Adverse Childhood Experiences (ACEs) and Environmental Exposures on Neurocognitive Outcomes in Children: Empirical Evidence, Potential Mechanisms, and Implications
Abstract
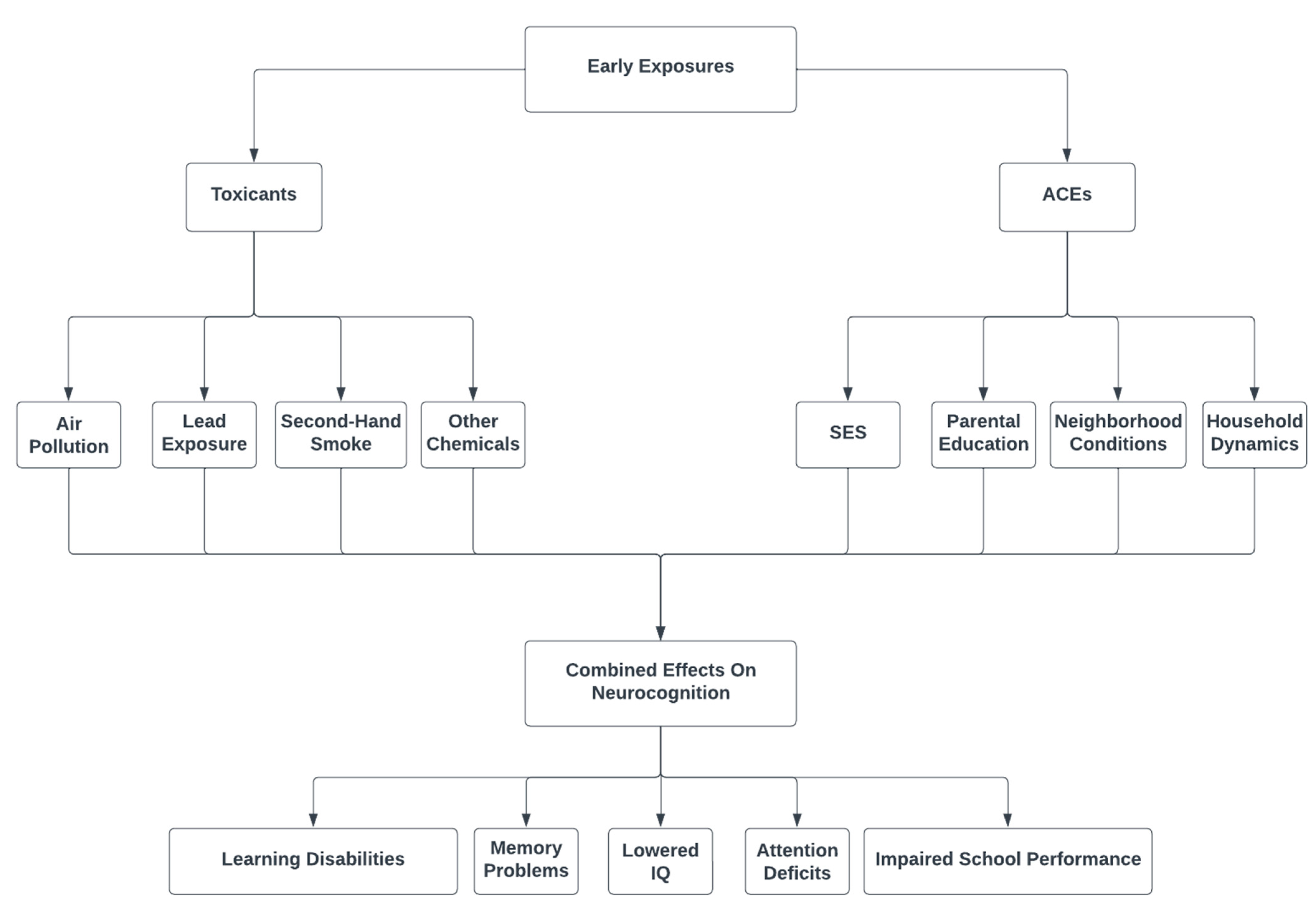
:1. Introduction
2. Methods
3. ACEs and Air Pollution
4. Potential Mechanisms of Action
5. ACEs and Lead Exposure
6. Potential Mechanism of Action
7. Other Sources of Environmental Toxicity
8. Potential Mechanism of Action
9. Implications and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Lewis, G. Environmental toxicity and poor cognitive outcomes in children and adults. J. Environ. Health 2014, 76, 130. [Google Scholar] [PubMed]
- Guxens, M.; Lubczyńska, M.J.; Muetzel, R.L.; Dalmau-Bueno, A.; Jaddoe, V.W.V.; Hoek, G.; Lugt, A.V.D.; Verhulst, F.C.; White, T.; Brunekreef, B.; et al. Air pollution exposure during fetal life, brain morphology, and cognitive function in school-age children. Biol. Psychiatry 2018, 84, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.M.; Kordas, K.; Golding, J.; Emond, A.M. Effects of low-level prenatal lead exposure on child IQ at 4 and 8 years in a UK birth cohort study. Neurotoxicology 2017, 62, 162–169. [Google Scholar] [CrossRef]
- Reuben, A.; Caspi, A.; Belsky, B.W.; Broadbent, J.; Harrington, H.; Sugden, K.; Houts, R.M.; Ramrakha, S.; Poulton, R.; Moffitt, T.E. Association of childhood blood lead levels with cognitive function and socioeconomic status at age 38 years and with IQ change and socioeconomic mobility between childhood and adulthood. JAMA 2017, 317, 1244–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidler, A.L.; Ritchie, S.J. The association between socioeconomic status and cognitive development in children is partly mediated by a chaotic home atmosphere. J. Cogn. Dev. 2018, 19, 486–508. [Google Scholar] [CrossRef]
- McDermott, C.L.; Seidlitz, J.; Nadig, A.; Liu, S.; Clasen, L.S.; Blumenthal, J.D.; Reardon, P.K.; Lalonde, F.; Greenstein, D.; Patel, R.; et al. Longitudinally mapping childhood socioeconomic status associations with cortical and subcortical morphology. J. Neurosci. 2019, 39, 1365–1373. [Google Scholar] [CrossRef] [Green Version]
- Webb, S.; Janus, M.; Duku, E.; Raos, R.; Brownell, M.; Forer, B.; Guhn, M.; Muhajarine, N. Neighbourhood socioeconomic status indices and early childhood development. SSM-Popul. Health 2017, 3, 48–56. [Google Scholar] [CrossRef]
- Boullier, M.; Blair, M. Adverse childhood experiences. Paediatr. Child Health 2018, 28, 132–137. [Google Scholar] [CrossRef]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Smith, M.L.; Herbert, A.; Hughes, A.; Northstone, K.; Howe, L.D. Socioeconomic position and adverse childhood experiences as risk factors for health-related behaviour change and employment adversity during the COVID-19 pandemic: Insights from a prospective cohort study in the UK. BMC Public Health 2022, 22, 1820. [Google Scholar] [CrossRef]
- Lyon-Scott, K.; Cohen-Cline, H. Associations between adverse childhood experiences and emergency department utilization in an adult medicaid population. Int. J. Environ. Res. Public Health 2022, 19, 10149. [Google Scholar] [CrossRef]
- Finkelhor, D.; Shattuck, A.; Turner, H.; Hamby, S. A Revised Inventory of Adverse Childhood Experiences. Child Abus. Negl. 2015, 48, 13–21. [Google Scholar] [CrossRef]
- Appleton, A.A.; Holdsworth, E.A.; Kubzansky, L.D. A Systematic Review of the Interplay Between Social Determinants and Environmental Exposures for Early-Life Outcomes. Curr. Environ. Health Rep. 2016, 3, 287–301. [Google Scholar] [CrossRef]
- Nkwata, A.K.; Zhang, M.; Song, X.; Giordani, B.; Ezeamama, A.E. Toxic Stress, Resilience and Race-Related Disparities in Neurocognitive and Quality of Life Change in a Nationally Representative Sample of Americans≥ 50 Years Old. Ph.D. Thesis, University of Georg, Athens, GA, USA, 2021. [Google Scholar]
- Myhre, O.; Låg, M.; Villanger, G.D.; Oftedal, B.; Øvrevik, J.; Holme, J.A.; Aase, H.; Paulsen, R.E.; Bal-Price, A.; Dirven, H. Early life exposure to air pollution particulate matter (PM) as risk factor for attention deficit/hyperactivity disorder (ADHD): Need for novel strategies for mechanisms and causalities. Toxicol. Appl. Pharmacol. 2018, 354, 196–214. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Zhang, X.; Song, H.; Li, Y. Association between exposure to air pollutants and attention-deficit hyperactivity disorder (ADHD) in children: A systematic review and meta-analysis. Int. J. Environ. Health Res. 2022, 32, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Banerjee, M.; Ray, M.R.; Lahiri, T. Attention-deficit hyperactivity disorder in children chronically exposed to high level of vehicular pollution. Eur. J. Pediatr. 2011, 170, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Mora-Tiscareño, A.; Franco-Lira, M.; Zhu, H.; Lu, Z.; Solorio, E.; Torres-Jardón, R.; D’Angiulli, A. Decreases in Short Term Memory, IQ, and Altered Brain Metabolic Ratios in Urban Apolipoprotein ε4 Children Exposed to Air Pollution. J. Alzheimer’s Dis. 2015, 45, 757–770. [Google Scholar] [CrossRef]
- Thygesen, M.; Holst, G.J.; Hansen, B.; Geels, C.; Kalkbrenner, A.; Schendel, D.; Brandt, J.; Pederson, C.B.; Dalsgaard, S. Exposure to air pollution in early childhood and the association with Attention-Deficit Hyperactivity Disorder. Environ. Res. 2020, 183, 108930. [Google Scholar] [CrossRef] [PubMed]
- Margolis, A.E.; Ramphal, B.; Pagliaccio, D.; Banker, S.; Selmanovic, E.; Thomas, L.V.; Factor-Litvak, P.; Perera, F.; Peterson, B.S.; Rundle, A.; et al. Prenatal exposure to air pollution is associated with childhood inhibitory control and adolescent academic achievement. Environ. Res. 2021, 202, 111570. [Google Scholar] [CrossRef]
- Beckwith, T.; Cecil, K.; Altaye, M.; Severs, R.; Wolfe, C.; Percy, Z.; Maloney, T.; Yolton, K.; LeMasters, G.; Brunst, K.; et al. Reduced gray matter volume and cortical thickness associated with traffic-related air pollution in a longitudinally studied pediatric cohort. PLoS ONE 2020, 15, e0228092. [Google Scholar] [CrossRef] [Green Version]
- Canfield, R.L.; Kreher, D.A.; Cornwell, C.; Henderson, C.R., Jr. Low-level lead exposure, executive functioning, and learning in early childhood. Child Neuropsychol. 2003, 9, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Vorvolakos, T.; Arseniou, S.; Samakouri, M. There is no safe threshold for lead exposure: Alpha literature review. Psychiatriki 2016, 27, 204–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.; Cai, B.; Dietrich, K.N.; Radcliffe, J.; Rogan, W.J. Lead exposure, IQ, and behavior in urban 5-to 7-year-olds: Does lead affect behavior only by lowering IQ? Pediatrics 2007, 119, e650–e658. [Google Scholar] [CrossRef] [Green Version]
- Ruiz JD, C.; Quackenboss, J.J.; Tulve, N.S. Contributions of a child’s built, natural, and social environments to their general cognitive ability: A systematic scoping review. PLoS ONE 2016, 11, e0147741. [Google Scholar] [CrossRef] [Green Version]
- Nilsen, F.M.; Tulve, N.S. A systematic review and meta-analysis examining the interrelationships between chemical and non-chemical stressors and inherent characteristics in children with ADHD. Environ. Res. 2020, 180, 108884. [Google Scholar] [CrossRef]
- Liu, J.; Portnoy, J.; Raine, A.; Gladieux, M.; McGarry, P.; Chen, A. Blood lead levels mediate the relationship between social adversity and child externalizing behavior. Environ. Res. 2022, 204, 112396. [Google Scholar] [CrossRef] [PubMed]
- Su, J.G.; Jerrett, M.; Nazelle, A.D.; Jennifer, W. Does exposure to air pollution in urban parks have socioeconomic, racial or ethnic gradients? Environ. Res. 2011, 111, 319–328. [Google Scholar] [CrossRef]
- Bateson, T.F.; Schwartz, J. Children’s Response to Air Pollutants. J. Toxicol. Environ. Health Part A 2007, 71, 238–243. [Google Scholar] [CrossRef]
- Clifford, A.; Lang, L.; Chen, R.; Anstey, K.J.; Seaton, A. Exposure to air pollution and cognitive functioning across the life course–a systematic literature review. Environ. Res. 2016, 147, 383–398. [Google Scholar] [CrossRef]
- Ritz, B.; Wilhelm, M. Ambient Air Pollution and Adverse Birth Outcomes: Methodologic Issues in an Emerging Field. Basic Clin. Pharmacol. Toxicol. 2008, 102, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Margai, F.; Henry, N. A Community-Based Assessment of Learning Disabilities Using Environmental and Contextual Risk Factors. Soc. Sci. Med. 2003, 56, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Perera, F.P.; Wheelock, K.; Wang, Y.; Tang, D.; Margolis, A.E.; Badia, G.; Cowell, W.; Miller, R.L.; Rauh, V.; Wang, S.; et al. Combined Effects of Prenatal Exposure to Polycyclic Aromatic Hydrocarbons and Material Hardship on Child ADHD Behavior Problems. Environ. Res. 2018, 160, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Loftus, C.; Hazlehurst, M.; Ni, Y.; Tylavsky, F.; Bush, N.; Szpiro, A.; Sathyanarayana, S.; Karr, C.; LeWinn, K. Early life exposure to outdoor air pollution and child behavior in the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) Study. Environ. Epidemiol. 2019, 3, 248–249. [Google Scholar]
- Vishnevetsky, J.; Tang, D.; Chang, H.-W.; Roen, E.L.; Wang, Y.; Rauh, V.; Wang, S.; Miller, R.L.; Herbstman, J.; Perera, F.P. Combined Effects of Prenatal Polycyclic Aromatic Hydrocarbons and Material Hardship on Child IQ. Neurotoxicol. Teratol. 2015, 49, 74–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullen, C.; Grineski, S.E.; Collins, T.W.; Mendoza, D.L. Effects of PM2.5 on Third Grade Students’ Proficiency in Math and English Language Arts. Int. J. Environ. Res. Public Health 2020, 17, 6931. [Google Scholar] [CrossRef]
- Votruba-Drzal, E.; Miller, P.; Betancur, L.; Spielvogel, B.; Kruzik, C.; Coley, R.L. Family and community resource and stress processes related to income disparities in school-aged children’s development. J. Educ. Psychol. 2021, 113, 1405. [Google Scholar] [CrossRef]
- Loftus, C.; Ni, Y.; Szpiro, A.A.; Hazlehurst, M.F.; Tylavsky, F.A.; Bush, N.R.; Sathyanarayana, S.; Carroll, K.N.; Young, M.; Karr, C.; et al. Exposure to ambient air pollution and early childhood behavior: A longitudinal cohort study. Environ. Res. 2020, 183, 109075. [Google Scholar] [CrossRef]
- Brockmeyer, S.; D’Angiulli, A. How air pollution alters brain development: The role of neuroinflammation. Transl. Neurosci. 2016, 7, 24–30. [Google Scholar] [CrossRef]
- Rivas-Arancibia, S.; Guevara-Guzmán, R.; López-Vidal, Y.; Rodríguez-Martínez, E.; Zanardo-Gomes, M.; Angoa-Pérez, M.; Raisman-Vozari, R. Oxidative stress caused by ozone exposure induces loss of brain repair in the hippocampus of adult rats. Toxicol. Sci. 2010, 113, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Calderón-Garcidueñas, L.; Mora-Tiscareño, A.; Styner, M.; Gómez-Garza, G.; Zhu, H.; Torres-Jardón, R.; Carlos, E.; Solorio-López, E.; Medina-Cortina, H.; Kavanaugh, M.; et al. White matter hyperintensities, systemic inflammation, brain growth, and cognitive functions in children exposed to air pollution. J. Alzheimer’s Dis. 2012, 31, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.C.; Kim, B.-N.; Hong, Y.-C.; Shin, M.-S.; Yoo, H.J.; Kim, J.-W.; Bhang, S.-Y.; Cho, I.H.; Kim, H.-W. Effect of environmental exposure to lead and tobacco smoke on inattentive and hyperactive symptoms and neurocognitive performance in children. J. Child Psychol. Psychiatry 2010, 51, 1050–1057. [Google Scholar] [CrossRef]
- Shannon, M. Severe lead poisoning in pregnancy. Ambul. Pediatr. 2003, 3, 37–39. [Google Scholar] [CrossRef]
- Béranger, R.; Garlantézec, R.; Maner-Idrissi, G.L.; Lacroix, A.; Rouget, F.; Trowbridge, J.; Warembourg, C.; Monfort, C.; Gléau, F.L.; Jourdin, M.; et al. Prenatal exposure to glycol ethers and neurocognitive abilities in 6-year-old children: The PELAGIE cohort study. Environ. Health Perspect. 2017, 125, 684–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cory-Slechta, D.A.; Virgolini, M.B.; Thiruchelvam, M.; Weston, D.D.; Bauter, M.R. Maternal stress modulates the effects of developmental lead exposure. Environ. Health Perspect. 2004, 112, 717–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chilton, M.; Knowles, M.; Rabinowich, J.; Arnold, K.T. The relationship between childhood adversity and food insecurity: ‘It’s like a bird nesting in your head’. Public Health Nutr. 2015, 18, 2643–2653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrie, J.; Rolf, K.; Troesken, W. Lead Exposure and the Perpetuation of Low Socioeconomic Status. Economics 2015. [Google Scholar]
- Mielke, H.W.; Reagan, P.L. Soil is an important pathway of human lead exposure. Environ. Health Perspect. 1998, 106 (Suppl. 1), 217–229. [Google Scholar]
- Jones, D.H.; Yu, X.; Guo, Q.; Duan, X.; Jia, C. Racial Disparities in the Heavy Metal Contamination of Urban Soil in the Southeastern United States. Int. J. Environ. Res. Public Health 2022, 19, 1105. [Google Scholar] [CrossRef]
- Ramesh, R.M.; Khiratkar, A.G.; Sindhu, K.N.; Rose, A.; John, S.M.; Bhagat, P.R.; Kang, G.; Mohan, V.R. Risk Factors and Hazards in the Household Environment for Elevated Blood Lead Levels in Urban Preschool Children of Vellore: A Case–Control Approach in the MAL-ED Birth Cohort. Indian J. Pediatr. 2022, 89, 125–132. [Google Scholar] [CrossRef]
- Wasserman, G.A.; Liu, X.; Popovac, D.; Factor-Litvak, P.; Kline, J.; Waternaux, C.; LoIacono, N.; Graziano, J.H. The Yugoslavia Prospective Lead Study: Contributions of Prenatal and Postnatal Lead Exposure to Early Intelligence. Neurotoxicology Teratol. 2000, 22, 811–818. [Google Scholar] [CrossRef]
- Shamsudin, S.B.; Majid, A.A. Association of blood lead levels and working memory ability of primary school children surrounding ex-copper mining area in Ranau, Sabah (Malaysia). Acta Sci. Malays. 2017, 1, 01–03. [Google Scholar] [CrossRef]
- Arnold, O.M.; Liu, J. Lead Exposure is Associated With Poor Working Memory in 12-Year Old Chinese Children. In ISEE Conference Abstracts, Proceedings of the 32nd Annual Conference of the International Society for Environmental Epidemiology Online Conference, 24–27 August 2020; ISEE: Herndon, VA, USA, 2020; Volume 2020, p. 2020. [Google Scholar]
- Goodlad, J.K.; Marcus, D.K.; Fulton, J.J. Lead and attention-deficit/hyperactivity disorder (ADHD) symptoms: A meta-analysis. Clin. Psychol. Rev. 2013, 33, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Bressler, J.; Kim, K.A.; Chakraborti, T.; Goldstein, G. Molecular mechanisms of lead neurotoxicity. Neurochem. Res. 1999, 24, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Neal, A.P.; Worley, P.F.; Guilarte, T.R. Lead exposure during synaptogenesis alters NMDA receptor targeting via NMDA receptor inhibition. Neurotoxicology 2011, 32, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Morris, R. Synaptic plasticity and learning: Selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J. Neurosci. 1989, 9, 3040–3057. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.F.; Pereira, E.F.R.; Mike, A.; Albuquerque, E.X. Pb2+ via protein kinase C inhibits nicotinic cholinergic modulation of synaptic transmission in the hippocampus. J. Pharmacol. Exp. Ther. 2004, 311, 700–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, G.W.; Kantrowitz, E. Socioeconomic status and health: The potential role of environmental risk exposure. Annu. Rev. Public Health 2002, 23, 303–331. [Google Scholar] [CrossRef] [Green Version]
- Meyer, S.; Eddleston, M.; Bailey, B.; Desel, H.; Gottschling, S.; Gortner, L. Unintentional household poisoning in children. Klin. Pädiatrie 2007, 219, 254–270. [Google Scholar] [CrossRef] [Green Version]
- Rauh, V.A.; Landrigan, P.J.; Claudio, L. Housing and health: Intersection of poverty and environmental exposures. Ann. N. Y. Acad. Sci. 2008, 1136, 276–288. [Google Scholar] [CrossRef]
- Jiang, H.; Justice, L.M.; Purtell, K.M.; Bates, R. Exposure to Environmental Toxicants and Early Language Development for Children Reared in Low-Income Households. Clin. Pediatr. 2020, 59, 557–565. [Google Scholar] [CrossRef]
- Stein, J.L.; Gunier, R.B.; Harley, K.; Kogut, K.; Bradman, A.; Eskenazi, B. Early Childhood Adversity Potentiates the Adverse Association between Prenatal Organophosphate Pesticide Exposure and Child IQ: The CHAMACOS Cohort. NeuroToxicology 2016, 56, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Vitória, P.D.; Nunes, C.; Precioso, J. Parents’ educational level and second-hand tobacco smoke exposure at home in a sample of Portuguese children. Rev. Port. De Pneumol. Engl. Ed. 2017, 23, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Yang, I.; Hall, Y.A.; Ashford, K.; Paul, S.; Polivka, B.; Ridner, S.L. Pathways from socioeconomic status to prenatal smoking: A test of the reserve capacity model. Nurs. Res. 2017, 66, 2. [Google Scholar] [CrossRef] [Green Version]
- Anderko, L.; Braun, J.; Auinger, P. Contribution of Tobacco Smoke Exposure to Learning Disabilities. J. Obstet. Gynecol. Neonatal. Nurs. 2010, 39, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ingrum, A. High School Dropout Determinants: The Effect of Socioeconomic Status and Learning Disabilities; Honors Projects. 24; Illinois Wesleyan University: Bloomington, IL, USA, 2006. [Google Scholar]
- Azar, N.; Booji, L.; Muckle, G.; Arbuckle, T.E.; Séguin, J.R.; Asztalos, E.; Fraser, W.D.; Lanphear, B.P.; Bouchard, M.F. Prenatal exposure to polybrominated diphenyl ethers (PBDEs) and cognitive ability in early childhood. Environ. Int. 2021, 146, 106296. [Google Scholar] [CrossRef] [PubMed]
- Cowell, W.J.; Sjödin, A.; Jones, R.; Wang, Y.; Wang, S.; Herbstman, J.B. Determinants of prenatal exposure to polybrominated diphenyl ethers (PBDEs) among urban, minority infants born between 1998 and 2006. Environ. Pollut. 2018, 233, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Cowell, W.J.; Margolis, A.; Rauh, V.A.; Sjödin, A.; Jones, R.; Wang, Y.; Garcia, W.; Perera, F.; Wang, S.; Herbstman, J.B. Associations between prenatal and childhood PBDE exposure and early adolescent visual, verbal and working memory. Environ. Int. 2018, 118, 9–16. [Google Scholar] [CrossRef]
- Liu, J.; Schelar, E. Pesticide Exposure and Child Neurodevelopment. Workplace Health Saf. 2012, 60, 235–242. [Google Scholar] [CrossRef]
- Herbstman, J.B.; Mall, J.K. Developmental exposure to polybrominated diphenyl ethers and neurodevelopment. Curr. Environ. Health Rep. 2014, 1, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Morris, C.V.; DiNieri, J.A.; Szutorisz, H.; Hurd, Y.L. Molecular mechanisms of maternal cannabis and cigarette use on human neurodevelopment. Eur. J. Neurosci. 2011, 34, 1574–1583. [Google Scholar] [CrossRef] [Green Version]
- Walsh, D.; McCartney, G.; Smith, M.; Armour, G. Relationship between childhood socioeconomic position and adverse childhood experiences (ACEs): A systematic review. J. Epidemiol. Community Health 2019, 73, 1087–1093. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S. Environmental regulations on air pollution in China and their impact on infant mortality. J. Health Econ. 2015, 42, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, M.P.; Shoaff, J.; Kioumourtzoglou, M.A.; Korrick, S.; Rifas-Shiman, S.L.; Hivert, M.F.; Oken, E.; James, P. Early Life Exposure to Green Space and Mid-Childhood Cognition in the Project Viva Cohort, Massachusetts. Am. J. Epidemiol. 2022, 191, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Dockx, Y.; Bijnens, E.M.; Luyten, L.; Peusens, M.; Provost, E.; Rasking, L.; Sleurs, H.; Hogervorst, J.; Plusquin, M.; Casas, L.; et al. Early life exposure to residential green space impacts cognitive functioning in children aged 4 to 6 years. Environ. Int. 2022, 161, 107094. [Google Scholar] [CrossRef] [PubMed]
- Strife, S.; Downey, L. Childhood development and access to nature: A new direction for Environmental Inequality Research. Organ. Environ. Natl. Libr. Med. 2009, 22, 99–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigolon, A.; Browning, M.H.; Lee, K.; Shin, S. Access to urban green space in cities of the Global South: A systematic literature review. Urban Sci. 2018, 2, 67. [Google Scholar] [CrossRef] [Green Version]
- Halabicky, O.M.; Pinto-Martin, J.A.; Compton, P.; Liu, J. Low level lead exposure in early childhood and parental education on adolescent IQ and working memory: A cohort study. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 168–176. [Google Scholar] [CrossRef] [PubMed]
| Toxicant | Exposure Source | Mechanistic Pathway of Toxicity | Cognitive Outcome |
|---|---|---|---|
| Air pollution | Vehicle emissions Industrial factory sites Construction sites | White matter hyperintensities and demyelination Neuroinflammation | Learning disabilities Lowered IQ Memory and attention deficits |
| Lead | Lead-based paints Direct ingestion Maternal smoking Lead-based water pipes | Impeding synaptic transmission through mimicking of calcium Reduction of NMDA receptors Phosphorylation of PKC | Working memory deficits Lowered IQ ADHD |
| Chemical pesticides | Household Polluted neighborhoods | Reduction of cholinergic neurons | Impaired cognition Lowered IQ |
| Tobacco smoke | Maternal and household cigarette smoking | DNA methylation | Parent-reported learning disabilities |
| Polybrominated diphenyl ethers | Household products and appliances | Deficits and excesses in thyroid hormones Oxidative stress | Impaired memory Lowered IQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gladieux, M.; Gimness, N.; Rodriguez, B.; Liu, J. Adverse Childhood Experiences (ACEs) and Environmental Exposures on Neurocognitive Outcomes in Children: Empirical Evidence, Potential Mechanisms, and Implications. Toxics 2023, 11, 259. https://doi.org/10.3390/toxics11030259
Gladieux M, Gimness N, Rodriguez B, Liu J. Adverse Childhood Experiences (ACEs) and Environmental Exposures on Neurocognitive Outcomes in Children: Empirical Evidence, Potential Mechanisms, and Implications. Toxics. 2023; 11(3):259. https://doi.org/10.3390/toxics11030259
Chicago/Turabian StyleGladieux, Margaret, Nathan Gimness, Bianca Rodriguez, and Jianghong Liu. 2023. "Adverse Childhood Experiences (ACEs) and Environmental Exposures on Neurocognitive Outcomes in Children: Empirical Evidence, Potential Mechanisms, and Implications" Toxics 11, no. 3: 259. https://doi.org/10.3390/toxics11030259
APA StyleGladieux, M., Gimness, N., Rodriguez, B., & Liu, J. (2023). Adverse Childhood Experiences (ACEs) and Environmental Exposures on Neurocognitive Outcomes in Children: Empirical Evidence, Potential Mechanisms, and Implications. Toxics, 11(3), 259. https://doi.org/10.3390/toxics11030259







