Assessing the Occurrence and Distribution of Microplastics in Surface Freshwater and Wastewaters of Latvia and Lithuania
Abstract
:1. Introduction
2. Materials and Methods
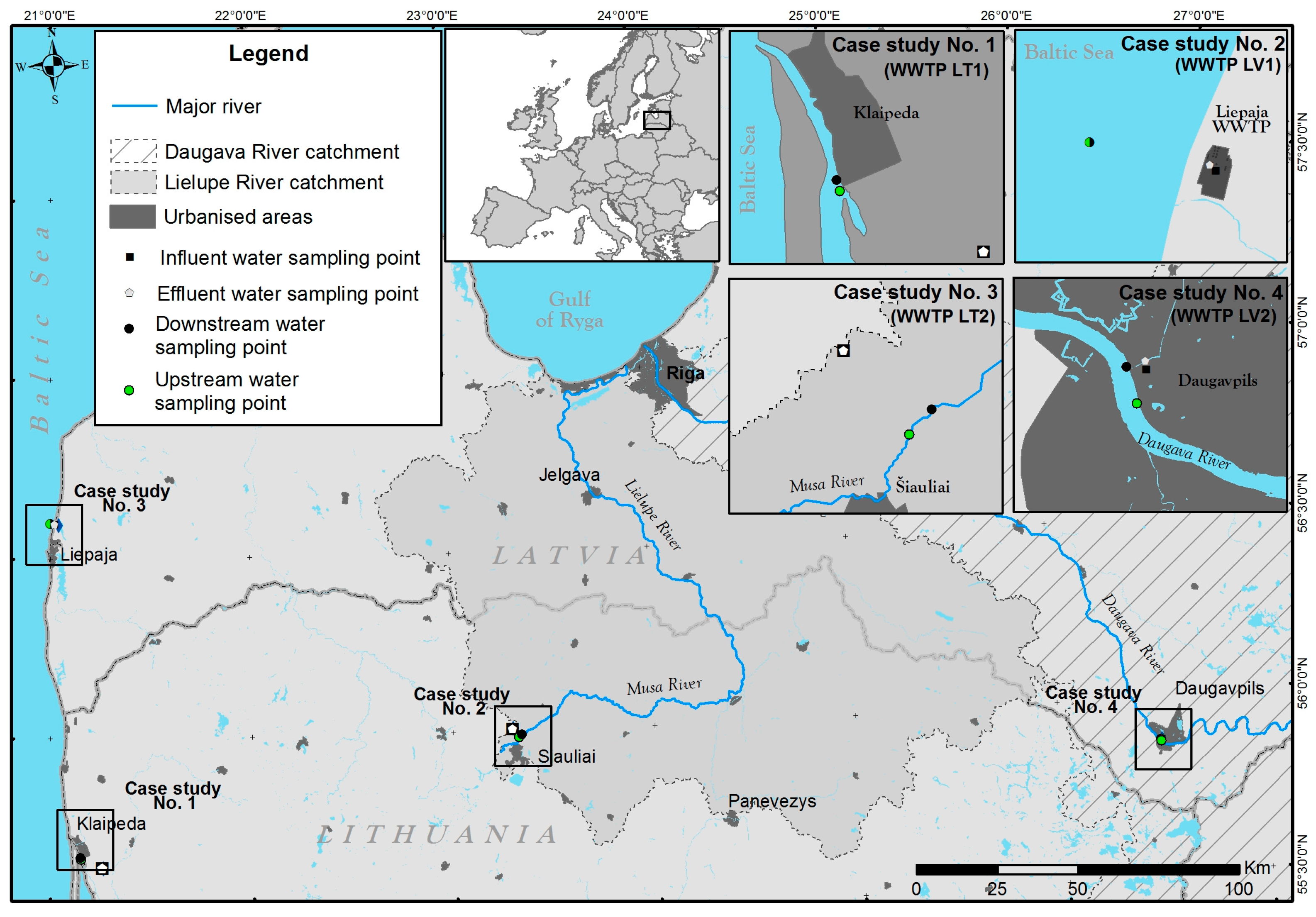
2.1. Sample Collection and Sample Preparation
2.2. Sample Preparation
2.3. Laboratory Analysis
2.3.1. Optical Microscopy Analysis of the Microplastics
2.3.2. Micro-Raman Spectroscopy Analysis of the Microplastics
2.3.3. Quality Assurance and Quality Control
3. Results
3.1. Optical Microscopy Results
3.2. Micro-Raman Spectroscopy Results
4. Discussion
4.1. Study Limitations
4.2. Microplastic Comparison in Latvia, Lithuania, and Other Aquatic Environments
| Location | Abundance (Particles/L) | Shape | Size | Color | Polymer | Reference |
|---|---|---|---|---|---|---|
| Latvia | 3.50 ± 2.38 1 | Fiber | <0.25 mm 0.5–1 mm 1–5 mm | Red Blue Black | PET PVC NL PS | This study |
| Lithuania | 7.50 ± 6.45 1 | Fiber | <0.25 mm 0.25–0.5 mm 0.5–1 mm 1–5 mm | Red Blue Black | PET PVC NL PS | This study |
| China | 10.5 ± 2.5 1 | Fragments | 0.01–0.1 mm 0.1–1 mm 1–5 mm | Transparent White Blue Black Yellow | PE PS PP PVC | [35] |
| China | 654 1 | Fibers Fragments | 50–100 μm 100–200 μm 200–500 μm 500–5000 μm | - | - | [36] |
| Portugal | 231 1 | Fragments Spherule Fibers | - | Black Blue Brown White | - | [37] |
| Iran | 0.027 ± 0.042 1 | Spherule Fibers | 0.05–0.5 mm 0.5–1 mm 1–2.5 mm 2.5–5 mm >5 mm | Red | - | [38] |
| Lithuania | 33.75 ± 40.08 2 | Fiber Fragment | <0.25 mm 0.25–0.5 mm 0.5–1 mm 1–5 mm | Transparent Yellow Red Blue Black | PET PVC NL PS | This study |
| Latvia | 11.67 ± 12.58 2 | Fiber | <0.25 mm 0.25–0.5 mm 0.5–1 mm 1–5 mm | Blue Black | PET PVC NL PS | This study |
| Lithuania | 2982 ± 54 2 | Fiber Fragment Pellet | 20–50 μm 50–100 µm 100–200 µm 200–500 µm 500–1000 µm | Black White Transparent Brown Yellow Blue Other | PET PS PP | [34] |
| Lithuania | 1244 ± 21 2 | Fiber Fragment Pellet | 20–50 μm 50–100 µm 100–200 µm 200–500 µm 500–1000 µm | Black White Transparent Brown Yellow Blue Other | PET PS PP | [34] |
4.3. Challenges and Potential Solutions for Removing Microplastics from Wastewater
4.4. Untested Hypotheses and Potential Avenues for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sulistyowati, L.; Riani, E.; Cordova, M.R. The occurrence and abundance of microplastics in surface water of the midstream and downstream of the Cisadane River, Indonesia. Chemosphere 2022, 291, 133071. [Google Scholar] [CrossRef] [PubMed]
- Galgani, L.; Loiselle, S.A. Plastic pollution impacts on marine carbon biogeochemistry. Environ. Pollut. 2021, 268, 115598. [Google Scholar] [CrossRef] [PubMed]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Rese. Oceans 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Moyo, M.; Falayi, T. Generation and management of microplastic waste. In Impact of Plastic Waste on the Marine Biota; Springer: Singapore, 2022; pp. 3–20. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Mchugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, R.; Dzingelevičienė, R.; Abbasi, S.; Szultka-Młyńska, M.; Buszewski, B. Determination of the pharmaceuticals–nano/microplastics in aquatic systems by analytical and instrumental methods. Environ. Monit. Assess. 2022, 194, 93. [Google Scholar] [CrossRef]
- Golgoli, M.; Khiadani, M.; Shafieian, A.; Sen, T.K.; Hartanto, Y.; Johns, M.L.; Zargar, M. Microplastics fouling and interaction with polymeric membranes: A review. Chemosphere 2021, 283, 131185. [Google Scholar] [CrossRef]
- Aigars, J.; Barone, M.; Suhareva, N.; Putna-Nimane, I.; Dimante-Deimantovica, I. Occurrence and spatial distribution of microplastics in the surface waters of the Baltic Sea and the Gulf of Riga. Mar. Pollut. Bull. 2021, 172, 112860. [Google Scholar] [CrossRef]
- Beer, S.; Garm, A.; Huwer, B.; Dierking, J.; Nielsen, T.G. No increase in marine microplastic concentration over the last three decades–a case study from the Baltic Sea. Sci. Total Environ. 2018, 621, 1272–1279. [Google Scholar] [CrossRef]
- Bollmann, U.E.; Simon, M.; Vollertsen, J.; Bester, K. Assessment of input of organic micropollutants and microplastics into the Baltic Sea by urban waters. Mar. Pollut. Bull. 2019, 148, 149–155. [Google Scholar] [CrossRef]
- Ferrero, L.; Scibetta, L.; Markuszewski, P.; Mazurkiewicz, M.; Drozdowska, V.; Makuch, P.; Jutrzenka-Trzebiatowska, P.; Zaleska-Medynska, A.; Andò, S.; Saliu, F.; et al. Airborne and marine microplastics from an oceanographic survey at the Baltic Sea: An emerging role of air-sea interaction? Sci. Total Environ. 2022, 824, 153709. [Google Scholar] [CrossRef] [PubMed]
- Halbach, M.; Vogel, M.; Tammen, J.K.; Rüdel, H.; Koschorreck, J.; Scholz-Böttcher, B.M. 30 years trends of microplastic pollution: Mass-quantitative analysis of archived mussel samples from the North and Baltic Seas. Sci. Total Environ. 2022, 826, 154179. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Buhhalko, N.; Lind, K.; Lips, I.; Liblik, T.; Väli, G.; Lips, U. Spatiotemporal Variability of Microplastics in the Eastern Baltic Sea. Front. Mar. Sci. 2022, 9, 686. [Google Scholar] [CrossRef]
- Narloch, I.; Gackowska, A.; Wejnerowska, G. Microplastic in the Baltic Sea: A review of distribution processes, sources, analysis methods and regulatory policies. Environ. Pollut. 2022, 315, 120453. [Google Scholar] [CrossRef] [PubMed]
- Piehl, S.; Hauk, R.; Robbe, E.; Richter, B.; Kachholz, F.; Schilling, J.; Lenz, R.; Fischer, D.; Fischer, F.; Labrenz, M.; et al. Combined approaches to predict microplastic emissions within an urbanized estuary (Warnow, southwestern Baltic Sea). Front. Environ. Sci. 2021, 9, 616765. [Google Scholar] [CrossRef]
- Schernewski, G.; Radtke, H.; Hauk, R.; Baresel, C.; Olshammar, M.; Osinski, R.; Oberbeckmann, S. Transport and behavior of microplastics emissions from urban sources in the Baltic Sea. Front. Environ. Sci. 2020, 8, 579361. [Google Scholar] [CrossRef]
- Tamminga, M.; Hengstmann, E.; Fischer, E.K. Microplastic analysis in the South Funen Archipelago, Baltic Sea, implementing manta trawling and bulk sampling. Mar. Pollut. Bull. 2018, 128, 601–608. [Google Scholar] [CrossRef]
- Wang, Z.M.; Wagner, J.; Ghosal, S.; Bedi, G.; Wall, S. SEM/EDS and optical microscopy analyses of microplastics in ocean trawl and fish guts. Sci. Total Environ. 2017, 603, 616–626. [Google Scholar] [CrossRef]
- Ojaveer, H.; Jaanus, A.; MacKenzie, B.R.; Martin, G.; Olenin, S.; Radziejewska, T.; Telesh, I.; Zettler, M.L.; Zaiko, A. Status of biodiversity in the Baltic Sea. PLoS ONE 2010, 5, e12467. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.; Cho, S.H.; Kim, K.H.; Kwon, E.E. Progress in quantitative analysis of microplastics in the environment: A review. Chem. Eng. J. 2021, 422, 130154. [Google Scholar] [CrossRef]
- Sierra, I.; Chialanza, M.R.; Faccio, R.; Carrizo, D.; Fornaro, L.; Pérez-Parada, A. Identification of microplastics in wastewater samples by means of polarized light optical microscopy. Environ. Sci. Pollut. Res. 2020, 27, 7409–7419. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, R.; Dzingelevičienė, R.; Bradauskaitė, A.; Lajevardipour, A.; Mlynska-Szultka, M.; Dzingelevičius, N.; Raugelė, S.; Razbadauskas, A.; Abbasi, S.; Rees, R.M.; et al. Pharmaceutical and Microplastic Pollution before and during the COVID-19 Pandemic in Surface Water, Wastewater, and Groundwater. Water 2022, 14, 3082. [Google Scholar] [CrossRef]
- Käppler, A.; Fischer, D.; Oberbeckmann, S.; Schernewski, G.; Labrenz, M.; Eichhorn, K.J.; Voit, B. Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal. Bioanal. Chem. 2016, 408, 8377–8391. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Cowger, W.; Steinmetz, Z.; Gray, A.; Munno, K.; Lynch, J.; Hapich, H.; Primpke, S.; De Frond, H.; Rochman, C.; Herodotou, O. Microplastic spectral classification needs an open source community: Open specy to the rescue! Anal. Chem. 2021, 93, 7543–7548. [Google Scholar] [CrossRef] [PubMed]
- Lӧder, M.G.; Imhof, H.K.; Ladehoff, M.; Lӧschel, L.A.; Lorenz, C.; Mintenig, S.; Piehl, S.; Primpke, S.; Schrank, I.; Laforsch, C.; et al. Enzymatic purification of microplastics in environmental samples. Environ. Sci. Technol. 2017, 51, 14283–14292. [Google Scholar] [CrossRef]
- Long, Z.; Pan, Z.; Wang, W.; Ren, J.; Yu, X.; Lin, L.; Lin, H.; Chen, H.; Jin, X. Microplastic abundance, characteristics, and removal in wastewater treatment plants in a coastal city of China. Water Res. 2019, 155, 255–265. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Int-Veen, I.; Löder, M.G.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Peeken, I.; Primpke, S.; Beyer, B.; Gütermann, J.; Katlein, C.; Krumpen, T.; Bergmann, M.; Hehemann, L.; Gerdts, G. Arctic sea ice is an important temporal sink and means of transport for microplastic. Nat. Commun. 2018, 9, 1505. [Google Scholar] [CrossRef] [Green Version]
- Brahney, J.; Hallerud, M.; Heim, E.; Hahnenberger, M.; Sukumaran, S. Plastic rain in protected areas of the United States. Science 2020, 368, 1257–1260. [Google Scholar] [CrossRef]
- Bayo, J.; Olmos, S.; López-Castellanos, J. Microplastics in an urban wastewater treatment plant: The influence of physicochemical parameters and environmental factors. Chemosphere 2020, 238, 124593. [Google Scholar] [CrossRef] [PubMed]
- Kashfi, F.S.; Ramavandi, B.; Arfaeinia, H.; Mohammadi, A.; Saeedi, R.; De-la-Torre, G.E.; Dobaradaran, S. Occurrence and exposure assessment of microplastics in indoor dusts of buildings with different applications in Bushehr and Shiraz cities, Iran. Sci. Total Environ. 2022, 829, 154651. [Google Scholar] [CrossRef] [PubMed]
- Uogintė, I.; Pleskytė, S.; Pauraitė, J.; Lujanienė, G. Seasonal variation and complex analysis of microplastic distribution in different WWTP treatment stages in Lithuania. Environ. Monit. Assess. 2022, 194, 829. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Tang, Y.; Dang, M.; Wang, S.; Jin, H.; Liu, Y.; Jing, H.; Zheng, C.; Yi, S.; Cai, Z. Spatial-temporal distribution of microplastics in surface water and sediments of Maozhou River within Guangdong-Hong Kong-Macao Greater Bay Area. Sci. Total Environ. 2020, 717, 135187. [Google Scholar] [CrossRef]
- Han, M.; Niu, X.; Tang, M.; Zhang, B.T.; Wang, G.; Yue, W.; Kong, X.; Zhu, J. Distribution of microplastics in surface water of the lower Yellow River near estuary. Sci. Total Environ. 2020, 707, 135601. [Google Scholar] [CrossRef]
- Prata, J.C.; Godoy, V.; da Costa, J.P.; Calero, M.; Martín-Lara, M.A.; Duarte, A.C.; Rocha-Santos, T. Microplastics and fibers from three areas under different anthropogenic pressures in Douro river. Sci. Total Environ. 2021, 776, 145999. [Google Scholar] [CrossRef]
- Pashaei, R.; Loiselle, S.A.; Leone, G.; Tamasi, G.; Dzingelevičienė, R.; Kowalkowski, T.; Gholizadeh, M.; Consumi, M.; Abbasi, S.; Buszewski, B.; et al. Determination of nano and microplastic particles in hypersaline lakes by multiple methods. Environ. Monit. Assess. 2021, 193, 668. [Google Scholar] [CrossRef]
- Pizzichetti, A.R.P.; Pablos, C.; Álvarez-Fernández, C.; Reynolds, K.; Stanley, S.; Marugán, J. Evaluation of membranes performance for microplastic removal in a simple and low-cost filtration system. Case Stud. Chem. Environ. Eng. 2021, 3, 100075. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Hadibarata, T. Microplastics removal through water treatment plants: Its feasibility, efficiency, future prospects and enhancement by proper waste management. Environ. Chall. 2021, 5, 100264. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zhou, C.; Hu, T.; Zeng, G.; Zhang, Y. Advanced oxidation processes for the elimination of microplastics from aqueous systems: Assessment of efficiency, perspectives and limitations. Sci. Total Environ. 2022, 842, 156723. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pashaei, R.; Sabaliauskaitė, V.; Suzdalev, S.; Balčiūnas, A.; Putna-Nimane, I.; Rees, R.M.; Dzingelevičienė, R. Assessing the Occurrence and Distribution of Microplastics in Surface Freshwater and Wastewaters of Latvia and Lithuania. Toxics 2023, 11, 292. https://doi.org/10.3390/toxics11040292
Pashaei R, Sabaliauskaitė V, Suzdalev S, Balčiūnas A, Putna-Nimane I, Rees RM, Dzingelevičienė R. Assessing the Occurrence and Distribution of Microplastics in Surface Freshwater and Wastewaters of Latvia and Lithuania. Toxics. 2023; 11(4):292. https://doi.org/10.3390/toxics11040292
Chicago/Turabian StylePashaei, Reza, Viktorija Sabaliauskaitė, Sergej Suzdalev, Arūnas Balčiūnas, Ieva Putna-Nimane, Robert M. Rees, and Reda Dzingelevičienė. 2023. "Assessing the Occurrence and Distribution of Microplastics in Surface Freshwater and Wastewaters of Latvia and Lithuania" Toxics 11, no. 4: 292. https://doi.org/10.3390/toxics11040292
APA StylePashaei, R., Sabaliauskaitė, V., Suzdalev, S., Balčiūnas, A., Putna-Nimane, I., Rees, R. M., & Dzingelevičienė, R. (2023). Assessing the Occurrence and Distribution of Microplastics in Surface Freshwater and Wastewaters of Latvia and Lithuania. Toxics, 11(4), 292. https://doi.org/10.3390/toxics11040292











