Aflatoxin-B1-Exposure-Induced Hepatic Injury Could Be Alleviated by Polydatin through Reducing Oxidative Stress, Inhibiting Inflammation and Improving Mitophagy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Sample Collection
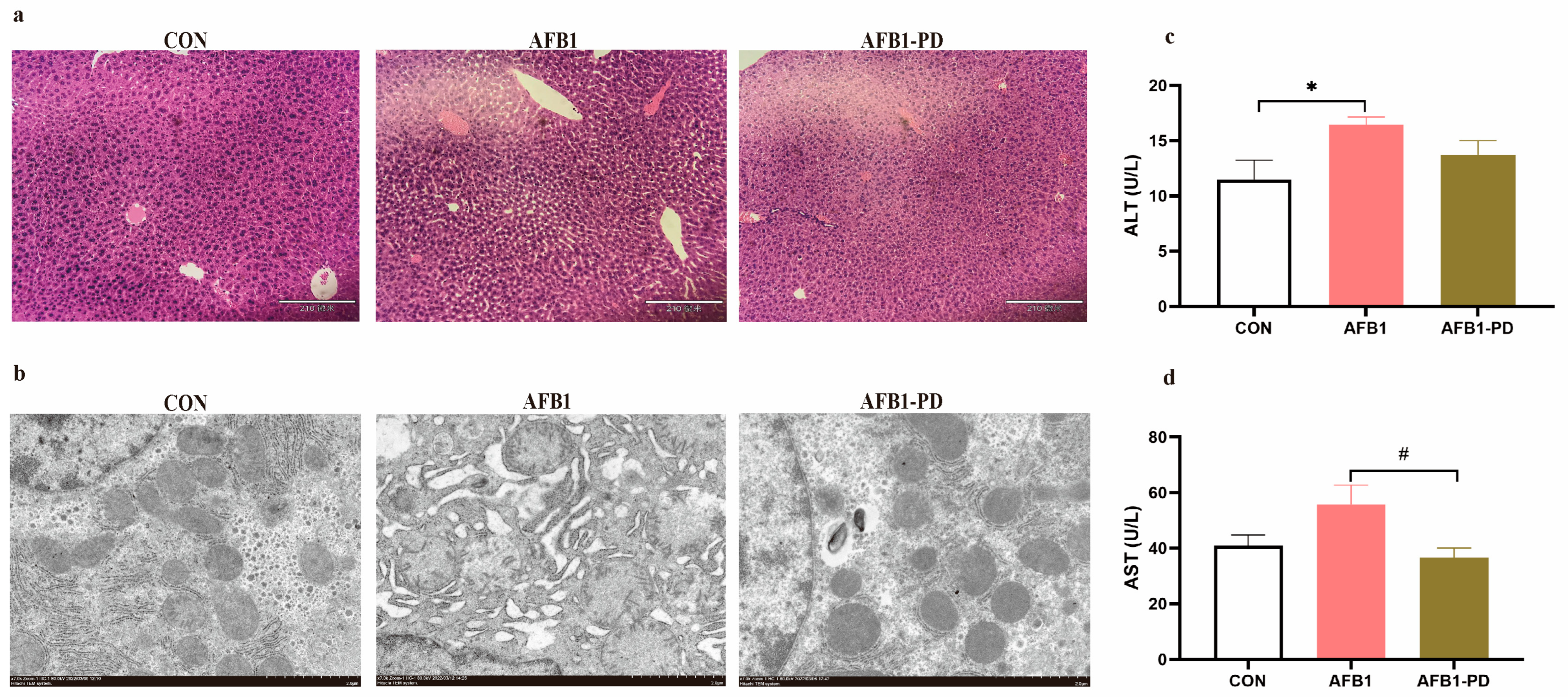
2.3. Haematoxylin and Eosin (H&E) Staining
2.4. Transmission Electron Microscope
2.5. Detection of Serum Transaminase Activities
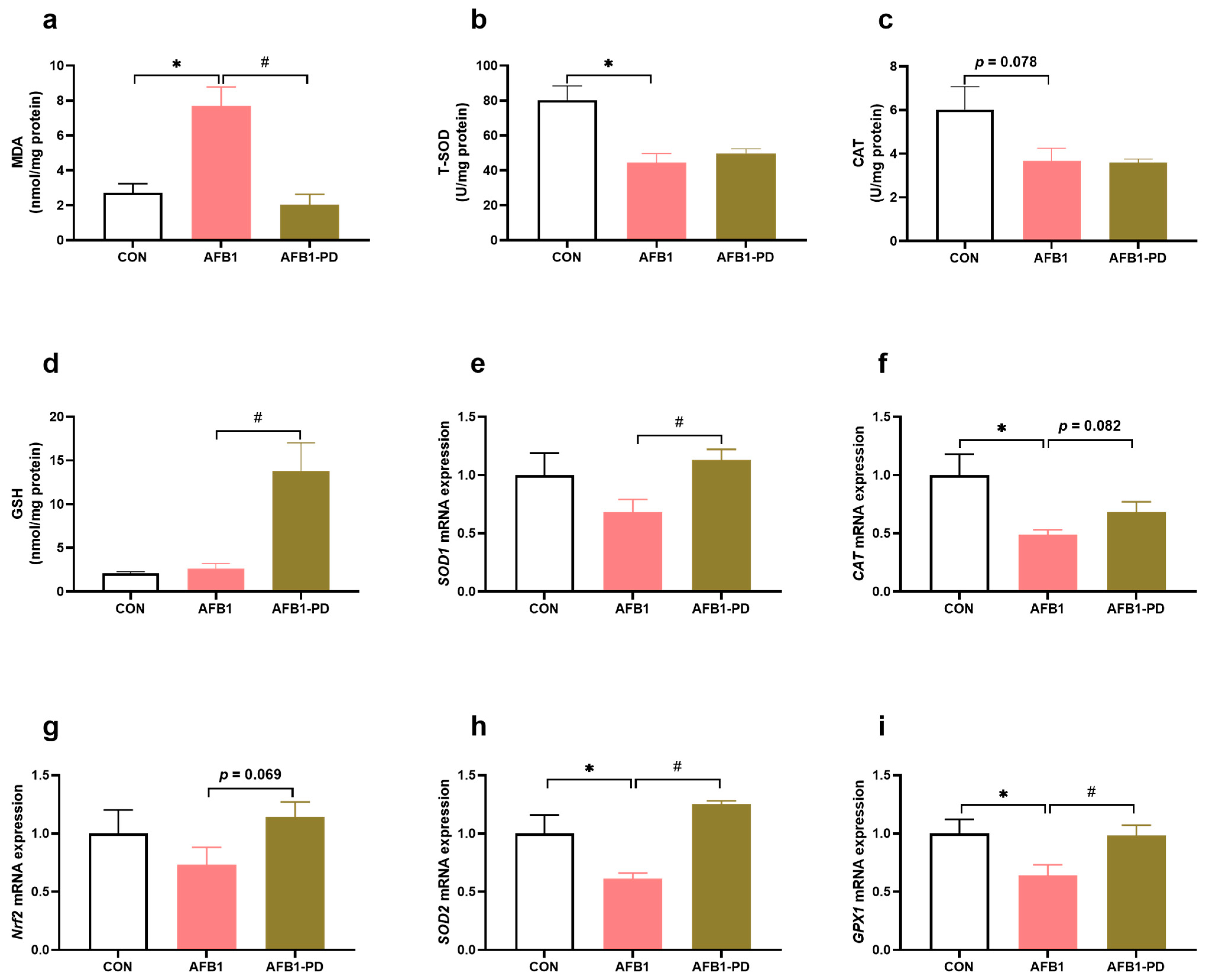
2.6. Oxidative Status Analysis
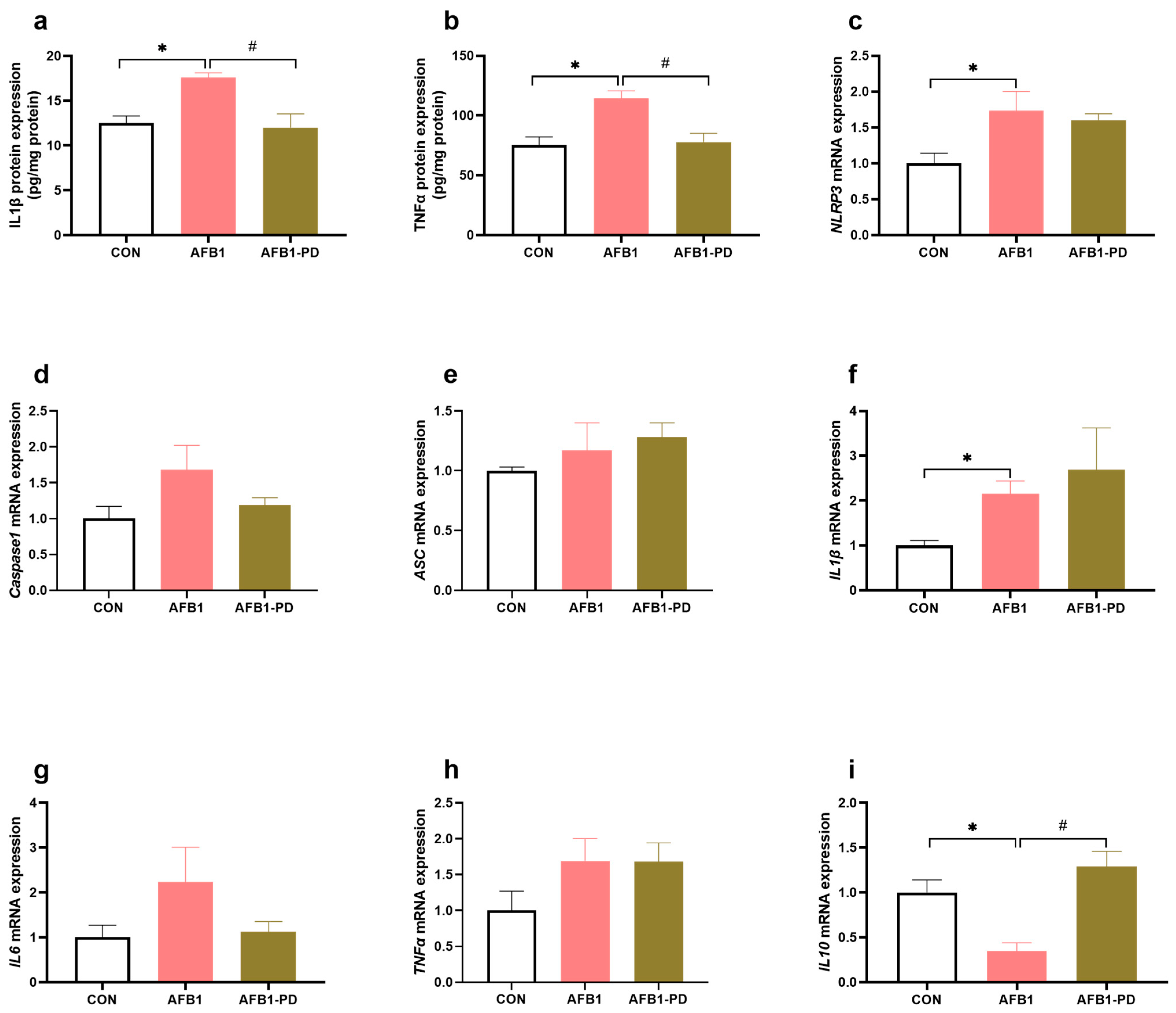
2.7. Cytokines Level Measurement
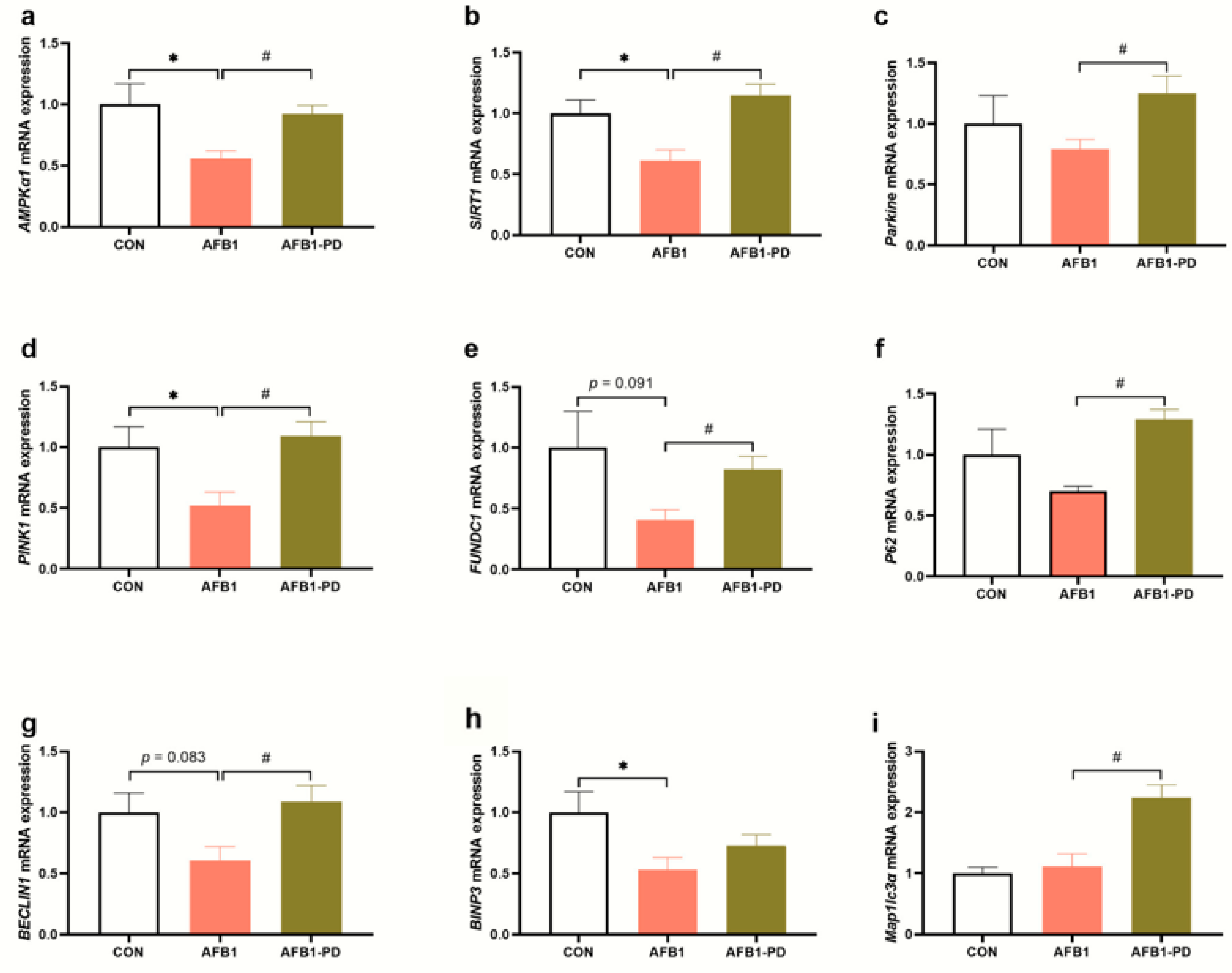
2.8. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR) Analysis
2.9. Statistical Analysis
3. Results
3.1. PD Alleviates Hepatic Injury in AFB1 Mice
3.2. PD Reduces Hepatic Oxidative Stress in AFB1 Mice
3.3. PD Inhibits Hepatic Inflammation in AFB1 Mice
3.4. PD Improves Hepatic Mitophagy in AFB1 Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yunus, A.W.; Razzazi-Fazeli, E.; Bohm, J. Aflatoxin B1 in affecting broiler’s performance, immunity, and gastrointestinal tract: A review of history and contemporary issues. Toxins 2011, 3, 566–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.; Nyamagoud, S.B.; SreeHarsha, N.; Mishra, A.; Gubbiyappa, S.K.; Singh, Y. Sitagliptin protects liver against aflatoxin B1-induced hepatotoxicity through upregulating Nrf2/ARE/HO-1 pathway. BioFactors 2020, 46, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Damiano, S.; Jarriyawattanachaikul, W.; Girolami, F.; Longobardi, C.; Nebbia, C.; Andretta, E.; Lauritano, C.; Dabbou, S.; Avantaggiato, G.; Schiavone, A.; et al. Curcumin Supplementation Protects Broiler Chickens Against the Renal Oxidative Stress Induced by the Dietary Exposure to Low Levels of Aflatoxin B1. Front. Vet. Sci. 2022, 8, 822227. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, Y.; Cao, Z.; Zhang, J.; Huang, W. AFB1-induced mice liver injury involves mitochondrial dysfunction mediated by mitochondrial biogenesis inhibition. Ecotoxicol. Environ. Saf. 2021, 216, 112213. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Wang, X.; Li, S.; Li, R.; Zhang, X. Curcumin confers hepatoprotection against AFB1-induced toxicity via activating autophagy and ameliorating inflammation involving Nrf2/HO-1 signaling pathway. Mol. Biol. Rep. 2018, 45, 1775–1785. [Google Scholar] [CrossRef]
- Taranu, I.; Hermenean, A.; Bulgaru, C.; Pistol, G.C.; Ciceu, A.; Grosu, I.A.; Marin, D.E. Diet containing grape seed meal by-product counteracts AFB1 toxicity in liver of pig after weaning. Ecotoxicol. Environ. Saf. 2020, 203, 110899. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Tian, J.; Muhammad, I.; Liu, M.; Wu, C.; Xu, C.; Zhang, X. Ferulic acid prevents aflatoxin B1-induced liver injury in rats via inhibiting cytochrome P450 enzyme, activating Nrf2/GST pathway and regulating mitochondrial pathway. Ecotoxicol. Environ. Saf. 2021, 224, 112624. [Google Scholar] [CrossRef]
- Pauletto, M.; Tolosi, R.; Giantin, M.; Guerra, G.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Insights into Aflatoxin B1 Toxicity in Cattle: An In Vitro Whole-Transcriptomic Approach. Toxins 2020, 12, 429. [Google Scholar] [CrossRef]
- Wang, Q.; Jia, F.; Guo, C.; Wang, Y.; Zhang, X.; Cui, Y.; Song, M.; Cao, Z.; Li, Y. PINK1/Parkin-mediated mitophagy as a protective mechanism against AFB1-induced liver injury in mice. Food Chem. Toxicol. 2022, 164, 113043. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Liu, M.; Zhou, X.; Wang, M.; Cao, K.; Jin, S.; Shan, A.; Feng, X. Curcumin mitigates aflatoxin B1-induced liver injury via regulating the NLRP3 inflammasome and Nrf2 signaling pathway. Food Chem. Toxicol. 2022, 161, 112823. [Google Scholar] [CrossRef]
- Jin, S.; Yang, H.; Wang, Y.; Pang, Q.; Jiao, Y.; Shan, A.; Feng, X. Dietary Curcumin Alleviated Aflatoxin B1-Induced Acute Liver Damage in Ducks by Regulating NLRP3-Caspase-1 Signaling Pathways. Foods 2021, 10, 3086. [Google Scholar] [CrossRef]
- Du, Q.H.; Peng, C.; Zhang, H. Polydatin: A review of pharmacology and pharmacokinetics. Pharm. Biol. 2013, 51, 1347–1354. [Google Scholar] [CrossRef]
- Zhao, X.J.; Yu, H.W.; Yang, Y.Z.; Wu, W.Y.; Chen, T.Y.; Jia, K.K.; Kang, L.L.; Jiao, R.Q.; Kong, L.D. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018, 18, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Huang, Q.H.; Wu, X.; Wu, J.Z.; Liang, J.L.; Lin, G.S.; Xu, L.Q.; Lai, X.P.; Su, Z.R.; Chen, J.N. Polydatin protects against acetaminophen-induced hepatotoxicity in mice via anti-oxidative and anti-apoptotic activities. Food Funct. 2018, 9, 5891–5902. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Kuang, G.; Jiang, W.; Jiang, R.; Jiang, D. Polydatin promotes apoptosis through upregulation the ratio of Bax/Bcl-2 and inhibits proliferation by attenuating the β-catenin signaling in human osteosarcoma cells. Am. J. Transl. Res. 2016, 8, 922–931. [Google Scholar] [PubMed]
- Lai, Y.; Zhou, C.; Huang, P.; Dong, Z.; Mo, C.; Xie, L.; Lin, H.; Zhou, Z.; Deng, G.; Liu, Y.; et al. Polydatin alleviated alcoholic liver injury in zebrafish larvae through ameliorating lipid metabolism and oxidative stress. J. Pharmacol. Sci. 2018, 138, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Zhang, Q.; Duan, H.; Ye, X.; Liu, J.; Peng, W.; Wu, C. Polydatin: A Critical Promising Natural Agent for Liver Protection via Antioxidative Stress. Oxid. Med. Cell. Longev. 2022, 2022, 9218738. [Google Scholar] [CrossRef]
- Chen, X.; Chan, H.; Zhang, L.; Liu, X.; Ho, I.H.T.; Zhang, X.; Ho, J.; Hu, W.; Tian, Y.; Kou, S.; et al. The phytochemical polydatin ameliorates non-alcoholic steatohepatitis by restoring lysosomal function and autophagic flux. J. Cell. Mol. Med. 2019, 23, 4290–4300. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Chen, Y.; Pei, Z.; Gao, H.; Shi, W.; Sun, M.; Xu, Q.; Zhao, J.; Meng, W.; Xiao, K. Protective effects of polydatin against sulfur mustard-induced hepatic injury. Toxicol. Appl. Pharmacol. 2019, 367, 1–11. [Google Scholar] [CrossRef]
- Gao, Y.; Dai, X.; Li, Y.; Li, G.; Lin, X.; Ai, C.; Cao, Y.; Li, T.; Lin, B. Role of Parkin-mediated mitophagy in the protective effect of polydatin in sepsis-induced acute kidney injury. J. Transl. Med. 2020, 18, 114. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zhao, Z.; Duan, C.; Wang, C.; Zhao, Y.; Yang, G.; Gao, L.; Niu, C.; Xu, J.; Li, S. Lactobacillus plantarum C88 protects against aflatoxin B1-induced liver injury in mice via inhibition of NF-κB–mediated inflammatory responses and excessive apoptosis. BMC Microbiol. 2019, 19, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Aarag, B.; Magdy, M.; AlAjmi, M.F.; Khalifa, S.A.M.; El-Seedi, H.R. Melittin Exerts Beneficial Effects on Paraquat-Induced Lung Injuries in Mice by Modifying Oxidative Stress and Apoptosis. Molecules 2019, 24, 1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.; Song, Z.; Li, S.; Yan, E.; Zhang, H.; Zhang, L.; Wang, C.; Wang, T. Effects of resveratrol on intestinal oxidative status and inflammation in heat-stressed rats. J. Therm. Biol. 2019, 85, 102415. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, R.; Liu, Y.; Zhang, X.; Zhang, M.; Zeng, Z.; Wu, L.; Gao, X.; Lan, T.; Wang, Y. Polydatin protects against carbon tetrachloride-induced liver fibrosis in mice. Arch. Biochem. Biophys. 2017, 629, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tan, Y.; Zhang, N.; Yao, F. Polydatin supplementation ameliorates diet-induced development of insulin resistance and hepatic steatosis in rats. Mol. Med. Rep. 2015, 11, 603–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Liu, Y.; Xu, W.; Dai, X.; Liu, R.; Gao, Y.; Chen, Z.; Li, Y. Polydatin mediates Parkin-dependent mitophagy and protects against mitochondria-dependent apoptosis in acute respiratory distress syndrome. Lab. Investig. 2019, 99, 819–829. [Google Scholar] [CrossRef]
- Gao, X.; Xu, J.; Jiang, L.; Liu, W.; Hong, H.; Qian, Y.; Li, S.; Huang, W.; Zhao, H.; Yang, Z.; et al. Morin alleviates aflatoxin B1-induced liver and kidney injury by inhibiting heterophil extracellular traps release, oxidative stress and inflammatory responses in chicks. Poult. Sci. 2021, 100, 101513. [Google Scholar] [CrossRef]
- Shen, H.M.; Shi, C.Y.; Shen, Y.; Ong, C.N. Detection of elevated reactive oxygen species level in cultured rat hepatocytes treated with aflatoxin B1. Free Radical Biol. Med. 1996, 21, 139–146. [Google Scholar] [CrossRef]
- Hayes, J.D.; Pulford, D.J.; Ellis, E.M.; McLeod, R.; James, R.F.L.; Seidegård, J.; Mosialou, E.; Jernström, B.; Neal, G.E. Regulation of rat glutathione S-transferase A5 by cancer chemopreventive agents: Mechanisms of inducible resistance to aflatoxin B1. Chem.-Biol. Interact. 1998, 111–112, 51–67. [Google Scholar] [CrossRef]
- Soetikno, V.; Sari, F.R.; Lakshmanan, A.P.; Arumugam, S.; Harima, M.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2–keap1 pathway. Mol. Nutr. Food Res. 2013, 57, 1649–1659. [Google Scholar] [CrossRef]
- Muhammad, I.; Wang, H.; Sun, X.; Wang, X.; Han, M.; Lu, Z.; Cheng, P.; Hussain, M.A.; Zhang, X. Dual Role of Dietary Curcumin Through Attenuating AFB1-Induced Oxidative Stress and Liver Injury via Modulating Liver Phase-I and Phase-II Enzymes Involved in AFB1 Bioactivation and Detoxification. Front. Pharmacol. 2018, 9, 554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.H.; Xu, L.Q.; Liu, Y.H.; Wu, J.Z.; Wu, X.; Lai, X.P.; Li, Y.C.; Su, Z.R.; Chen, J.N.; Xie, Y.L. Polydatin Protects Rat Liver against Ethanol-Induced Injury: Involvement of CYP2E1/ROS/Nrf2 and TLR4/NF-κB p65 Pathway. Evid. Based Complement. Alternat. Med. 2017, 2017, 7953850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Kim, J.S. Mitophagy: Therapeutic Potentials for Liver Disease and Beyond. Toxicol. Res. 2014, 30, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; McKeen, T.; Zhang, J.; Ding, W.X. Role and Mechanisms of Mitophagy in Liver Diseases. Cells 2020, 9, 837. [Google Scholar] [CrossRef] [Green Version]
- Yoshii, S.R.; Mizushima, N. Autophagy machinery in the context of mammalian mitophagy. Biochim. Biophys. Acta 2015, 1853, 2797–2801. [Google Scholar] [CrossRef] [Green Version]
- Di Sante, G.; Pestell, T.G.; Casimiro, M.C.; Bisetto, S.; Powell, M.J.; Lisanti, M.P.; Cordon-Cardo, C.; Castillo-Martin, M.; Bonal, D.M.; Debattisti, V.; et al. Loss of Sirt1 Promotes Prostatic Intraepithelial Neoplasia, Reduces Mitophagy, and Delays Park2 Translocation to Mitochondria. Am. J. Pathol. 2015, 185, 266–279. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; Xiao, L.; Liu, G.; Sun, L.; He, L. STC-1 ameliorates renal injury in diabetic nephropathy by inhibiting the expression of BNIP3 through the AMPK/SIRT3 pathway. Lab. Investig. 2019, 99, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Xiao, H.; Li, X.; Zhu, J.; Gao, J.; Wang, L.; Hu, C. AMPK-PINK1/Parkin Mediated Mitophagy Is Necessary for Alleviating Oxidative Stress-Induced Intestinal Epithelial Barrier Damage and Mitochondrial Energy Metabolism Dysfunction in IPEC-J2. Antioxidants 2021, 10, 2010. [Google Scholar] [CrossRef] [PubMed]
- Bak, D.H.; Na, J.; Im, S.I.; Oh, C.T.; Kim, J.Y.; Park, S.K.; Han, H.J.; Seok, J.; Choi, S.Y.; Ko, E.J.; et al. Antioxidant effect of human placenta hydrolysate against oxidative stress on muscle atrophy. J. Cell. Physiol. 2019, 234, 1643–1658. [Google Scholar] [CrossRef]

| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| SIRT1 | ACTGGAGCTGGGGTTTCTGT | CACAGAGACGGCTGGAACTG |
| AMPKα1 | ACCTGAGAACGTCCTGCTTG | GGCCTGCGTACAATCTTCCT |
| PINK1 | TGTGGAATATCTCGGCAGGT | GATGTTAGGGTGTGGGGCAA |
| Parkin | GCCAGAGGTCCAGCAGTTAAA | CACCACTCATCCGGTTTGGA |
| BNIP3 | TTCTCACTGTGACAGCCCAC | GGTCGACTTGACCAATCCCA |
| FUNDC1 | CTTCTTCTTCAGGCAACAGACT | GCAAAAAGCCTCCCACGAAG |
| P62 | GGACAGCCAGAGGAACAGAT | CTCAATCAGCCGGGGATCAG |
| Map1lc3α | TTGGTCAAGATCATCCGGCG | GAAGGTTTCTTGGGAGGCGT |
| BECLIN1 | GCCTCTGAAACTGGACACGA | TGTAGACATCATCCTGGCTGG |
| Nrf2 | GCCCTCAGCATGATGGACTT | AACTTGTACCGCCTCGTCTG |
| GPX1 | AGTCCACCGTGTATGCCTTC | CCTCAGAGAGACGCGACATT |
| SOD1 | CATGGCGATGAAAGCGGTG | GCACTGGTACAGCCTTGTGTA |
| SOD2 | AACGCCACCGAGGAGAAGTA | TCCAGCAACTCTCCTTTGGGT |
| CAT | TGATCTGACCAAGGTTTGGC | AGTGTCCGGGTAGGCAAAAA |
| NLRP3 | TCTCCCGCATCTCCATTTGT | CTGTCCCGCATTTTAGTCCG |
| Caspase1 | CAGGAGGGAATATGTGGG | CACCTTGGGCTTGTCTTT |
| ASC | TGACAGTGCAACTGCGAGAA | GTGAGCTCCAAGCCATACGA |
| IL10 | CCAAGGTGTCTACAAGGCCA | GCTCTGTCTAGGTCCTGGAGT |
| IL6 | CTCCCAACAGACCTGTCTATAC | CCATTGCACAACTCTTTTCTCA |
| IL1β | GTCTTTCCCGTGGACCTTC | ATCTCGGAGCCTGTAGTGC |
| TNFα | GCGACGTGGAACTGGCAGAAG | GCCACAAGCAGGAATGAGAAGAGG |
| β-actin | TATAAAACCCGGCGGCGCA | GTCATCCATGGCGAACTGGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, K.; Niu, J.; Zheng, X.; Qiao, Y.; Zhang, J.; Guo, R.; Dong, G.; Song, Z.; Huang, J.; Wang, J.; et al. Aflatoxin-B1-Exposure-Induced Hepatic Injury Could Be Alleviated by Polydatin through Reducing Oxidative Stress, Inhibiting Inflammation and Improving Mitophagy. Toxics 2023, 11, 309. https://doi.org/10.3390/toxics11040309
Cheng K, Niu J, Zheng X, Qiao Y, Zhang J, Guo R, Dong G, Song Z, Huang J, Wang J, et al. Aflatoxin-B1-Exposure-Induced Hepatic Injury Could Be Alleviated by Polydatin through Reducing Oxidative Stress, Inhibiting Inflammation and Improving Mitophagy. Toxics. 2023; 11(4):309. https://doi.org/10.3390/toxics11040309
Chicago/Turabian StyleCheng, Kang, Jingyi Niu, Xiaotong Zheng, Yining Qiao, Jinyan Zhang, Rui Guo, Guorun Dong, Zhihua Song, Jin Huang, Jinrong Wang, and et al. 2023. "Aflatoxin-B1-Exposure-Induced Hepatic Injury Could Be Alleviated by Polydatin through Reducing Oxidative Stress, Inhibiting Inflammation and Improving Mitophagy" Toxics 11, no. 4: 309. https://doi.org/10.3390/toxics11040309
APA StyleCheng, K., Niu, J., Zheng, X., Qiao, Y., Zhang, J., Guo, R., Dong, G., Song, Z., Huang, J., Wang, J., & Zhang, Y. (2023). Aflatoxin-B1-Exposure-Induced Hepatic Injury Could Be Alleviated by Polydatin through Reducing Oxidative Stress, Inhibiting Inflammation and Improving Mitophagy. Toxics, 11(4), 309. https://doi.org/10.3390/toxics11040309







