Enrichment Characteristics of Hazardous Trace Elements in Feed Coal and Coal Ash in Huaibei Area under Leaching
Abstract
:1. Introduction
1.1. Se
1.2. Hg
1.3. Pb
2. Sampling and Testing Methods
2.1. Sampling
2.2. Methods
2.2.1. Proximate Analysis and Ultimate Analysis of FC
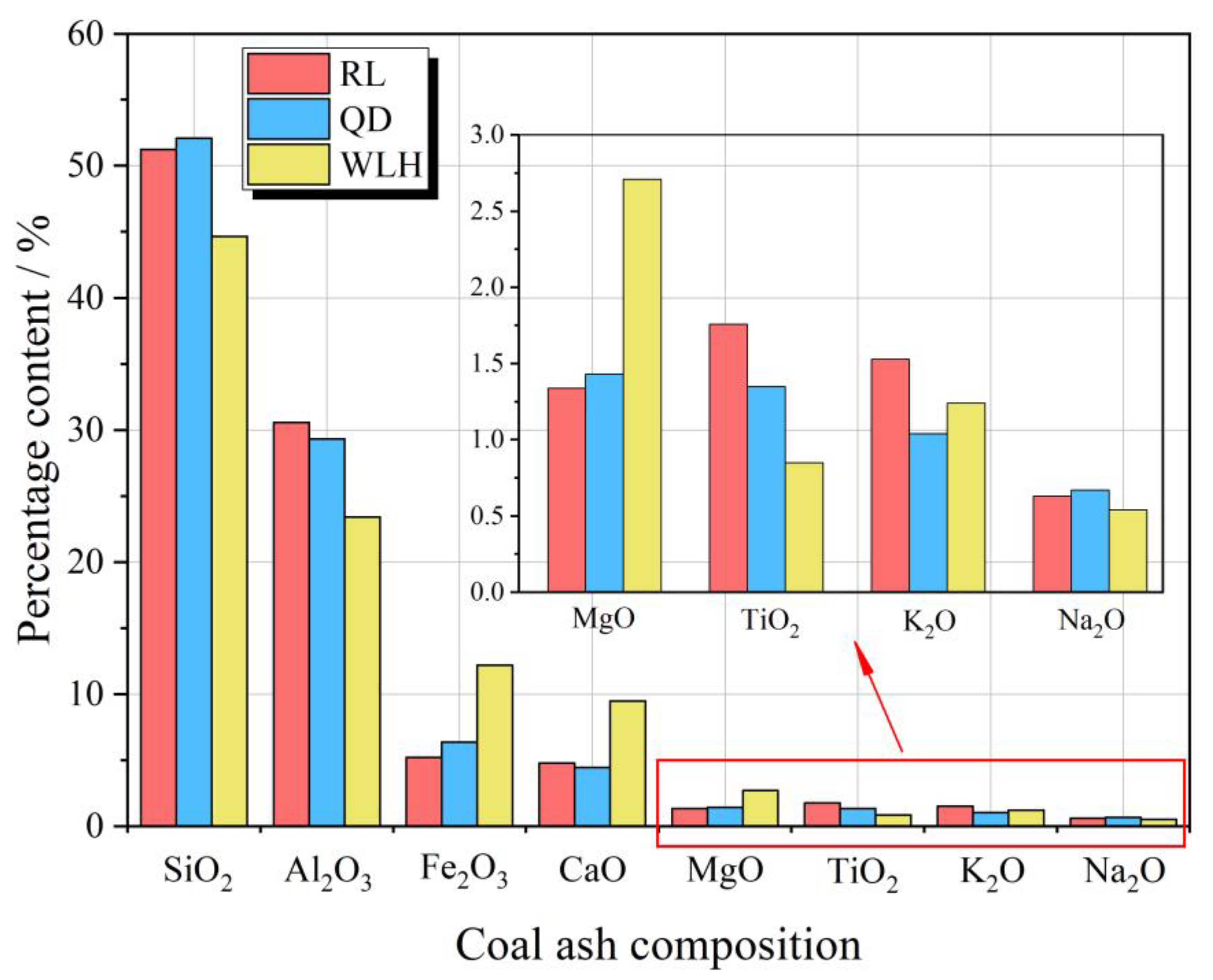
2.2.2. Major Element Oxides of FC
2.2.3. Composition Analysis of Minerals in FC
2.2.4. Sequential Chemical Extraction of FC
2.2.5. Leaching Test of FC and CA
2.2.6. Determination of HEs in FC and CA
3. Results and Discussion
3.1. Basic Characteristics of Coal Quality in FC
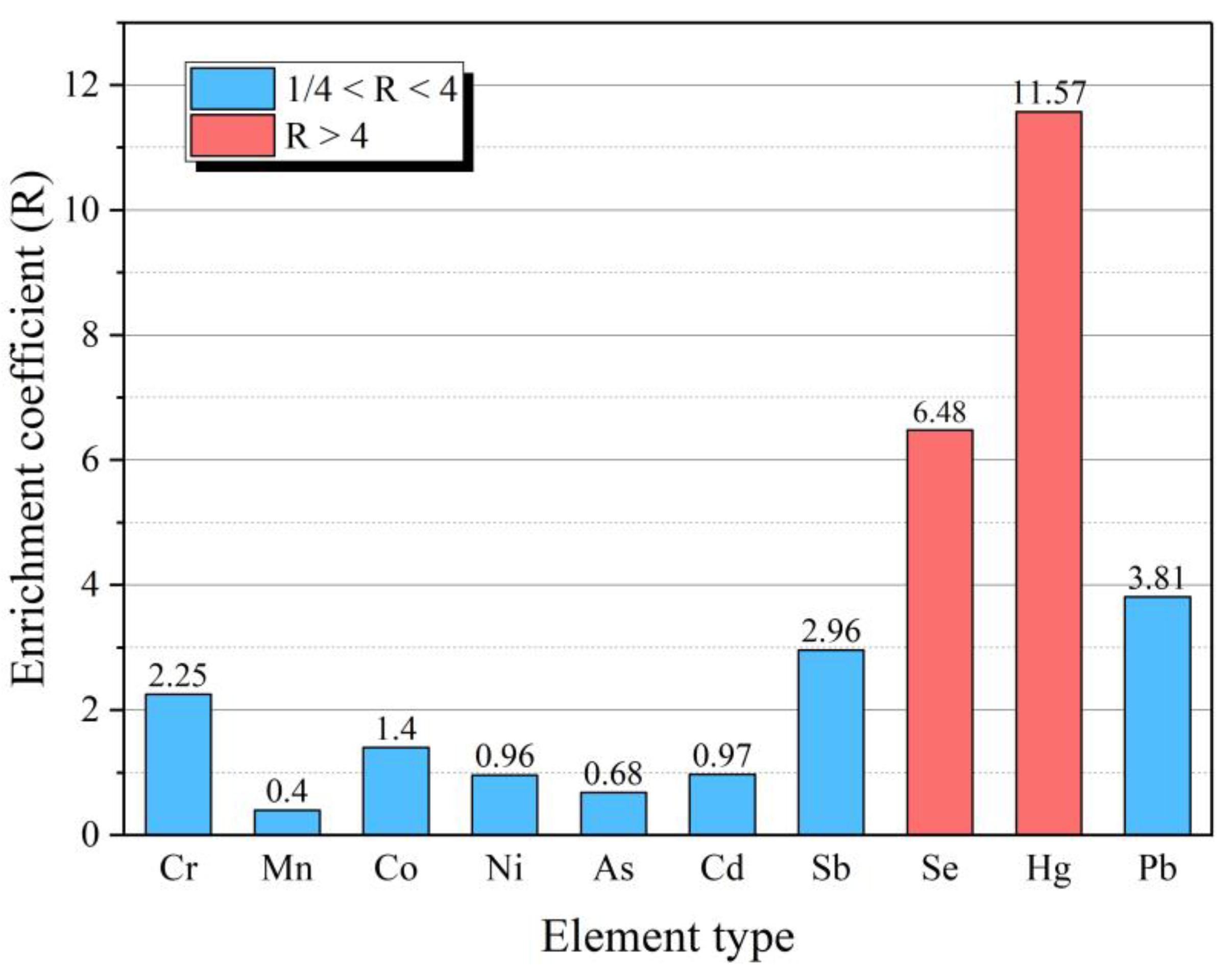
3.2. Enrichment Characteristics of Typical HEs in FC
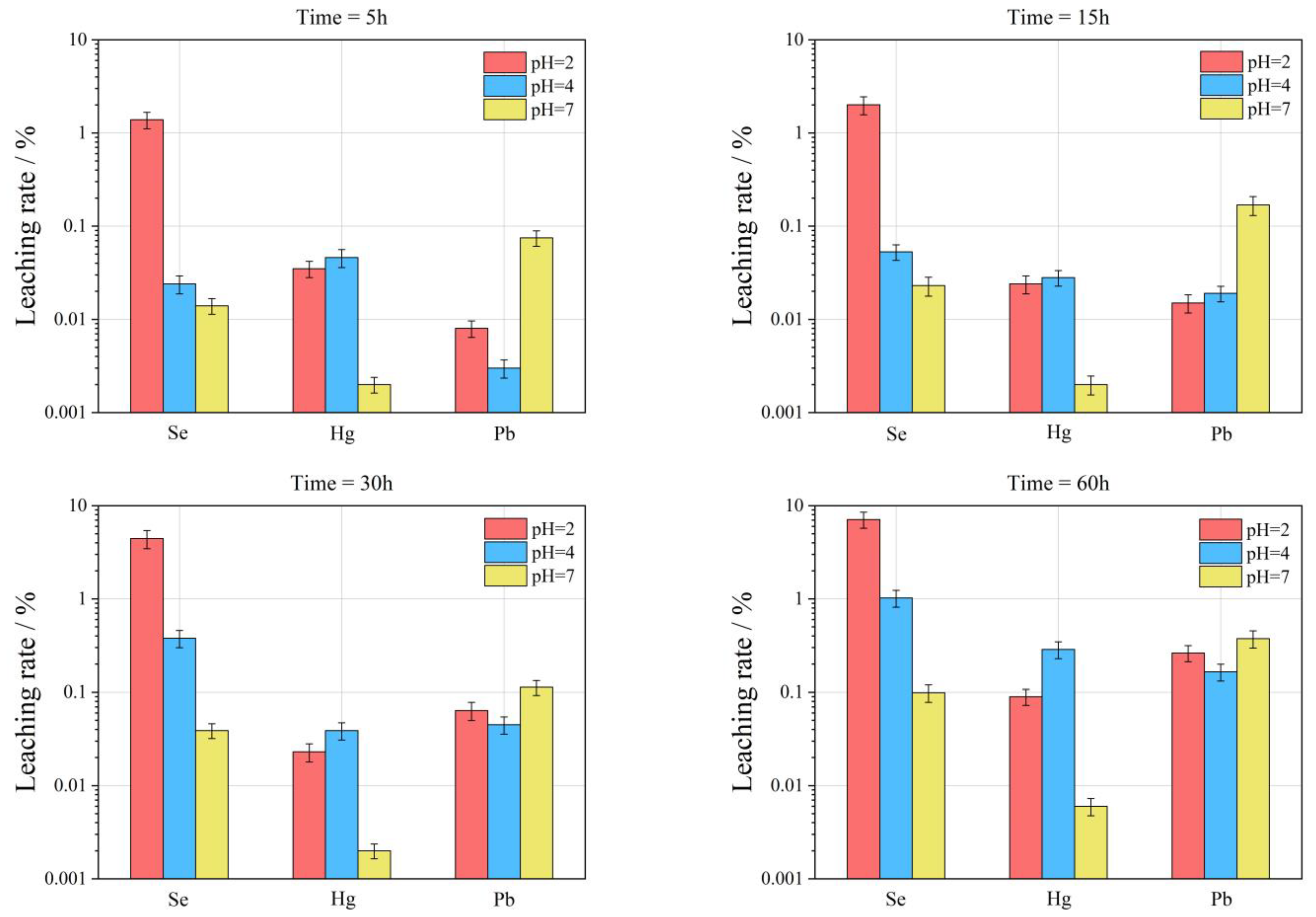
3.3. Leaching Characteristics of Se, Hg, and Pb in FC and CA
3.3.1. Calculation of Relative Leaching Rate
3.3.2. Data Accuracy Analysis
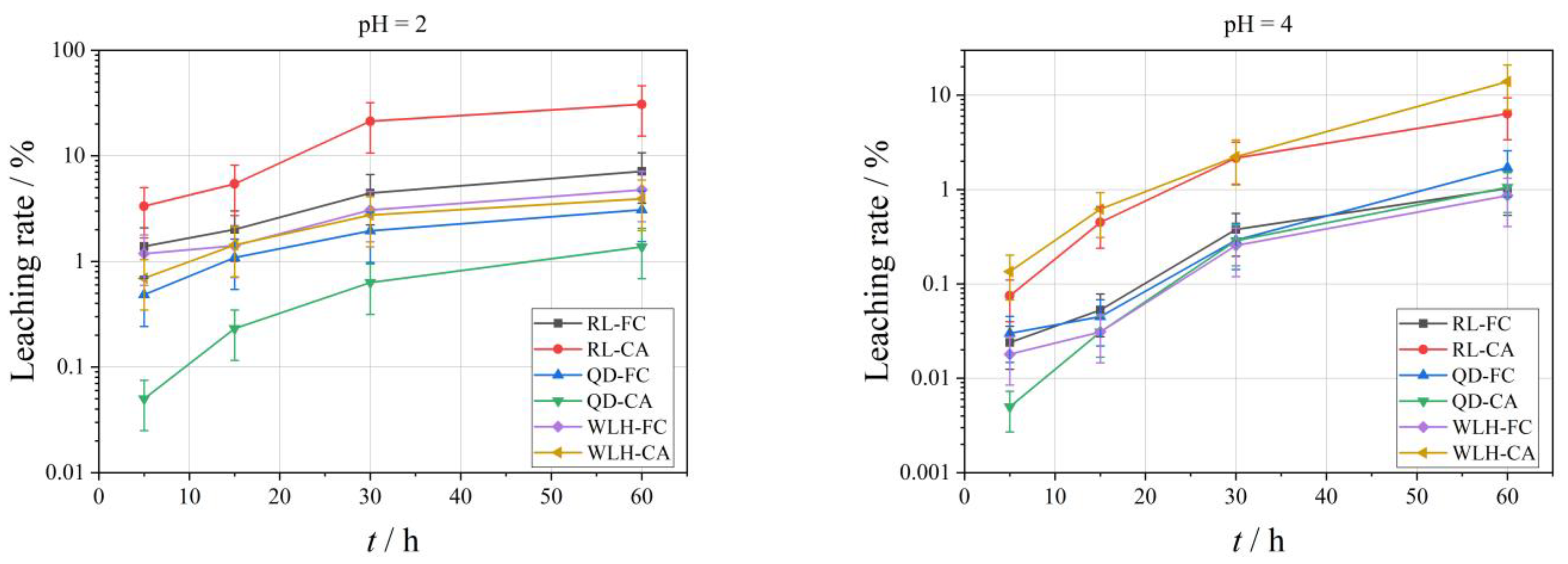
3.3.3. Leaching Characteristics of Se Element in FC and CA
- (1)
- With the decrease of acidity of leaching filtrate, the leaching effect of Se is characterized by increasing gradually, that is, the pH value rises from 2 to 7, and the maximum leaching rate (Lse, max) of Se is reduced from 30.73% to 0.42%.
- (2)
- Compared with FC, the relative leaching rate (Lse) of Se in CA is relatively high, i.e., the Lse, max in FC is 0.19–7.12% under different pH conditions; the Lse, max in CA is 0.13–30.73%.
- (3)
- The Lse, max in different types of samples is also different, and the overall performance is RL-CA > RL-FC > WLH-CA > WLH-FC > QD-FC > QD-CA.
- (4)
- With the increase of leaching time, the overall trend of Lse in FC and CA are increasing. Generally, the Lse is relatively high before 30 h, and then increases slowly from 30 h to 60 h with a trend of continuous growth.
3.3.4. Leaching Characteristics of Hg Element in FC and CA
- (1)
- With the decrease in acidity of the leaching filtrate, the leaching effect of the Hg element is not obvious, that is, the maximum leaching rate (LHg, max) of Hg is 0.99% when pH = 2; the LHg, max is 3.99% when pH = 4; the LHg, max is 0.68% when pH = 7.
- (2)
- Relative to FC, the leaching rate of Hg in CA is relatively big (same order of Se), i.e., the LHg, max in FC is 0.006–0.29% under different pH conditions; the LHg, max in CA is 0.11–3.99%.
- (3)
- With the increase in leaching time, the LHg in FC shows a decreasing trend in the first 30 h when pH = 2, and an increasing trend from 30 to 60 h. When pH = 4, the LHg in FC shows a decreasing trend in the first 15 h and an increasing trend from 15 to 60 h; when pH = 7, there is an overall increasing trend from 5 to 60 h.
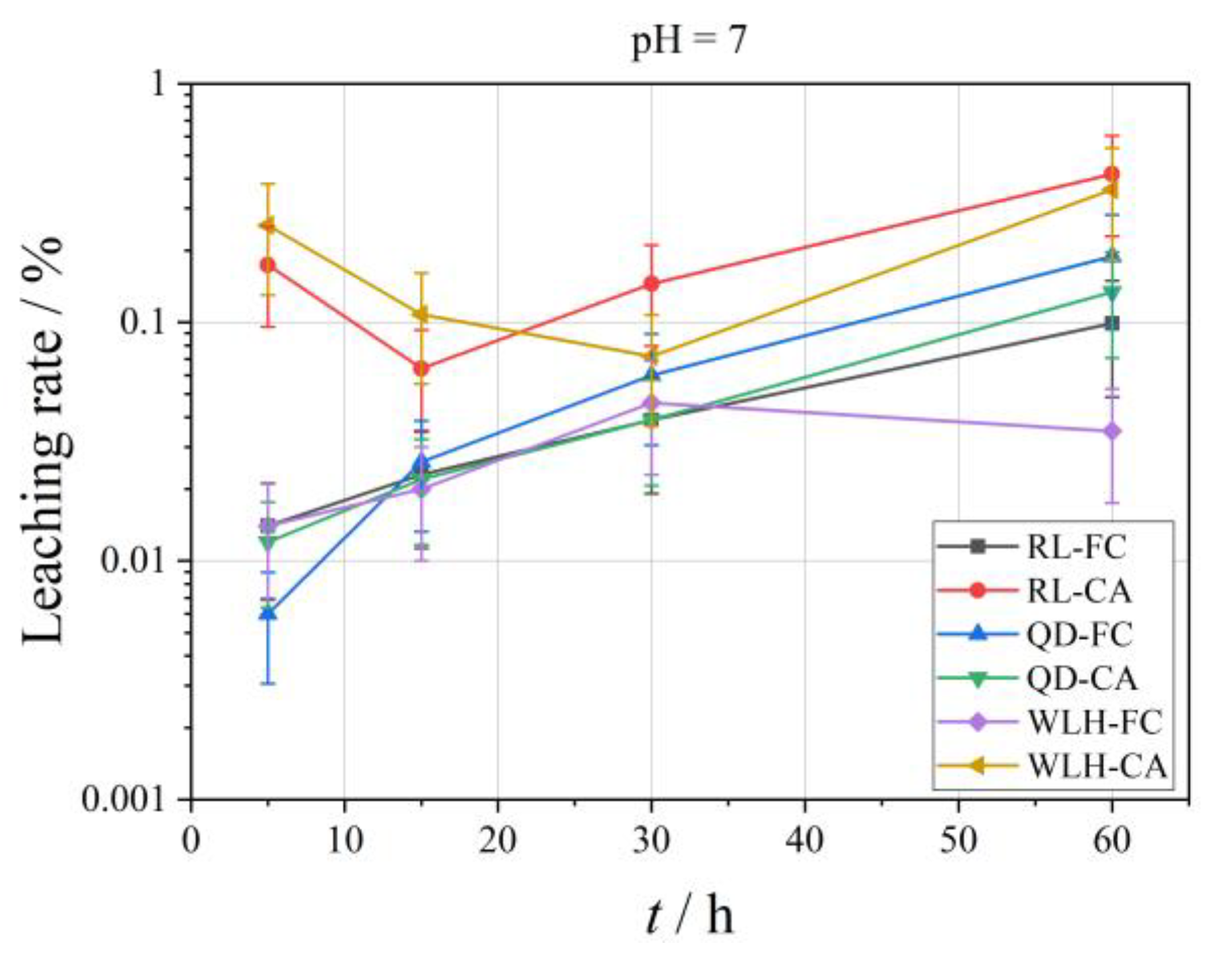
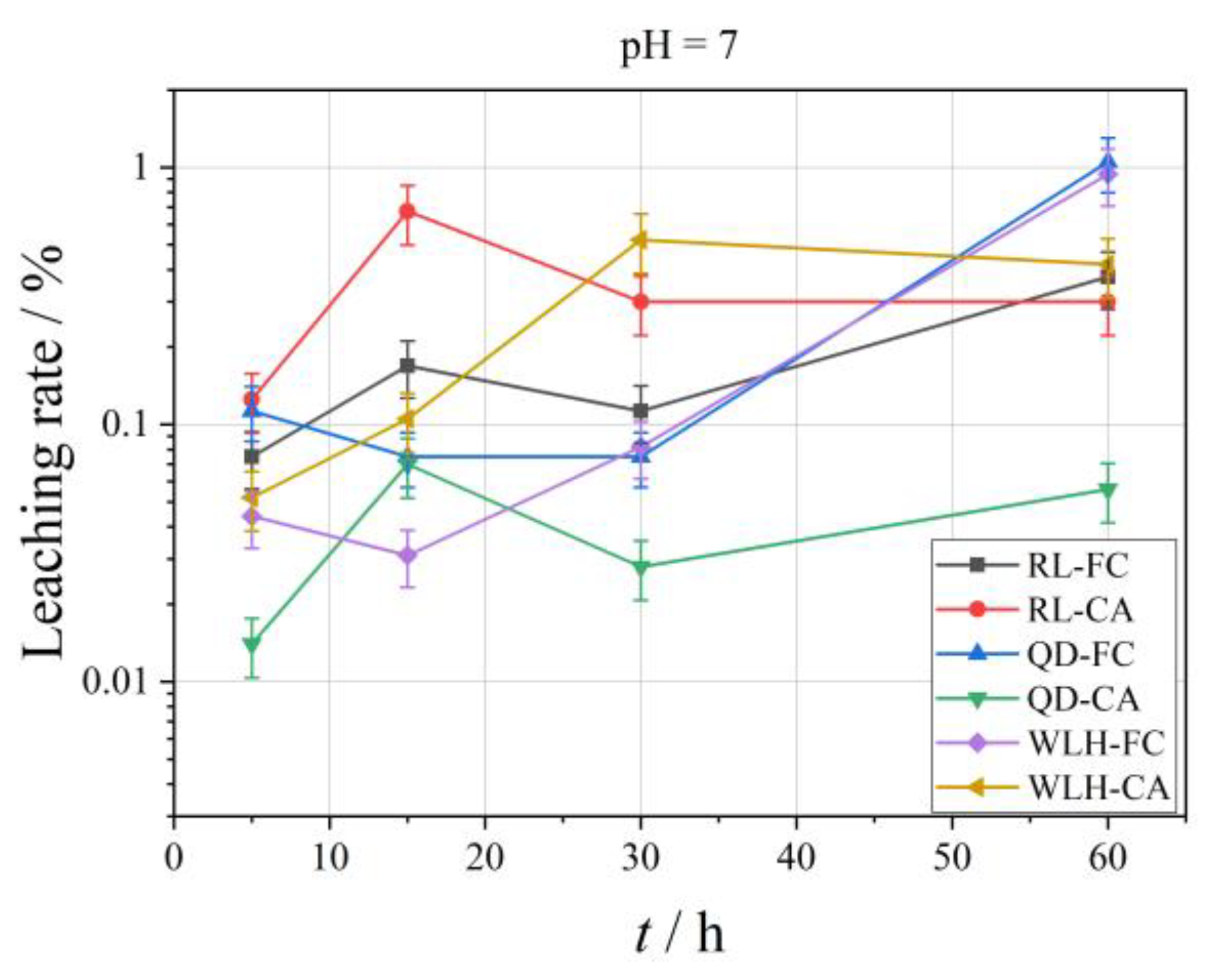
3.3.5. Leaching Characteristics of Pb Element in FC and CA
- (1)
- The LPb in different pH leaching filtrates is not high, and the maximum leaching rate (LPb, max) of Pb was less than 2%. When pH = 2, the LPb, max is 0.26%; when pH = 4, the LPb, max is 0.36%; when pH = 7, the LPb, max is 1.05%.
- (2)
- Compared with FC, the LPb in CA is relatively high (one order of magnitude higher), and under different pH conditions, the LPb, max in FC is 0.03–1.05%; the LPb, max in CA is 0.05–0.68%. The specific properties are different at different pH values and at different time periods at the same pH, as can be seen from the figure.
- (3)
- At pH = 2, LPb shows an increasing trend for RL-FC, RL-CA and QD-FC, a decreasing trend for QD-CA, WLH-FC, and WLH-CA in the time range of 5–15 h, and an increasing trend for all of them in the time range of 15–60 h. LPb shows an increasing trend in the time range of 5–60 h at pH = 4. Under the condition of pH = 7, LPb shows a trend of increasing from 5 to 15 h, decreasing from 15 to 30 h, and increasing from 30 to 60 h for RL-FC, RL-CA, and QD-CA; however, LPb shows an overall increasing trend for QD-FC, WLH-FC, and WLH-CA.
3.4. Analysis of Factors Affecting Leaching Behavior of Se, Hg, and Pb in FC and CA
3.4.1. The Relationship between the Content of Se, Hg, and Pb and Their Modes of Occurrence
Se
Hg
Pb
3.4.2. The Relationship between Mineral Composition of FC and Leaching of Se, Hg, and Pb
3.4.3. The Relationship between pH Value, Leaching Time, and Leaching of Se, Hg, and Pb
4. Conclusions
- (1)
- The content of Cr, Co, Se, Sb, Hg, and Pb in FC from Huaibei coalfield was higher than that of Chinese coals and World coals. Except for Se and Hg, the content of other elements was at “Normal” levels, but the content of Pb was also larger. The above results may be related to the unique stratigraphy of the Huaibei coalfield, where the coal-bearing strata are the upper and lower Shi Box Formation and Shanxi Formation of the Paleozoic Permian, and the upper part of the coal-bearing strata is covered by loose layers of the Cenozoic.
- (2)
- The general trend indicated that the leaching rate of the same element increased with the increase in leaching time. With the decrease in acidity of the leaching solution, the leaching rate of the Se element was gradually increasing, while the leaching rates of Hg and Pb element were not obvious. This situation may be related to the state of occurrence of selenium, which can adsorb selenate as well as combine with iron oxide. In addition, the leaching of mercury seems to be controlled by the adsorption of the aquatic phase. In acidic and alkaline fly ash samples, lead is highly insoluble and hardly moves, which may be the reason for the insignificant leaching rate of mercury and lead.
- (3)
- The leaching rate of the Se element had a great relationship with its modes of occurrence. The difference in the Hg content in the ion-exchange state may be the main reason for the difference in the Hg leaching rate. The content of Pb had little effect on its leaching rate, while its modes of occurrence (residue state) determined its leaching rate. This may be due to the low metamorphic grade and low sulfide content of the coal samples in the study area. It can also be seen that the higher the Hg content in FC and CA, the greater the leaching rate of Hg and the difference in the Hg content in the ion exchange state may be an important reason for the difference in the leaching behavior of Hg; the occurrence state of Pb determines that the LPb in FC and CA is not high, and the content of Pb element in coal has little effect on the leaching and precipitation of this element.
- (4)
- The LSe was controlled by the pH value and leaching time of the leaching solution at the same time, specifically, the lower the pH value and the longer the leaching time, the more the LSe; the LHg and LPb mainly depended on leaching time. Therefore, the low pH of the coalfield environment and the increase in the leaching rate of Se, Hg, and Pb contained in the coal seam over time will lead to an increase in heavy metal content in the surrounding environmental soil and damage the ecological environment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Qian, F.; Chen, D. Evaluation of the use of coal-based synthetic natural gas for haze prevention in China. J. Environ. Sci. China 2015, 35, 2615–2622. [Google Scholar] [CrossRef]
- Li, W.; Ma, Z.; Huang, Q.; Jiang, X. Distribution and leaching characteristics of heavy metals in a hazardous waste incinerator. Fuel 2018, 233, 427–441. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Chou, C.; Finkelman, R.; Seredin, V.; Zhou, Y. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- De Vallejuelo, S.; Gredilla, A.; da Boit, K.; Teixeira, E.; Sampaio, C.H.; Madariaga, J.; Silva, L. Nanominerals and potentially hazardous elements from coal cleaning rejects of abandoned mines: Environmental impact and risk assessment. Chemosphere 2017, 169, 725–733. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Dreher, K.; Jaskot, R.; Lehmann, J.; Richards, J.; McGee Andrew, J.; Costa, G. Soluble transition metals mediate residual oil fly ash induced acute lunginjury. J. Toxicol. Environ. Health A 2010, 50, 285–305. [Google Scholar] [CrossRef]
- Kostova, I.; Vassileva, C.; Dai, S.; Hower, J. Mineralogy, geochemistry and mercury content characterization of fly ashs from the Maritza 3 and Varna thermoelectric power plants, Bulgaria. Fuel 2016, 186, 674–684. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, B.; Aposhian, H.; Zhou, Y.; Chen, M.; Zhang, A.; Waalkes, M. Chronic arsenic poisoning from burning high-arsenic-containing coal in Guizhou, China. J. Peripher. Nerv. Syst. 2002, 110, 119–122. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Burken, J.; Ban, H.; Ladwig, K. The leaching characteristics of selenium from coal fly ashes. J. Environ. Qual. 2007, 36, 1784–1792. [Google Scholar] [CrossRef]
- Yin, L.; Zhou, Y.; Xu, Q.; Zhu, Z.; Du, W.; An, Z. Mercury emission from coal-fired power plants in China. Proc. Chin. Soc. Electr. Eng. 2013, 33, 110. [Google Scholar] [CrossRef]
- Neupane, G.; Donahoe, R. Leachability of elements in alkaline and acidic coal fly ash samples during batch and column leaching tests. Fuel 2013, 104, 758–770. [Google Scholar] [CrossRef]
- Dutta, B.; Khanra, S.; Mallick, D. Leaching of elements from coal fly ash: Assessment of its potential for use filling abandoned coal mines. Fuel 2009, 88, 1314–1323. [Google Scholar] [CrossRef]
- Khanra, S.; Mallick, D.; Dutta, S.; Chaudhuri, S. Studies on the phase mineralogy and leaching characteristics of coal fly ash. Water Air Soil Pollut. 1998, 107, 251–275. [Google Scholar] [CrossRef]
- Zheng, L.; Ding, S.; Liu, C. Leaching characteristics of environmentally sensitive trace elements in different types of coal gangue. J. Cent. South Univ. (Nat. Sci. Ed.) 2016, 47, 703–710. [Google Scholar] [CrossRef]
- Hassett, D.; Pfughoeft-hassett, D.; Heenink, L. Leaching of CCBs: Observations from over 25 years of research. Fuel 2005, 84, 1378–1383. [Google Scholar] [CrossRef]
- Christenson, H.; Pope, J.; Trumm, D.; Newman, N.; Blanco, I.; Kerr, G.; Young, M.; Uster, B. Manganese and trace element removal from new zealand coal mine drainage using limestone leaching beds. N. Zeal. J Geol. Geophys. 2019, 62, 217–228. [Google Scholar] [CrossRef]
- Nugteren, H.; Janssen-Jurkovícová, M.; Scarlett, B. Improvement of environmental quality of coal fly ash by applying forced leaching. Fuel 2001, 80, 873–877. [Google Scholar] [CrossRef]
- Ward, C.; French, D.; Riley, K.; Stephenson, L.; Farrell, O.; Li, Z. Element leachability from a coal stockpile and associated coastal sand deposits. Fuel Process. Technol. 2011, 92, 817–824. [Google Scholar] [CrossRef]
- Iwashita, A.; Sakaguchi, Y.; Nakajima, T.; Takanashi, H.; Ohki, A.; Kambara, S. Leaching characteristics of boron and selenium for various coal fly ashes. Fuel 2005, 84, 479–485. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, J.; Qiao, Y.; Xu, M. Combustion characteristics of Enshi stone soal and leaching characteristics of heavy metal of Se element. J. Huazhong Univ. Sci. Technol. (Nat. Sci.Ed.) 2013, 41, 125–128. [Google Scholar]
- Ward, C.R.; French, D.; Jankowski, J.; Dubikova, M.; Li, Z.; Riley, K.W. Element mobility from fresh and long-stored acidic fly ashes associated with an Australian power station. Int. J. Coal Geol. 2009, 80, 224–236. [Google Scholar] [CrossRef]
- Jones, D. The Leaching of Major and Trace Elements from Coal Ash. In Environmental Aspects of Trace Elements in Coal; Energy & Environment, Volume 2; Swaine, D.J., Goodarzi, F., Eds.; Springer: Dordrecht, The Netherland, 1995. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.; Blsson, M. Sequential extraction procedure for the speciation particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Fu, B.; Liu, G.; Mian, M.; Sun, M.; Wu, D. Characteristics and speciation of heavy metals in fly ash and FGD gypsum from Chinese coal-fired power plants. Fuel 2019, 251, 593–602. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, L.; Duzgoren-Aydin, N.; Gao, L.; Liu, J.; Peng, Z. Health effects of arsenic, fluorine, and selenium from indoor burning of Chinese coal. Rev. Environ. Contam. Toxicol. 2007, 189, 89–106. [Google Scholar] [CrossRef]
- Ren, D.; Zhao, F.; Dai, S.; Zhang, J. Trace Element Geochemistry of Coal; Science Press: Beijing, China, 2006; (In Chinese with an English Abstract). [Google Scholar]
- Dai, S.; Wang, X.; Zhou, Y.; Hower, J.; Li, D.; Chen, W.; Zhu, X.; Zou, J. Chemical and mineralogical compositions of silicic, mafic, and alkali tonsteins in the late Permian coals from the Songzao Coalfield, Chongqing, Southwest China. Chem. Geol. 2011, 282, 29–44. [Google Scholar] [CrossRef]
- Swaine, D. Trace elements in coal and their dispersal during combustion. Fuel Process. Technol. 1994, 39, 121–137. [Google Scholar] [CrossRef]
- Ketris, M.; Yudovich, Y. Estimations of Clarkes for Carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Zhao, F. Experimental Study on Distribution and Occurrence Mechanism of Hazardous Trace Elements in Coal and Leaching of Coal Products; China University of Mining and Technology: Beijing, China, 1997; (In Chinese with an English Abstract). [Google Scholar]
- Ren, D.; Xu, D.; Zhao, F. A preliminary study on the enrichment mechanism and occurrence of hazardous trace elements in the Tertiary lignite from the Shenbei coalfield, China. Int. J. Coal Geol. 2004, 57, 187–196. [Google Scholar] [CrossRef]
- Yudovich, Y.; Ketris, M. Selenium in coal: A review. Int. J. Coal Geol. 2006, 67, 112–126. [Google Scholar] [CrossRef]
- Riley, K.; French, D.; Lambropoulos, N.; Farrell, O.; Wood, R.; Huggins, F. Origin and occurrence of selenium in some Australian coals. Int. J. Coal Geol. 2007, 72, 72–80. [Google Scholar] [CrossRef]
- Freyer, D.; Voigt, W. Crystallization and phase stability of CaSO4 and CaSO4-based salts. Monatshefte Chem. 2003, 134, 693–719. [Google Scholar] [CrossRef]
- Finkelman, R.; Palmer, C.; Wang, P. Quantification of the modes of occurrence of 42 elements in coal. Int. J. Coal Geol. 2018, 185, 138–160. [Google Scholar] [CrossRef]
- Pflughoeft-Hassett, D. Leaching Characteristics of Fly Ash–Activated Carbon from Mercury Control Technologies; Energy & Environmental Research Center: Grand Forks, ND, USA, 2004; p. 15. [Google Scholar]
- Characterization of Mercuryenriched Coal Combustion Residues from Electric Utilities Using Enhanced Sorbents for Mercury Control; Contract No. EP-C-04-023 Work Assignment; United States Enviromantal Protection Agency: Washington, DC, USA, 2006; p. 131.
- Izquierdo, M.; Koukouzas, N.; Touliou, S.; Panopoulos, K.; Querol, X.; Itskos, G. Geochemical controls on leaching of lignite-fired combustion by-products from Greece. Appl. Geochem. 2011, 26, 1599–1606. [Google Scholar] [CrossRef]
- Nathan, Y.; Dvorachek, M.; Pelly, I.; Mimran, U. Characterization of coal fly ash from Israel. Fuel 1999, 78, 205–213. [Google Scholar] [CrossRef]
- Praharaj, T.; Powell, M.; Hart, B.; Tripathy, S. Leachability of elements from sub-bituminous coal fly ash from India. Environ. Int. 2002, 27, 609–615. [Google Scholar] [CrossRef]
- Kim, A.; Kazonich, G.; Dahlberg, M. Relative solubility of cations in class F fly ash. Rev. Environ. Sci. Biol. 2003, 37, 4507–4511. [Google Scholar] [CrossRef]
- Moreno, N.; Querol, X.; Andrés, J.; Stanton, K.; Towler, M.; Nugteren, H.; Janssen-Jurkovicová, M.; Jones, R. Physico-chemical characteristics of European pulverized coal combustion fly ashes. Fuel 2005, 84, 1351–1363. [Google Scholar] [CrossRef]
- Warren, C.; Dudas, M. Leaching behaviour of selected trace elements in chemically weathered alkaline fly ash. Sci. Total Environ. 1988, 76, 229–246. [Google Scholar] [CrossRef]
- Tewalt, S.; Bragg, L.; Finkelman, R. Mercury in U.S. Coal—Abundance, Distribution, and Modes of Occurrence. U.S. Geological Survey Fact Sheet. 2001, FS–095–01. Available online: http://pubs.usgs.gov/fs/fs095-01/index.html (accessed on 23 March 2023).
- Hower, J.; Robertson, J. Clausthalite in coal. Int. J. Coal Geol. 2003, 53, 219–225. [Google Scholar] [CrossRef]







| Abbreviation | Description |
|---|---|
| CA | coal ash |
| FC | feed coal |
| HEs | hazardous elements |
| LSe | the relative leaching rate of Se |
| LPb | the relative leaching rate of Pb |
| LHg | the relative leaching rate of Hg |
| Vdaf | volatile matter |
| Mad | moisture content |
| Ad | ash yield |
| Cdaf | carbon |
| Hdaf | hydrogen |
| Ndaf | nitrogen |
| St, d | total sulfur |
| Odaf | oxygen |
| RL | Renlou coal mine |
| QD | Qidong coal mine |
| WLH | Wolonghu coal mine |
| Sample | Coal Seam | Proximate Analysis (wt. %) | Ultimate Analysis (wt. %) | Total Sulfur Content (wt. %) | Major Elements Analysis (wt. %) | ||||||||||||
| Mad | Ad | Vdaf | Cdaf | Hdaf | Ndaf | Odaf | St, d | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | TiO2 | K2O | Na2O | ||
| RL | 7-2 | 2.06 | 19.38 | 35.44 | 85.53 | 5.65 | 1.35 | 6.98 | 0.38 | 51.23 | 30.57 | 5.22 | 4.78 | 1.34 | 1.76 | 1.53 | 0.63 |
| QD | 9 | 0.68 | 21.38 | 32.59 | 83.98 | 5.34 | 1.38 | 8.22 | 0.56 | 52.08 | 29.32 | 6.38 | 4.45 | 1.43 | 1.35 | 1.04 | 0.67 |
| WLH | 10 | 2.2 | 15.69 | 12.64 | 82.35 | 6.92 | 1.24 | 8.83 | 0.51 | 44.64 | 23.42 | 12.20 | 9.47 | 2.71 | 0.85 | 1.24 | 0.54 |
| Sample | Coal Mine | Coal Seam | Cr (μg/g) | Mn (μg/g) | Co (μg/g) | Ni (μg/g) | As (μg/g) | Se (μg/g) | Cd (μg/g) | Sb (μg/g) | Hg (μg/g) | Pb (μg/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QD-1 | Qidong | 3-2 | 25.14 | 67.80 | 15.40 | 18.00 | nd | nd | 0.13 | 0.92 | 0.48 | 15.28 |
| QD-2 | Qidong | 6 | 43.41 | 22.33 | 9.92 | 22.68 | 26.31 | 5.54 | 0.55 | 3.30 | 0.11 | 16.11 |
| QD-3 | Qidong | 7 | 99.50 | 47.50 | 33.03 | 62.24 | 11.26 | 6.86 | 0.74 | 5.28 | 0.14 | 37.82 |
| QD-4 | Qidong | 9 | 26.02 | 9.60 | 7.58 | 23.30 | 2.77 | 1.99 | 0.11 | 0.81 | 0.04 | 4.95 |
| ZXZ-1 | Zhuxianzhuang | 8 | 28.64 | 12.57 | 2.45 | 14.63 | 6.67 | 3.20 | 0.12 | 0.49 | 0.06 | 7.10 |
| ZXZ-2 | Zhuxianzhuang | 10 | 23.31 | 8.82 | 9.50 | 16.77 | 4.45 | 2.31 | 0.21 | 1.44 | 0.05 | 11.46 |
| RL-1 | Renlou | 7-2 | 36.36 | 20.33 | 16.26 | 36.08 | 11.66 | 5.98 | 0.25 | 3.41 | 0.12 | 10.05 |
| RL-2 | Renlou | 8-2 | 27.05 | 25.51 | 9.83 | 21.79 | 6.02 | 5.50 | 0.35 | 2.73 | 0.11 | 10.42 |
| WLH-1 | Wolonghu | 8 | 35.43 | 5.82 | 6.43 | 19.56 | 13.06 | 6.93 | 0.16 | 0.84 | 0.14 | 14.29 |
| LH-1 | Linhuan | 7 | nd | 11.33 | 6.23 | 12.23 | 2.16 | 14.63 | nd | 10.99 | nd | 19.75 |
| LH-2 | Linhuan | 8 | nd | 30.23 | 4.07 | 7.23 | 2.38 | 16.29 | nd | 6.86 | nd | 121.26 |
| LH-3 | Linhuan | 9 | nd | 4.29 | 0.48 | 7.57 | 3.64 | 12.94 | nd | 7.36 | nd | 62.61 |
| TT-1 | Tongting | 9 | nd | nd | 4.21 | 13.35 | 1.49 | 11.33 | nd | 8.45 | nd | 79.10 |
| TY-1 | Taoyuan | 8 | nd | 29.03 | 1.62 | 4.25 | 4.55 | 20.40 | nd | 35.39 | nd | 26.00 |
| TY-2 | Taoyuan | 10 | nd | 4.73 | nd | 1.97 | 2.28 | 17.48 | nd | 20.78 | nd | 28.02 |
| QN-1 | Qinan | 6-1 | nd | 8.56 | 7.75 | 13.24 | 3.69 | 12.10 | nd | 8.85 | nd | 134.83 |
| QN-2 | Qinan | 7-1 | nd | nd | 4.24 | 6.78 | 3.77 | 9.69 | nd | 9.35 | nd | 9.14 |
| QN-3 | Qinan | 7-2 | nd | nd | nd | 2.70 | 2.11 | 11.12 | nd | 9.88 | nd | 38.90 |
| XT-1 | Xutuan | 7-1 | nd | 17.44 | 6.18 | 10.71 | 3.60 | 15.39 | nd | 15.96 | nd | 23.17 |
| XT-2 | Xutuan | 7-2 | nd | 16.78 | 6.32 | 10.51 | 4.06 | 17.27 | nd | 24.72 | nd | 15.73 |
| Elements | Measured Data of Huaibei Coalfield (μg/g) | Collecting Data of Huaibei Coalfield a (μg/g) | Chinese Coals b (μg/g) | World Coals c (μg/g) |
|---|---|---|---|---|
| Cr | ||||
| Mn | nd | 271.2 * | 50 * | |
| Co | nd | |||
| Ni | nd | |||
| As | ||||
| Se | nd | |||
| Cd | nd | 0.46 * | 0.30 * | |
| Sb | nd | 2.56 * | 3.0 * | |
| Hg | nd | 1.37 * | 0.012 * | |
| Pb | nd |
| Sample Number | Se (μg/g) | Hg (μg/g) | Pb (μg/g) | |
|---|---|---|---|---|
| FC | RL-FC | 5.98 | 0.12 | 10.05 |
| QD-FC | 1.99 | 0.04 | 4.95 | |
| WLH-FC | 2.31 | 0.15 | 11.46 | |
| CA | RL-CA | 1.32 | 0.10 | 2.98 |
| QD-CA | 1.25 | 0.06 | 1.97 | |
| WLH-CA | 0.98 | 0.05 | 3.36 | |
| Leachate | pH = 2 | pH = 4 | pH = 7 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaching Time | Time (h) | Time (h) | Time (h) | ||||||||||
| 5 | 15 | 30 | 60 | 5 | 15 | 30 | 60 | 5 | 15 | 30 | 60 | ||
| Se | RL-FC | 1.386 | 2.007 | 4.438 | 7.115 | 0.024 | 0.053 | 0.378 | 1.025 | 0.014 | 0.023 | 0.039 | 0.099 |
| RL-CA | 3.335 | 5.432 | 21.263 | 30.729 | 0.075 | 0.450 | 2.154 | 6.364 | 0.174 | 0.064 | 0.145 | 0.418 | |
| QD-FC | 0.485 | 1.085 | 1.946 | 3.089 | 0.030 | 0.045 | 0.291 | 1.708 | 0.006 | 0.026 | 0.060 | 0.189 | |
| QD-CA | 0.050 | 0.231 | 0.631 | 1.374 | 0.005 | 0.031 | 0.288 | 1.057 | 0.012 | 0.022 | 0.039 | 0.134 | |
| WLH-FC | 1.186 | 1.406 | 3.069 | 4.763 | 0.018 | 0.031 | 0.255 | 0.865 | 0.014 | 0.020 | 0.046 | 0.035 | |
| WLH-CA | 0.693 | 1.431 | 2.754 | 3.924 | 0.135 | 0.621 | 2.232 | 13.968 | 0.255 | 0.108 | 0.072 | 0.360 | |
| Hg | RL-FC | 0.035 | 0.024 | 0.023 | 0.090 | 0.046 | 0.028 | 0.039 | 0.287 | 0.002 | 0.002 | 0.002 | 0.006 |
| RL-CA | 0.106 | 0.113 | 0.094 | 1.313 | 0.456 | 0.319 | 0.656 | 3.338 | 0.025 | 0.066 | 0.056 | 0.113 | |
| QD-FC | 0.038 | 0.024 | 0.018 | 0.069 | 0.047 | 0.031 | 0.034 | 0.268 | 0.002 | 0.002 | 0.002 | 0.008 | |
| QD-CA | 0.125 | 0.107 | 0.214 | 0.986 | 0.636 | 0.118 | 0.536 | 3.986 | 0.032 | 0.032 | 0.129 | 0.514 | |
| WLH-FC | 0.038 | 0.018 | 0.015 | 0.074 | 0.037 | 0.025 | 0.013 | 0.194 | 0.003 | 0.003 | 0.002 | 0.007 | |
| WLH-CA | 0.294 | 0.315 | 0.432 | 0.576 | 0.702 | 0.108 | 0.432 | 3.672 | 0.036 | 0.045 | 0.162 | 0.684 | |
| Pb | RL-FC | 0.008 | 0.015 | 0.064 | 0.263 | 0.003 | 0.019 | 0.045 | 0.165 | 0.075 | 0.169 | 0.113 | 0.375 |
| RL-CA | 0.013 | 0.045 | 0.060 | 0.210 | 0.015 | 0.068 | 0.315 | 0.360 | 0.125 | 0.675 | 0.300 | 0.300 | |
| QD-FC | 0.007 | 0.009 | 0.049 | 0.068 | 0.003 | 0.038 | 0.071 | 0.060 | 0.113 | 0.075 | 0.075 | 1.050 | |
| QD-CA | 0.006 | 0.004 | 0.020 | 0.051 | 0.001 | 0.003 | 0.034 | 0.146 | 0.014 | 0.070 | 0.028 | 0.056 | |
| WLH-FC | 0.005 | 0.002 | 0.025 | 0.020 | 0.002 | 0.016 | 0.023 | 0.025 | 0.044 | 0.031 | 0.082 | 0.941 | |
| WLH-CA | 0.016 | 0.005 | 0.031 | 0.105 | 0.009 | 0.031 | 0.084 | 0.251 | 0.052 | 0.105 | 0.523 | 0.419 | |
| Sample Number | RL-FC | QD-FC | WLH-FC | |||
|---|---|---|---|---|---|---|
| Data/(μg/g) | Ti (i = 1, 2, 3) | Data/(μg/g) | Ti (i=1, 2, 3) | Data/(μg/g) | Ti (i = 1, 2, 3) | |
| Results | 4.123 | 2.081 | 1.656 | 2.189 | 3.159 | 0.748 |
| 4.426 | 0.079 | 1.965 | 0.143 | 3.267 | 1.646 | |
| 4.765 | 2.160 | 2.217 | 2.046 | 2.781 | 2.394 | |
| Average of the data | 4.438 | 1.946 | 3.069 | |||
| SD of the data | 0.262 | 0.229 | 0.208 | |||
| Correlation Coefficients (r) | Sample Type | |
|---|---|---|
| FC | CA | |
| Correlation Coefficients with Maximum Leaching Rate (Lmax) | ||
| Se | 0.938 | 0.518 |
| Hg | 0.807 | 0.665 |
| Pb | −0.451 | 0.647 |
| Correlation Coefficients (r) | Total Sulfur | Oxide | ||
|---|---|---|---|---|
| St, d | Fe2O3 | CaO | ||
| Correlation Coefficients with Maximum Leaching Rate (Lmax) | ||||
| Se | FC | −0.988 | −0.250 | −0.038 |
| CA | −0.995 | −0.483 | −0.286 | |
| Hg | FC | −0.981 | −0.568 | −0.380 |
| CA | −0.928 | −0.026 | 0.187 | |
| Pb | FC | 0.396 | 0.504 | 0.309 |
| CA | 0.191 | −0.229 | −0.017 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Lu, J.; Wu, J.; Li, B.; Nyasha, N.K. Enrichment Characteristics of Hazardous Trace Elements in Feed Coal and Coal Ash in Huaibei Area under Leaching. Toxics 2023, 11, 308. https://doi.org/10.3390/toxics11040308
Wang D, Lu J, Wu J, Li B, Nyasha NK. Enrichment Characteristics of Hazardous Trace Elements in Feed Coal and Coal Ash in Huaibei Area under Leaching. Toxics. 2023; 11(4):308. https://doi.org/10.3390/toxics11040308
Chicago/Turabian StyleWang, Degao, Jianwei Lu, Jian Wu, Bo Li, and Ndhlovu Kataza Nyasha. 2023. "Enrichment Characteristics of Hazardous Trace Elements in Feed Coal and Coal Ash in Huaibei Area under Leaching" Toxics 11, no. 4: 308. https://doi.org/10.3390/toxics11040308
APA StyleWang, D., Lu, J., Wu, J., Li, B., & Nyasha, N. K. (2023). Enrichment Characteristics of Hazardous Trace Elements in Feed Coal and Coal Ash in Huaibei Area under Leaching. Toxics, 11(4), 308. https://doi.org/10.3390/toxics11040308






