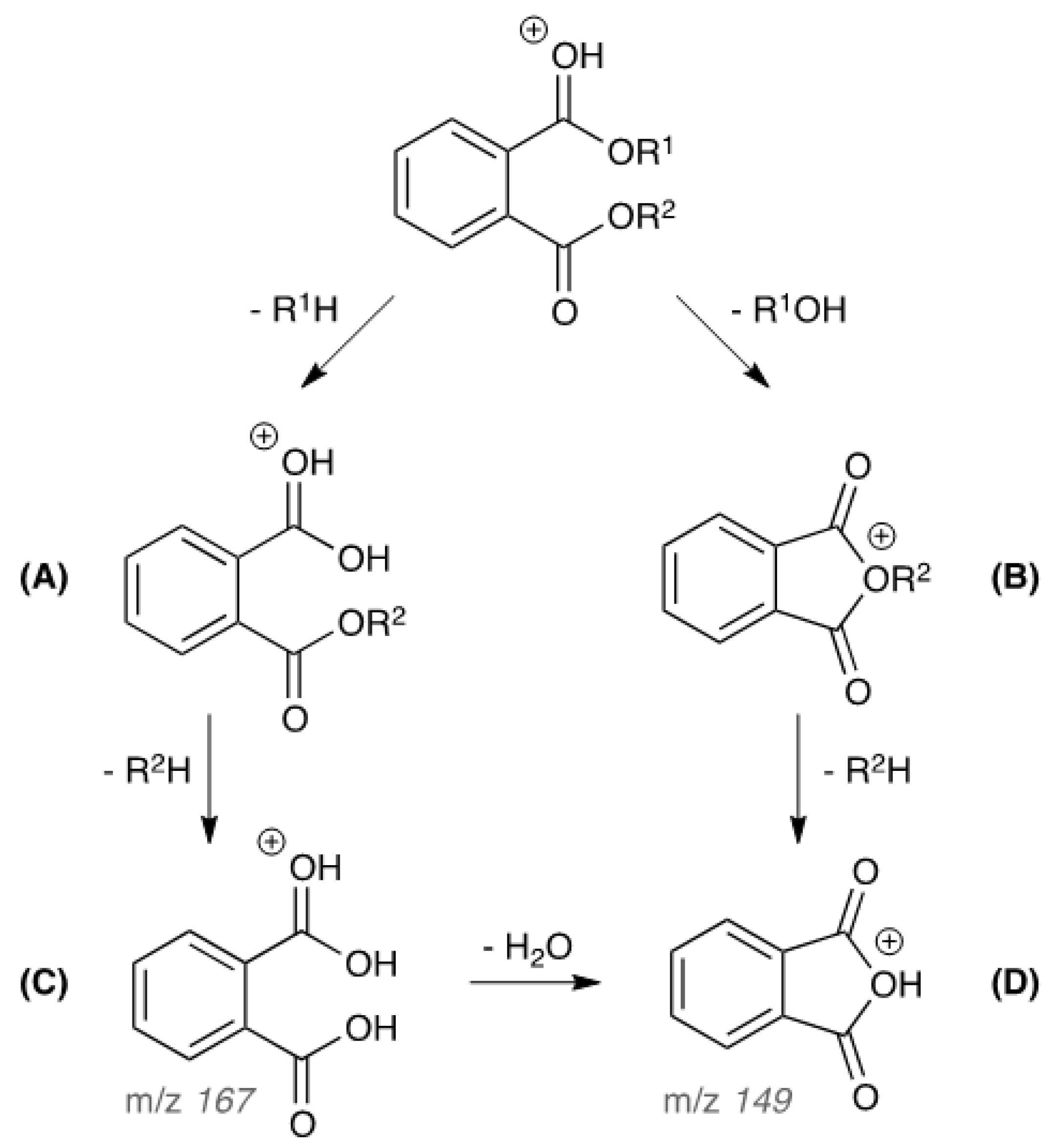
3.1. Optimizing APGC-MS/MS Conditions for Phthalates Analysis
3.2. Matrix Effect (ME)
3.3. Method Detection Limit (MDL) and Method Quantitation Limit (MQL)
3.4. Accuracy
3.5. Analysis of Phthalates in Different Types of Vegetable Oil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, X.L. Phthalate Esters in Foods: Sources, Occurrence, and Analytical Methods. Compr. Rev. Food Sci. Food Saf. 2009, 9, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Sungur, S.; Okur, R.; Turgut, F.H.; Ustun, I.; Gokce, C. Migrated phthalate levels into edible oils. Food Addit. Contam. B Surveill 2015, 8, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical Review on the Presence of Phthalates in Food and Evidence of Their Biological Impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.E.; Braun, J.; Trasande, L.; Dills, R.; Sathyanarayana, S. Phthalates and diet: A review of the food monitoring and epidemiology data. Environ. Health 2014, 13, 43. [Google Scholar] [CrossRef]
- Ventric, P.; Ventric, D.; Russo, E.; Sarro, G.D. Phthalates: European regulation, chemistry, pharmacokinetic and related toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 88–96. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2018/2005 of 17 December 2018 amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Bis(2-ethylhexyl) Phthalate (DEHP), Dibutyl Phthalate (DBP), Benzyl Butyl Phthalate (BBP) and Diisobutyl Phthalate (DIBP). OJ L 322. 18 December 2018; pp. 14–19. Available online: https://www.legislation.gov.uk/eur/2018/2005/contents (accessed on 23 December 2022).
- Luo, Q.; Liu, Z.H.; Yin, H.; Dang, Z.; Wu, P.X.; Zhu, N.W.; Lin, Z.; Liu, Y. Global review of phthalates in edible oil: An emerging and nonnegligible exposure source to human. Sci. Total Environ. 2020, 704, 135369. [Google Scholar] [CrossRef]
- Wang, S.Y.; Wng, M.Q.; Yang, E.Q.; Chen, X.M.; Pan, F.G. Review on Occurrence, Sources of Contamination, and Mitigation Strategies of Phthalates in Vegetable Oils. Eur. J. Lipid Sci. Technol. 2022, 124, 2100086. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the update of the risk assessment of di-butylphthalate(DBP), butyl-benzyl-phthalate (BBP), bis(2-ethylhexyl)phthalate (DEHP), di-isononylphthalate (DINP) anddi-isodecylphthalate (DIDP) for use in food contact materials. EFSA J. 2019, 17, 5838. [Google Scholar]
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. OJ L 12. 15 January 2011, pp. 1–89. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:012:0001:0089:en:PDF (accessed on 23 December 2022).
- GB 9685-2016; National Food Safety Standard: Standard for the Use of Additives in Food Contact Materials and Articles. The National Health and Family Planning Commission (NHFPC) of the People’s Republic of China: Beijing, China, 2016.
- China State Administration for Market Regulation, Guiding Opinions of the State Administration for Market Regulation on Prevention and Control of Pollution Risks of “Plasticizers” in Foods, 2019, No.214. Available online: https://gkml.samr.gov.cn/nsjg/spscs/201911/t20191103_323790.html (accessed on 29 June 2022).
- Tang, Z.; Gong, Z.; Jia, W.; Shen, W.; Han, Q.; Fang, F.; Peng, C. Occurrence and exposure risk assessment of phthalate esters in edible plant oils with a high-frequency import rate in west China. RSC Adv. 2022, 12, 7383–7390. [Google Scholar] [CrossRef] [PubMed]
- Sálamo, J.G.; Rodríguez, B.S.; Borges, J.H. Analytical methods for the determination of phthalates in food. Curr. Opin. Food Sci. 2018, 22, 122–136. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wang, Y.; Ruan, J.; Zhang, J.; Sun, C. Recent advances in analysis of phthalate esters in foods. TrAC Trends Anal. Chem. 2015, 72, 10–26. [Google Scholar] [CrossRef]
- Barp, L.; Purcaro, G.; Franchina, F.A.; Zoccali, M.; Sciarrone, D.; Tranchida, P.Q.; Mondello, L. Determination of phthalate esters in vegetable oils using direct immersion solid-phase microextraction and fast gas chromatography coupled with triple quadrupole mass spectrometry. Anal. Chim. Acta 2015, 887, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, M.M.; Liu, Y.L. Concentration and survey of phthalic acid esters in edible vegetable oils and oilseeds by gas chromatography-mass spectrometry in China. Food Control 2016, 68, 118–123. [Google Scholar] [CrossRef]
- Lamb, A.; Roberts, D.; Cojocariu, C. Routine determination of phthalates in vegetable oil by single quadrupole GC-MS. Thermo Fish. Sci. Appl. Note 2018, 10589. [Google Scholar]
- Horning, E.C.; Horning, M.G.; Carroll, D.I.; Dzidic, I.; Stillwell, R.N. New picogram detection system based on a mass spectrometer with an external ionization source at atmospheric pressure. Anal. Chem. 1973, 45, 936–943. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, J.; Yang, R.; Zhang, J.; Shao, B. Atmospheric pressure chemical ionization source as an advantageous technique for gas chromatography-tandem mass spectrometry. TrAC Trends Anal. Chem. 2020, 132, 116053. [Google Scholar] [CrossRef]
- Shida, S.S.; Nagata, M.; Nemoto, S.; Akiyama, H. Multi-residue determination of pesticides in green tea by gas chromatography-tandem mass spectrometry with atmospheric pressure chemical ionisation using nitrogen as the carrier gas. Food Addit. Contam. Part A 2021, 38, 125–135. [Google Scholar] [CrossRef]
- Fang, J.; Zhao, H.; Zhang, Y.; Lu, M.; Cai, Z. Atmospheric pressure chemical ionization in gas chromatography-mass spectrometry for the analysis of persistent organic pollutants. Trends Environ. Anal. Chem. 2020, 25, e00076. [Google Scholar] [CrossRef]
- Organitini, K.L.; Hailmovici, L.; Jobs, K.J.; Reiner, E.J.; Ladak, A.; Stevens, D.; Cochran, J.W.; Dorman, F.L. Comparison of Atmospheric Pressure Ionization Gas Chromatography-Triple Quadrupole Mass Spectrometry to Traditional High-Resolution Mass Spectrometry for the Identification and Quantification of Halogenated Dioxins and Furans. Anal. Chem. 2015, 87, 7902–7908. [Google Scholar] [CrossRef] [PubMed]
- Hammond, E.W. VEGETABLE OILS/Types and Properties. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 5899–5904. [Google Scholar] [CrossRef]
- Dupont, J. VEGETABLE OILS/Dietary Importance. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 5921–5925. [Google Scholar] [CrossRef]
- Raposo, F.; Barceló, D. Challenges and strategies of matrix effects using chromatography-mass spectrometry: An overview from research versus regulatory viewpoints. TrAC Trends Anal. Chem. 2021, 134, 116068. [Google Scholar] [CrossRef]
- Tan, J.; Lee, H.H.; Wong, L.; Fong, C.; Ong, A.; Lin, Q.; Xiao, Y. Simultaneous determination of neutral and acidic human milk oligosaccharides (HMOs) by liquid chromatography with tandem mass spectrometry (LC-MS/MS). Appl. Food Res. 2022, 2, 100153. [Google Scholar] [CrossRef]
- Wisconsin Department of Natural Resources Laboratory Certification Program, Analytical Detection Limit Guidance & Laboratory Guide for Determining Method Detectio Limits. 1996. Available online: http://www.iatl.com/content/file/LOD%20Guidance%20Document.pdf (accessed on 15 September 2022).
- EPA US Method 556.1: Determination of Carbonyl Compounds in Drinking Water by Fast Gas Chromatography. 1999. Available online: https://www.epa.gov/esam/epa-method-5561-determination-carbonyl-compounds-drinking-water-fast-gas-chromatography (accessed on 15 September 2022).

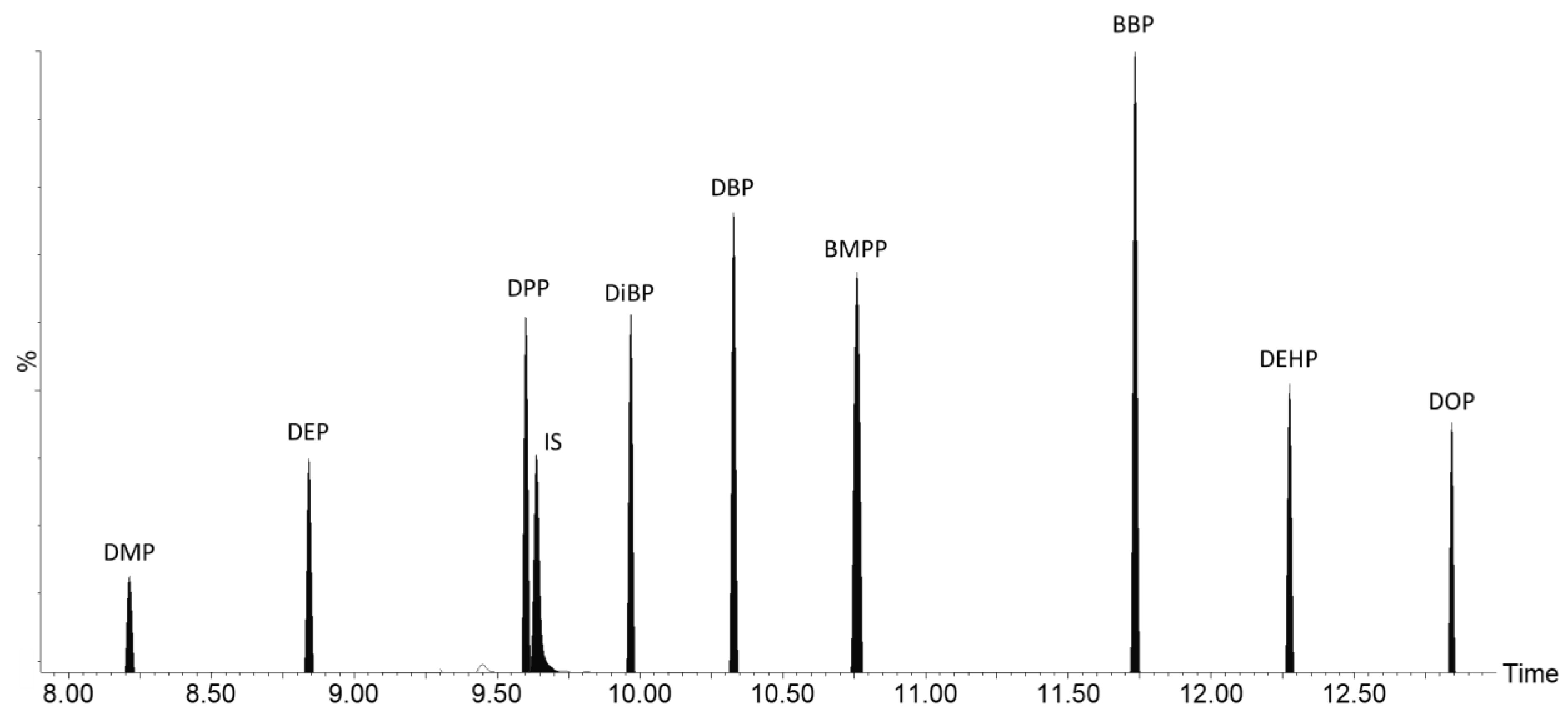
| Compound | RT (Min) | Precursor Ion | Product Ion | Collision Energy |
|---|---|---|---|---|
| DMP | 8.21 | 195.0 | 163.0 * | 7 |
| DEP | 8.84 | 223.1 | 149.0 | 15 |
| 223.1 | 177.1 * | 5 | ||
| DPP | 9.59 | 251.0 | 121.0 | 30 |
| 251.0 | 191.0 * | 5 | ||
| DiBP | 9.96 | 279.0 | 121.0 | 40 |
| 279.0 | 205.0 * | 5 | ||
| DBP | 10.32 | 279.0 | 121.0 | 40 |
| 279.0 | 205.0 * | 5 | ||
| BBP | 11.73 | 313.2 | 91.0 | 20 |
| 313.2 | 205.0 * | 5 | ||
| BMPP | 10.76 | 335.0 | 167.0 * | 10 |
| 335.0 | 251.0 | 5 | ||
| DEHP | 12.27 | 391.0 | 149.0 | 10 |
| 391.0 | 167.0 * | 10 | ||
| DOP | 12.84 | 391.4 | 149.0 | 20 |
| 391.4 | 261.2 * | 10 |
| Compound | Linearity (R2) | MDLsol (µg/L) a | MQLsol (µg/L) b | MQLoil (mg/kg) c |
|---|---|---|---|---|
| DMP | 0.998 | 0.71 | 2.45 | 0.025 |
| DEP | 0.997 | 1.67 | 5.75 | 0.058 |
| DPP | 0.999 | 0.94 | 3.24 | 0.032 |
| DiBP | 0.999 | 0.92 | 3.18 | 0.032 |
| DBP | 0.998 | 1.62 | 5.59 | 0.056 |
| BBP | 0.994 | 0.79 | 2.73 | 0.027 |
| BMPP | 0.997 | 0.43 | 1.48 | 0.015 |
| DEHP | 0.996 | 1.28 | 4.43 | 0.044 |
| DOP | 0.995 | 1.25 | 4.33 | 0.043 |
| Compound | 0.2 mg/kg | 1 mg/kg | ||||||
|---|---|---|---|---|---|---|---|---|
| Intra-Day (n = 7) | Inter-Day (n = 6) | Intra-Day (n = 7) | Inter-Day (n = 6) | |||||
| R * (%) | RSD (%) | R (%) | RSD (%) | R (%) | RSD (%) | R (%) | RSD (%) | |
| DMP | 104 | 4.41 | 105 | 8.66 | 106 | 4.82 | 98 | 4.56 |
| DEP | 99 | 12.0 | 101 | 7.05 | 114 | 3.67 | 109 | 4.76 |
| DPP | 112 | 3.54 | 103 | 8.19 | 113 | 2.92 | 106 | 5.29 |
| DiBP | 109 | 3.20 | 98 | 10.3 | 98 | 2.86 | 100 | 6.02 |
| DBP | 111 | 4.67 | 96 | 14.4 | 112 | 3.02 | 99 | 9.04 |
| BBP | 104 | 9.32 | 92 | 8.12 | 106 | 6.72 | 93 | 6.49 |
| BMPP | 108 | 7.28 | 95 | 8.92 | 107 | 3.72 | 94 | 7.96 |
| DEHP | 102 | 6.41 | 92 | 8.90 | 107 | 7.45 | 89 | 14.1 |
| DOP | 102 | 6.07 | 89 | 13.6 | 101 | 5.80 | 91 | 7.30 |
| Oil Sample | DMP | DEP | DPP | DiBP | DBP | BBP | BMPP | DEHP | DnOP |
|---|---|---|---|---|---|---|---|---|---|
| Palm Oil 1 | - a | - | - | - | - | - | - | 0.05 ± 0.004 | - |
| Palm Oil 2 | - | - | - | - | 0.12 ± 0.02 b | - | - | 0.13 ± 0.02 | - |
| Palm Oil 3 | - | - | - | - | - | - | - | 0.13 ± 0.02 | - |
| Soybean Cooking Oil | - | - | - | - | 0.13 ± 0.02 | - | - | 0.07 ± 0.01 | - |
| Olive Oil | - | - | - | - | - | - | - | 0.17 ± 0.03 | - |
| Coconut Oil | - | - | - | - | - | - | - | - | - |
| Canola Oil | - | - | - | - | - | - | - | 0.23 ± 0.04 | - |
| Corn Oil | - | - | - | - | 0.06 ± 0.004 | - | - | 0.39 ± 0.03 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Wong, W.Y.; Chan, L.Y.; Yong, C.K.; Abe, K.; Hancock, P.; Hird, S. Simultaneous Determination of Nine Phthalates in Vegetable Oil by Atmospheric Pressure Gas Chromatography with Tandem Mass Spectrometry (APGC-MS/MS). Toxics 2023, 11, 200. https://doi.org/10.3390/toxics11030200
Xiao Y, Wong WY, Chan LY, Yong CK, Abe K, Hancock P, Hird S. Simultaneous Determination of Nine Phthalates in Vegetable Oil by Atmospheric Pressure Gas Chromatography with Tandem Mass Spectrometry (APGC-MS/MS). Toxics. 2023; 11(3):200. https://doi.org/10.3390/toxics11030200
Chicago/Turabian StyleXiao, Yongjun, Wen Yee Wong, Li Yan Chan, Chee Keat Yong, Kosuke Abe, Peter Hancock, and Simon Hird. 2023. "Simultaneous Determination of Nine Phthalates in Vegetable Oil by Atmospheric Pressure Gas Chromatography with Tandem Mass Spectrometry (APGC-MS/MS)" Toxics 11, no. 3: 200. https://doi.org/10.3390/toxics11030200
APA StyleXiao, Y., Wong, W. Y., Chan, L. Y., Yong, C. K., Abe, K., Hancock, P., & Hird, S. (2023). Simultaneous Determination of Nine Phthalates in Vegetable Oil by Atmospheric Pressure Gas Chromatography with Tandem Mass Spectrometry (APGC-MS/MS). Toxics, 11(3), 200. https://doi.org/10.3390/toxics11030200








