Investigation of Microplastics (≥10 μm) in Meconium by Fourier Transform Infrared Microspectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Collection
2.3. Pretreating the Meconium Samples
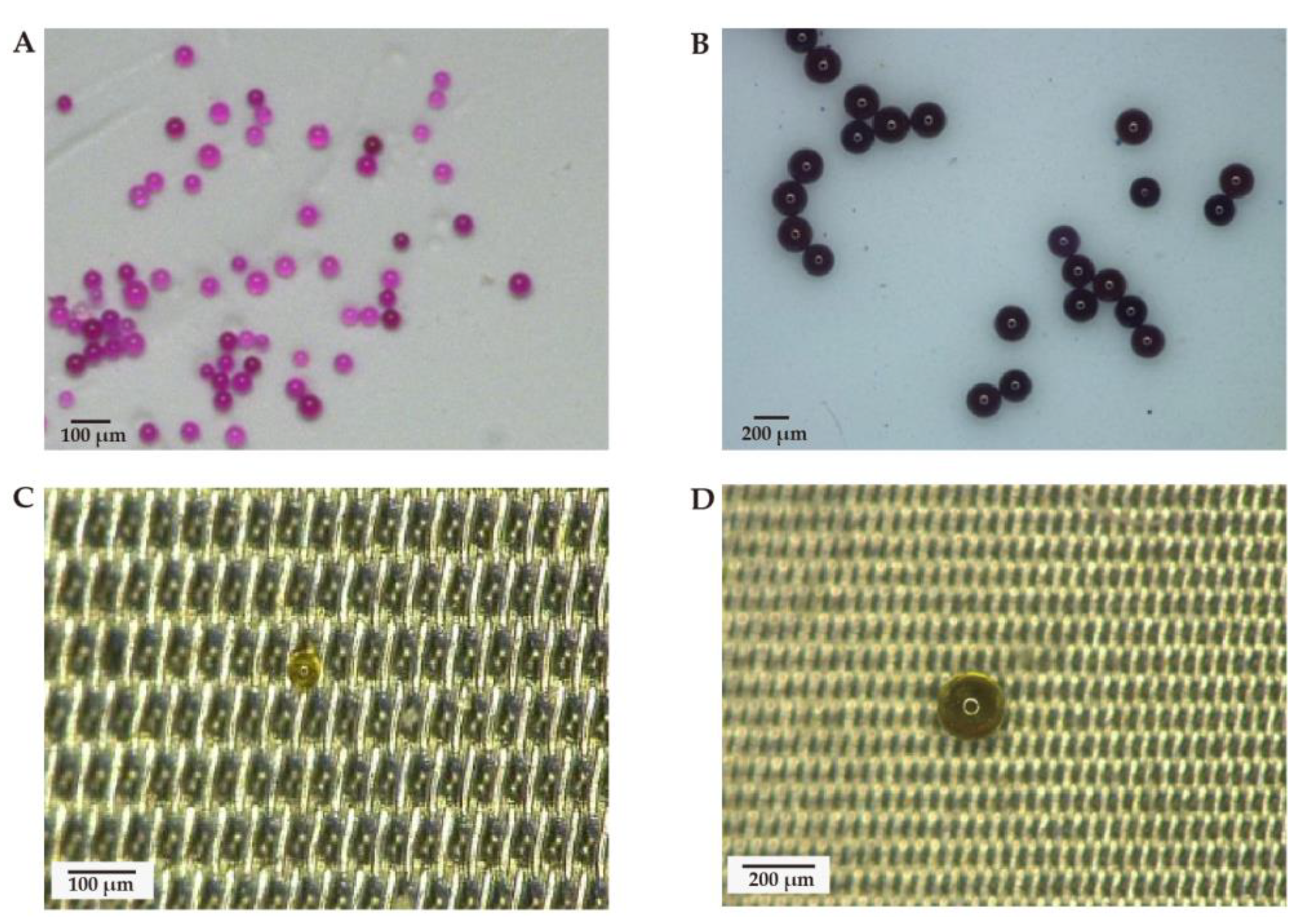
2.4. Recovery Experiment
2.5. Analysis of Samples by Ultra-Depth Three-Dimensional Microscope and Micro-FTIR
2.6. Quality Control
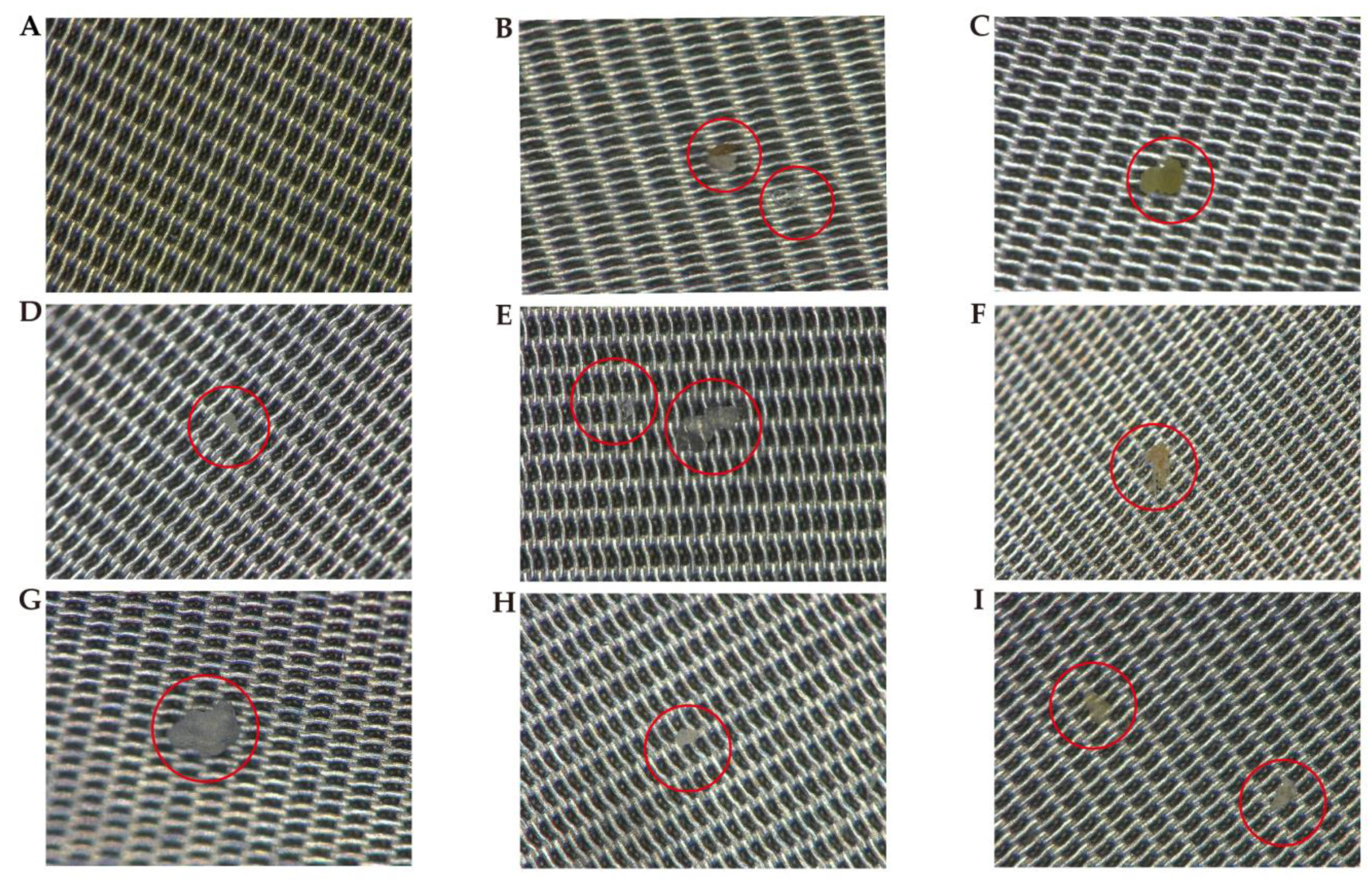
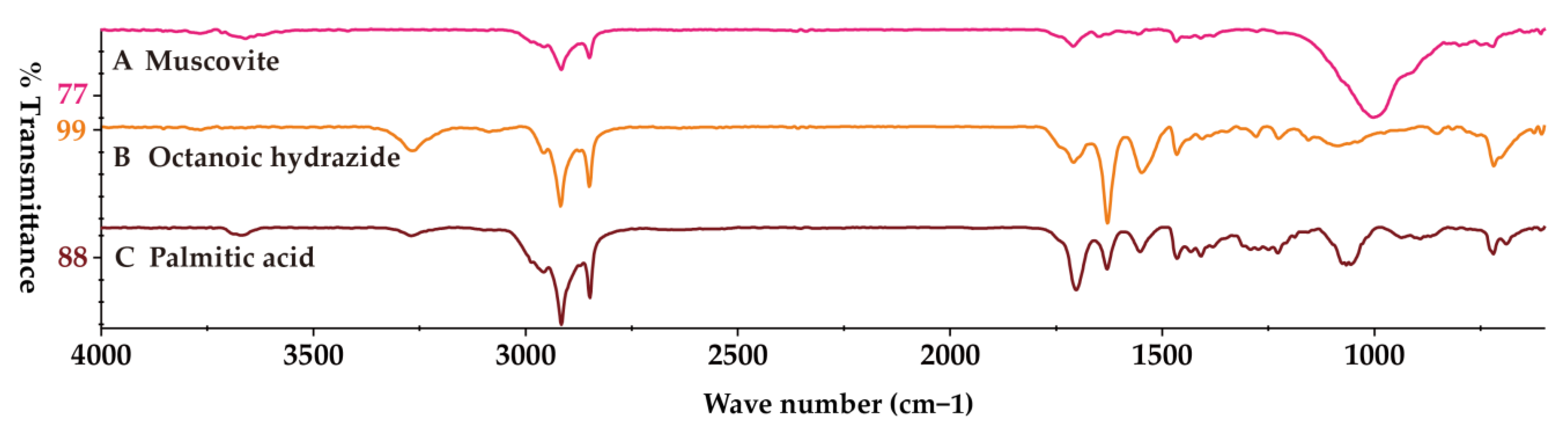
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okoffo, E.D.; Donner, E.; McGrath, S.P.; Tscharke, B.J.; O’Brien, J.W.; O’Brien, S.; Ribeiro, F.; Burrows, S.D.; Toapanta, T.; Rauert, C.; et al. Plastics in biosolids from 1950 to 2016: A function of global plastic production and consumption. Water Res. 2021, 201, 117367. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Silva, A.L.P.; da Costa, J.P.; Mouneyrac, C.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. Solutions and Integrated Strategies for the Control and Mitigation of Plastic and Microplastic Pollution. Int. J. Environ. Res. Public Health 2019, 16, 2411. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhong, Y.; Huang, Y.; Lin, X.; Liu, J.; Lin, L.; Hu, M.; Jiang, J.; Dai, M.; Wang, B.; et al. Underestimated health risks: Polystyrene micro- and nanoplastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis. Part. Fibre Toxicol. 2021, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017, 1, 116. [Google Scholar] [CrossRef]
- Praveena, S.M.; Shaifuddin, S.N.M.; Akizuki, S. Exploration of microplastics from personal care and cosmetic products and its estimated emissions to marine environment: An evidence from Malaysia. Mar. Pollut. Bull. 2018, 136, 135–140. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.B.; He, H.R.; Zhang, J.F.; Ma, G.S. You are what you eat: Microplastics in the feces of young men living in Beijing. Sci. Total Environ. 2021, 767, 144345. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Amato-Lourenco, L.F.; Carvalho-Oliveira, R.; Junior, G.R.; Dos Santos Galvao, L.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Amereh, F.; Amjadi, N.; Mohseni-Bandpei, A.; Isazadeh, S.; Mehrabi, Y.; Eslami, A.; Naeiji, Z.; Rafiee, M. Placental plastics in young women from general population correlate with reduced foetal growth in IUGR pregnancies. Environ. Pollut. 2022, 314, 120174. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, W.; Lin, T.; Liu, S.; Sun, Z.; Liu, F.; Yuan, Y.; Xiang, X.; Kuang, H.; Yang, B.; et al. Maternal exposure to polystyrene nanoplastics during gestation and lactation induces hepatic and testicular toxicity in male mouse offspring. Food Chem. Toxicol. 2022, 160, 112803. [Google Scholar] [CrossRef]
- Jeong, B.; Baek, J.Y.; Koo, J.; Park, S.; Ryu, Y.K.; Kim, K.S.; Zhang, S.; Chung, C.; Dogan, R.; Choi, H.S.; et al. Maternal exposure to polystyrene nanoplastics causes brain abnormalities in progeny. J. Hazard. Mater. 2022, 426, 127815. [Google Scholar] [CrossRef]
- Lu, K.; Lai, K.P.; Stoeger, T.; Ji, S.; Lin, Z.; Lin, X.; Chan, T.F.; Fang, J.K.-H.; Lo, M.; Gao, L.; et al. Detrimental effects of microplastic exposure on normal and asthmatic pulmonary physiology. J. Hazard. Mater. 2021, 416, 126069. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.H.; Shen, Y.; Roshdy, M.; Cheng, X.; Wang, G.; Yang, X. Polystyrene nanoplastics exposure caused defective neural tube morphogenesis through caveolae-mediated endocytosis and faulty apoptosis. Nanotoxicology 2021, 15, 885–904. [Google Scholar] [CrossRef]
- Zaheer, J.; Kim, H.; Ko, I.O.; Jo, E.K.; Choi, E.J.; Lee, H.J.; Shim, I.; Woo, H.J.; Choi, J.; Kim, G.H.; et al. Pre/post-natal exposure to microplastic as a potential risk factor for autism spectrum disorder. Environ. Int. 2022, 161, 107121. [Google Scholar] [CrossRef]
- Vizcaino, E.; Grimalt, J.O.; Glomstad, B.; Fernández-Somoano, A.; Tardón, A. Gestational weight gain and exposure of newborns to persistent organic pollutants. Environ. Health Perspect. 2014, 122, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.E.J.; Chan, S.Y.; Chakraborty, A.; Rajaraman, G.; Ricardo, S.; Benharouga, M.; Alfaidy, N.; Staud, F.; Murthi, P. Significance of the placental barrier in antenatal viral infections. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166244. [Google Scholar] [CrossRef]
- Concheiro, M.; Huestis, M.A. Drug exposure during pregnancy: Analytical methods and toxicological findings. Bioanalysis 2018, 10, 587–606. [Google Scholar] [CrossRef] [PubMed]
- Bearer, C.F. Meconium as a biological marker of prenatal exposure. Ambul. Pediatr. 2003, 3, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Bakdash, A.; Burger, P.; Goecke, T.W.; Fasching, P.A.; Reulbach, U.; Bleich, S.; Hastedt, M.; Rothe, M.; Beckmann, M.W.; Pragst, F.; et al. Quantification of fatty acid ethyl esters (FAEE) and ethyl glucuronide (EtG) in meconium from newborns for detection of alcohol abuse in a maternal health evaluation study. Anal. Bioanal. Chem. 2010, 396, 2469–2477. [Google Scholar] [CrossRef]
- Ostrea, E.M.; Bielawski, D.M.; Posecion, N.C. Meconium analysis to detect fetal exposure to neurotoxicants. Arch. Dis. Child. 2006, 91, 628–629. [Google Scholar] [CrossRef]
- Guillard, A.; Gaultier, E.; Cartier, C.; Devoille, L.; Noireaux, J.; Chevalier, L.; Morin, M.; Grandin, F.; Lacroix, M.Z.; Coméra, C.; et al. Basal Ti level in the human placenta and meconium and evidence of a materno-foetal transfer of food-grade TiO nanoparticles in an ex vivo placental perfusion model. Part. Fibre Toxicol. 2020, 17, 51. [Google Scholar] [CrossRef]
- Braun, T.; Ehrlich, L.; Henrich, W.; Koeppel, S.; Lomako, I.; Schwabl, P.; Liebmann, B. Detection of Microplastic in Human Placenta and Meconium in a Clinical Setting. Pharmaceutics 2021, 13, 921. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lin, G.; Liu, X.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. Detection of various microplastics in placentas, meconium, infant feces, breastmilk and infant formula: A pilot prospective study. Sci. Total Environ. 2022, 854, 158699. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, X.; Guo, J.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. The Association Between Microplastics and Microbiota in Placentas and Meconium: The First Evidence in Humans. Environ. Sci. Technol. 2022; online ahead of print. [Google Scholar]
- Kutralam-Muniasamy, G.; Shruti, V.C.; Pérez-Guevara, F.; Roy, P.D. Microplastic diagnostics in humans: “The 3Ps” Progress, problems, and prospects. Sci. Total Environ. 2023, 856, 159164. [Google Scholar] [CrossRef] [PubMed]
- Kuhlman, R.L. Letter to the editor, discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 167, 107400. [Google Scholar] [CrossRef]
- Yan, Z.; Zhao, H.; Zhao, Y.; Zhu, Q.; Qiao, R.; Ren, H.; Zhang, Y. An efficient method for extracting microplastics from feces of different species. J. Hazard. Mater. 2020, 384, 121489. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Zhao, S.; Ward, J.E.; Danley, M.; Mincer, T.J. Field-Based Evidence for Microplastic in Marine Aggregates and Mussels: Implications for Trophic Transfer. Environ. Sci. Technol. 2018, 52, 11038–11048. [Google Scholar] [CrossRef]
- Aung, T.; Batish, I.; Ovissipour, R. Prevalence of Microplastics in the Eastern Oyster Crassostrea virginica in the Chesapeake Bay: The Impact of Different Digestion Methods on Microplastic Properties. Toxics 2022, 10, 29. [Google Scholar] [CrossRef]
- Davidson, K.; Dudas, S.E. Microplastic Ingestion by Wild and Cultured Manila Clams (Venerupis philippinarum) from Baynes Sound, British Columbia. Arch. Environ. Contam. Toxicol. 2016, 71, 147–156. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Lam, T.W.L.; Fok, L.; Lin, L.; Xie, Q.; Li, H.X.; Xu, X.R.; Yeung, L.C. Spatial variation of floatable plastic debris and microplastics in the Pearl River Estuary, South China. Mar. Pollut. Bull. 2020, 158, 111383. [Google Scholar] [CrossRef]
- Yan, M.; Nie, H.; Xu, K.; He, Y.; Hu, Y.; Huang, Y.; Wang, J. Microplastic abundance, distribution and composition in the Pearl River along Guangzhou city and Pearl River estuary, China. Chemosphere 2019, 217, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef]
- Hu, J.; Qin, X.; Zhang, J.; Zhu, Y.; Zeng, W.; Lin, Y.; Liu, X. Polystyrene microplastics disturb maternal-fetal immune balance and cause reproductive toxicity in pregnant mice. Reprod. Toxicol. 2021, 106, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Paolinelli, F.; Avellini, C.; Salvolini, E.; Ciarmela, P.; Lorenzi, T.; Emanuelli, M.; Toti, P.; Giuliante, R.; Gesuita, R.; et al. IL-1β and TGF-β weaken the placental barrier through destruction of tight junctions: An in vivo and in vitro study. Placenta 2014, 35, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Beall, M.H.; van den Wijngaard, J.P.; van Gemert, M.J.; Ross, M.G. Amniotic fluid water dynamics. Placenta 2007, 28, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.A. Fetal swallowing and amniotic fluid volume. Obstet. Gynecol. 1966, 28, 606–610. [Google Scholar] [PubMed]
- Prata, J.C.; da Costa, J.P.; Girao, A.V.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Identifying a quick and efficient method of removing organic matter without damaging microplastic samples. Sci. Total Environ. 2019, 686, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.M.; Gerlach, M.J.; Adam, T.; Heimesaat, M.M.; Rossi, L.; Surette, M.G.; Sloboda, D.M.; Braun, T. Fetal meconium does not have a detectable microbiota before birth. Nat. Microbiol. 2021, 6, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.S.; Taylor, M.P.; Wilson, S.P. Quantification and exposure assessment of microplastics in Australian indoor house dust. Environ. Pollut. 2021, 283, 117064. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, T.; Zhu, L.; Xu, P.; Wang, X.; Gao, L.; Li, D. Analysis of suspended microplastics in the Changjiang Estuary: Implications for riverine plastic load to the ocean. Water Res. 2019, 161, 560–569. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J.; Zhang, H.; Shi, H.; Fei, Y.; Huang, S.; Tong, Y.; Wen, D.; Luo, Y.; Barceló, D. Microplastics in agricultural soils on the coastal plain of Hangzhou Bay, east China: Multiple sources other than plastic mulching film. J. Hazard. Mater. 2020, 388, 121814. [Google Scholar] [CrossRef] [PubMed]
- Perez-Guevara, F.; Kutralam-Muniasamy, G.; Shruti, V.C. Critical review on microplastics in fecal matter: Research progress, analytical methods and future outlook. Sci. Total Environ. 2021, 778, 146395. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Chan, K.L.A. ATR-FTIR spectroscopic imaging: Recent advances and applications to biological systems. Analyst 2013, 138, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Wick, P.; Malek, A.; Manser, P.; Meili, D.; Maeder-Althaus, X.; Diener, L.; Diener, P.A.; Zisch, A.; Krug, H.F.; von Mandach, U. Barrier capacity of human placenta for nanosized materials. Environ. Health Perspect. 2010, 118, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Fournier, S.B.; D’Errico, J.N.; Adler, D.S.; Kollontzi, S.; Goedken, M.J.; Fabris, L.; Yurkow, E.J.; Stapleton, P.A. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part. Fibre Toxicol. 2020, 17, 55. [Google Scholar] [CrossRef]
- Xia, T.; Lei, C.; Xu, C.; Peng, N.; Li, Y.; Yang, X.Y.; Cheng, Z.Z.; Gauthier, M.; Gu, H.Z.; Zou, T. Preparation and Antitumor Study of Two-Dimensional Muscovite Nanosheets. Langmuir 2020, 36, 14268–14275. [Google Scholar] [CrossRef]
- Qian, Y.; Si, J.M.; Wang, L.J.; Chen, S.J.; Zhu, Y.F. Mechanisms of muscovite on gastric mucosal protective effect. Zhongguo Zhong Yao Za Zhi 2004, 29, 781–785. [Google Scholar]
- Si, J.M.; Qian, Y.; Wu, J.G. The effect of muscovite on the quality of gastric ulcer healing. Zhongguo Zhong Yao Za Zhi 2005, 30, 1536–1541. [Google Scholar]
- Chen, H.Q.; Zou, S.H.; Yang, J.B.; Cai, J.; Zhang, Y.; Wang, Z.L. A survey and analysis of using traditional Chinese medicine during pregnancy. Int. J. Clin. Exp. Med. 2015, 8, 19496–19500. [Google Scholar]
- Amine, H.; Benomar, Y.; Taouis, M. Palmitic acid promotes resistin-induced insulin resistance and inflammation in SH-SY5Y human neuroblastoma. Sci. Rep. 2021, 11, 5427. [Google Scholar] [CrossRef] [PubMed]
- Bichler, A.; Daxenbichler, G.; Geir, W. Determination of amniotic fluid palmitic acid concentration for the estimation of fetal lung maturity. Clin. Chim. Acta 1977, 74, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Saliu, F.; Biale, G.; Raguso, C.; La Nasa, J.; Degano, I.; Seveso, D.; Galli, P.; Lasagni, M.; Modugno, F. Detection of plastic particles in marine sponges by a combined infrared micro-spectroscopy and pyrolysis-gas chromatography-mass spectrometry approach. Sci. Total Environ. 2022, 819, 152965. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Claveau-Mallet, D.; Hernandez, L.M.; Xu, E.G.; Farner, J.M.; Tufenkji, N. Separation and Analysis of Microplastics and Nanoplastics in Complex Environmental Samples. Acc. Chem. Res. 2019, 52, 858–866. [Google Scholar] [CrossRef] [PubMed]


| Chemical | Hydrogen Peroxide (H2O2) | Nitric Acid (HNO3) | Fenton’s Reagent + HNO3 | Petroleum Ether and Alcohol + HNO3 + H2O2 |
|---|---|---|---|---|
| Sample | Feces [7,30,31] Meconium [24] | Meconium [25] Oyster [32] and clam tissue [33] | Feces [29] | Meconium Present study |
| Method summary | 25 mL 30% H2O2 with 3 g human fecal samples for 20 days [7]. 30% H2O2 at 25 °C for two weeks [30]. 30% H2O2 and samples were mixed in a 1:1 v/v ratio, incubated in a sand bath (~75 °C) for 24 h and then at room temperature for 36 to 48 h [31]. H2O2 for 5 weeks to completely eliminate the organic matter [24]. | HNO3 is added to the samples, allowing to stand for 48 h, and then heat at 95 °C for at least 3 h [25]. HNO3 is added to the samples and incubate at 60 °C for 24 h [32]. Clam tissue digested in 40 mL of 69–71% HNO3 for 4 h in a hot water bath (~90 °C) [33]. | Phase 1: 140–700 mL Fenton’s reagent (H2O2: iron catalyst solution = 2.5:1) with human, chicken, and zebrafish feces, lasting less than 5 h below 40 °C. Phase 2: 65% HNO3 is added and incubated in 50 °C water bath for 30 min. | Phase 1: Petroleum ether and alcohol (4:1, v/v) remove lipids. Phase 2: HNO3 (5 mL/g meconium) is added and incubated in cold water bath overnight, followed by digestion for 4 h at 80 °C. Phase 3: 5 mL H2O2 is added for 30 min at 80 °C, followed by filtering the digestion solution. |
| Advantage | Easy to operate; extract MPs with no damage. | Efficient and feasible way for meconium digestion; time-saving. | Easy to operate; time-saving | Completely digest our meconium samples; easy to operate; time-saving. |
| Disadvantage | Could not completely digest our meconium samples; time-consuming | Could not completely digested our meconium samples; potentially damage MPs. | Could not completely digest our meconium samples. | Potentially damage MPs |
| Group | Added Targets/Item | Recovery Targets/Item | Recovery Rate (%) | |||
|---|---|---|---|---|---|---|
| 50 μm | 200 μm | 50 μm | 200 μm | 50 μm | 200 μm | |
| 1 | 293 | 87 | 258 | 65 | 88.05 | 74.71 |
| 2 | 255 | 63 | 181 | 31 | 70.98 | 49.21 |
| 3 | 726 | 34 | 690 | 26 | 95.04 | 76.47 |
| Average recovery rate (%) | - | - | - | - | 84.69 ± 12.38 | 66.80 ± 15.26 |
| Sample Description | Dry Weight/g | Results |
|---|---|---|
| Procedure blank control 1 | - | Negative |
| Procedure blank control 2 | - | Negative |
| Procedure blank control 3 | - | Negative |
| Meconium No. 1 | 1.8600 | Negative |
| Meconium No. 2 | 0.8013 | Negative |
| Meconium No. 3 | 2.1695 | Negative |
| Meconium No. 9 | 2.5295 | Negative |
| Meconium No. 10 | 2.8588 | Negative |
| Meconium No. 12 | 4.3440 | Negative |
| Meconium No. 14 | 3.0063 | Negative |
| Meconium No. 15 | 0.4038 | Negative |
| Meconium No. 16 | 0.9895 | Negative |
| Meconium No. 17 | 4.6835 | Negative |
| Meconium No. 18 | 1.4887 | Negative |
| Meconium No. 19 | 1.4463 | Negative |
| Meconium No. 29 | 2.1207 | Negative |
| Meconium No. 31 | 1.7712 | Negative |
| Meconium No. 32 | 0.7137 | Negative |
| Meconium No. 37 | 2.1840 | Negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, J.; Gao, X.; Du, J.; Sui, H.; Wu, J.; Zhong, Y.; Liang, B.; Huang, Y.; Ye, R.; et al. Investigation of Microplastics (≥10 μm) in Meconium by Fourier Transform Infrared Microspectroscopy. Toxics 2023, 11, 310. https://doi.org/10.3390/toxics11040310
Li Z, Wang J, Gao X, Du J, Sui H, Wu J, Zhong Y, Liang B, Huang Y, Ye R, et al. Investigation of Microplastics (≥10 μm) in Meconium by Fourier Transform Infrared Microspectroscopy. Toxics. 2023; 11(4):310. https://doi.org/10.3390/toxics11040310
Chicago/Turabian StyleLi, Zhiming, Jiamin Wang, Xia Gao, Jiaxin Du, Haixia Sui, Jieling Wu, Yizhou Zhong, Boxuan Liang, Yuji Huang, Rongyi Ye, and et al. 2023. "Investigation of Microplastics (≥10 μm) in Meconium by Fourier Transform Infrared Microspectroscopy" Toxics 11, no. 4: 310. https://doi.org/10.3390/toxics11040310
APA StyleLi, Z., Wang, J., Gao, X., Du, J., Sui, H., Wu, J., Zhong, Y., Liang, B., Huang, Y., Ye, R., Deng, Y., Yang, X., & Huang, Z. (2023). Investigation of Microplastics (≥10 μm) in Meconium by Fourier Transform Infrared Microspectroscopy. Toxics, 11(4), 310. https://doi.org/10.3390/toxics11040310








