Effects of Endocrine-Disrupting Heavy Metals on Human Health
Abstract
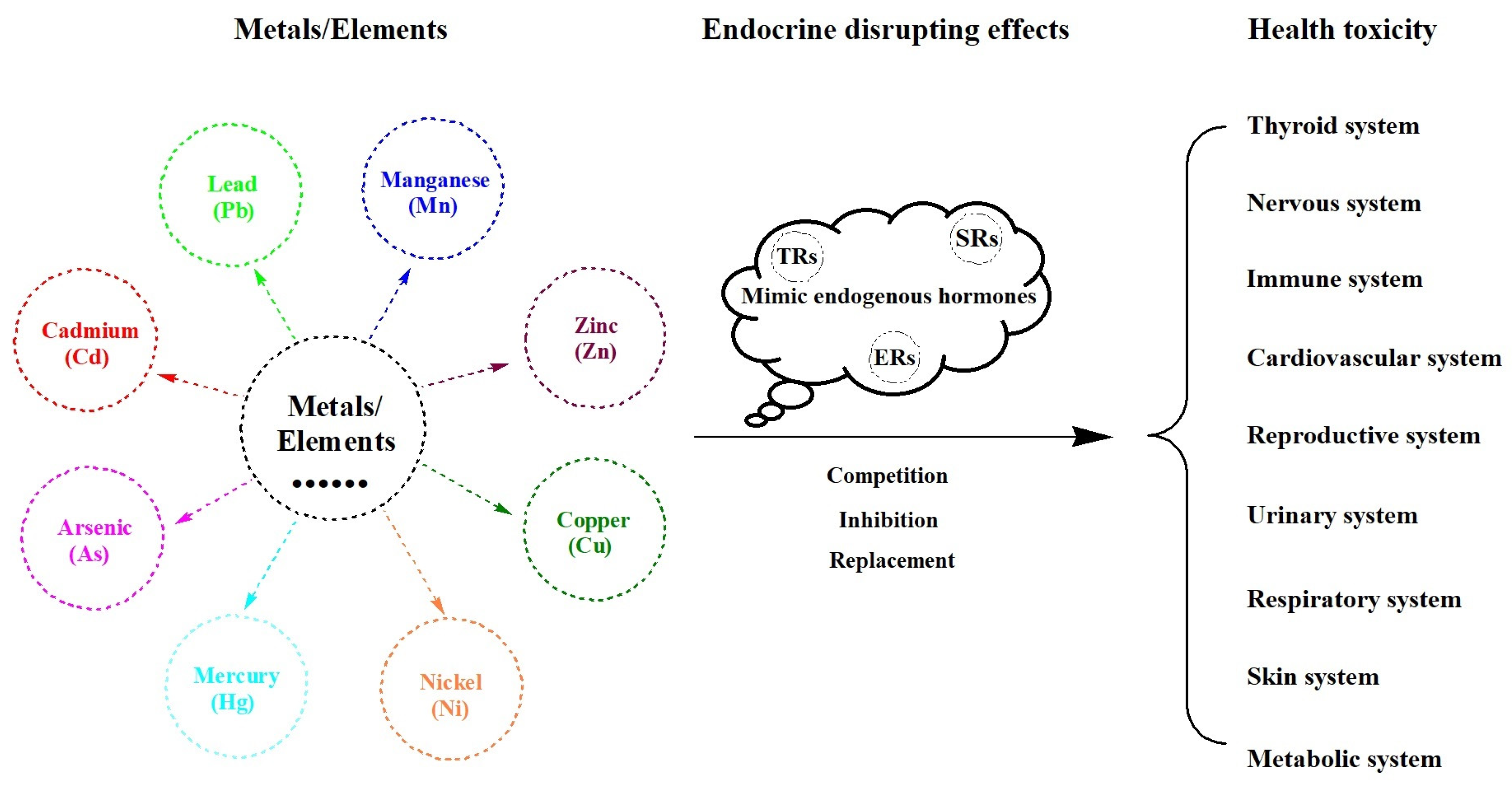
:1. Introduction
2. Health Effects of Heavy Metals
2.1. Lead (Pb)
2.2. Cadmium (Cd)
2.3. Arsenic (As)
2.4. Mercury (Hg)
2.5. Other Heavy Metals
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, X.; Huo, X.; Xu, X.; Liu, D.; Wu, W. E-waste lead exposure and children’s health in China. Sci. Total Environ. 2020, 734, 139286. [Google Scholar] [CrossRef] [PubMed]
- Bist, P.; Choudhary, S. Impact of Heavy Metal Toxicity on the Gut Microbiota and Its Relationship with Metabolites and Future Probiotics Strategy: A Review. Biol. Trace Elem. Res. 2022, 200, 5328–5350. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xu, X.; Boezen, H.M.; Huo, X. Children with health impairments by heavy metals in an e-waste recycling area. Chemosphere 2016, 148, 408–415. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. Int. 2016, 23, 8244–8259. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef]
- Bertram, M.G.; Gore, A.C.; Tyler, C.R.; Brodin, T. Endocrine-disrupting chemicals. Curr. Biol. 2022, 32, R727–R730. [Google Scholar] [CrossRef]
- Lisco, G.; Giagulli, V.A.; Iovino, M.; Guastamacchia, E.; Pergola, G.; Triggiani, V. Endocrine-Disrupting Chemicals: Introduction to the Theme. Endocr. Metab. Immune Disord Drug Targets 2022, 22, 677–685. [Google Scholar]
- Street, M.E.; Audouze, K.; Legler, J.; Sone, H.; Palanza, P. Endocrine Disrupting Chemicals: Current Understanding, New Testing Strategies and Future Research Needs. Int. J. Mol. Sci. 2021, 22, 933. [Google Scholar] [CrossRef]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef]
- Paschoalini, A.L.; Savassi, L.A.; Arantes, F.P.; Rizzo, E.; Bazzoli, N. Heavy metals accumulation and endocrine disruption in Prochilodus argenteus from a polluted neotropical river. Ecotoxicol. Environ. Saf. 2019, 169, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, L.; Zhao, Y.; Wang, Y. Changes in serum heavy metals in polycystic ovary syndrome and their association with endocrine, lipid-metabolism, inflammatory characteristics and pregnancy outcomes. Reprod. Toxicol. 2022, 111, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Guo, F.; Liu, K.; Ding, R.; Wang, Y. The effect of endocrine-disrupting chemicals on placental development. Front Endocrinol. 2023, 14, 1059854. [Google Scholar] [CrossRef] [PubMed]
- Macedo, S.; Teixeira, E.; Gaspar, T.B.; Boaventura, P.; Soares, M.A.; Miranda-Alves, L.; Soares, P. Endocrine-disrupting chemicals and endocrine neoplasia: A forty-year systematic review. Environ. Res. 2023, 218, 114869. [Google Scholar] [CrossRef] [PubMed]
- Midya, V.; Colicino, E.; Conti, D.V.; Berhane, K.; Garcia, E.; Stratakis, N.; Andrusaityte, S.; Basagana, X.; Casas, M.; Fossati, S.; et al. Association of Prenatal Exposure to Endocrine-Disrupting Chemicals With Liver Injury in Children. JAMA Netw. Open 2022, 5, e2220176. [Google Scholar] [CrossRef] [PubMed]
- Betts, K.S. CDC updates guidelines for children’s lead exposure. Environ. Health Perspect 2012, 120, a268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorvolakos, T.; Arseniou, S.; Samakouri, M. There is no safe threshold for lead exposure: A literature review. Psychiatriki 2016, 27, 204–214. [Google Scholar] [CrossRef] [Green Version]
- IARC of WHO. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Last Updated: 2022-09-07. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 17 March 2023).
- Doumouchtsis, K.K.; Doumouchtsis, S.K.; Doumouchtsis, E.K.; Perrea, D.N. The effect of lead intoxication on endocrine functions. J. Endocrinol. Investig. 2009, 32, 175–183. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, X.; Qin, Q.; Ye, K.; Wu, W.; Huo, X. Heavy metal exposure has adverse effects on the growth and development of preschool children. Environ. Geochem. Health 2019, 41, 309–321. [Google Scholar] [CrossRef]
- Zeng, X.; Zeng, Z.; Wang, Q.; Liang, W.; Guo, Y.; Huo, X. Alterations of the gut microbiota and metabolomics in children with e-waste lead exposure. J. Hazard. Mater. 2022, 434, 128842. [Google Scholar] [CrossRef]
- Kahn, L.G.; Liu, X.; Rajovic, B.; Popovac, D.; Oberfield, S.; Graziano, J.H.; Factor-Litvak, P. Blood lead concentration and thyroid function during pregnancy: Results from the Yugoslavia Prospective Study of Environmental Lead Exposure. Environ. Health Perspect 2014, 122, 1134–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javorac, D.; Baralic, K.; Maric, D.; Mandic-Rajcevic, S.; Dukic-Cosic, D.; Bulat, Z.; Djordjevic, A.B. Exploring the endocrine disrupting potential of lead through benchmark modelling—Study in humans. Environ. Pollut. 2023, 316, 120428. [Google Scholar] [CrossRef]
- Kresovich, J.K.; Argos, M.; Turyk, M.E. Associations of lead and cadmium with sex hormones in adult males. Environ. Res. 2015, 142, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, X.S.; Li, D.L.; Yu, Y.J. Effects of environmental lead pollution on blood lead and sex hormone levels among occupationally exposed group in an E-waste dismantling area. Biomed. Environ. Sci. 2013, 26, 474–484. [Google Scholar]
- Zhang, J.; Yang, Y.; Liu, W.; Liu, J. Potential endocrine-disrupting effects of metals via interference with glucocorticoid and mineralocorticoid receptors. Environ. Pollut. 2018, 242, 12–18. [Google Scholar] [CrossRef]
- Dundar, B.; Oktem, F.; Arslan, M.K.; Delibas, N.; Baykal, B.; Arslan, C.; Gultepe, M.; Ilhan, I.E. The effect of long-term low-dose lead exposure on thyroid function in adolescents. Environ. Res. 2006, 101, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, W.; Zhang, B.; Shen, X.; Hu, C.; Chen, X.; Jin, S.; Jiang, Y.; Liu, H.; Cao, Z.; et al. Maternal Heavy Metal Exposure, Thyroid Hormones, and Birth Outcomes: A Prospective Cohort Study. J. Clin. Endocrinol. Metab. 2019, 104, 5043–5052. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, B.; Lin, K.; Zhang, Y.; Xu, X.; Huo, X. Thyroid disruption and reduced mental development in children from an informal e-waste recycling area: A mediation analysis. Chemosphere 2018, 193, 498–505. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, C.; Xu, X.; Zhang, Y.; Huang, Y.; Huo, X. Elevated lead levels in relation to low serum Neuropeptide Y and adverse behavioral effects in preschool children with e-waste exposure. Chemosphere 2021, 269, 129380. [Google Scholar] [CrossRef]
- Axelrad, D.A.; Coffman, E.; Kirrane, E.F.; Klemick, H. The relationship between childhood blood lead levels below 5 microg/dL and childhood intelligence quotient (IQ): Protocol for a systematic review and meta-analysis. Environ. Int. 2022, 169, 107475. [Google Scholar] [CrossRef]
- Santa, M.M.; Hill, B.D.; Kline, J. Lead (Pb) neurotoxicology and cognition. Appl. Neuropsychol. Child. 2019, 8, 272–293. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Huo, X.; Zhang, S.; Xu, C.; Huang, Y.; Xu, X. Elevated levels of lead exposure and impact on the anti-inflammatory ability of oral sialic acids among preschool children in e-waste areas. Sci. Total Environ. 2020, 699, 134380. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Huo, X.; Zhang, Y.; Wang, Q.; Zhang, Y.; Xijin, X. Cardiovascular endothelial inflammation by chronic coexposure to lead (Pb) and polycyclic aromatic hydrocarbons from preschool children in an e-waste recycling area. Environ. Pollut. 2019, 246, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, X.; Lu, X.; Zeng, Z.; Faas, M.M.; Xu, X. Exposure to multiple heavy metals associate with aberrant immune homeostasis and inflammatory activation in preschool children. Chemosphere 2020, 257, 127257. [Google Scholar] [CrossRef]
- Lu, X.; Xu, X.; Zhang, Y.; Zhang, Y.; Wang, C.; Huo, X. Elevated inflammatory Lp-PLA2 and IL-6 link e-waste Pb toxicity to cardiovascular risk factors in preschool children. Environ. Pollut. 2018, 234, 601–609. [Google Scholar] [CrossRef]
- Nakhaee, S.; Rezayee, M.; Mansouri, B.; Hadianfar, A.; Zadeh, A.A.; Zardast, M.; Sefat, M.P.; Mehrpour, O. Comparison of Thyroid Function in Lead-Poisoned Patients and Healthy Individuals in Eastern Iran. Biol. Trace Elem. Res. 2022, 200, 3097–3102. [Google Scholar] [CrossRef]
- Fahim, Y.A.; Sharaf, N.E.; Hasani, I.W.; Ragab, E.A.; Abdelhakim, H.K. Assessment of Thyroid Function and Oxidative Stress State in Foundry Workers Exposed to Lead. J. Health Pollut. 2020, 10, 200903. [Google Scholar] [CrossRef]
- Tellez-Plaza, M.; Navas-Acien, A.; Menke, A.; Crainiceanu, C.M.; Pastor-Barriuso, R.; Guallar, E. Cadmium exposure and all-cause and cardiovascular mortality in the U.S. general population. Environ. Health Perspect 2012, 120, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Huff, J.; Lunn, R.M.; Waalkes, M.P.; Tomatis, L.; Infante, P.F. Cadmium-induced cancers in animals and in humans. Int. J. Occup. Environ. Health 2007, 13, 202–212. [Google Scholar] [CrossRef] [Green Version]
- IARC of WHO. Exposure to Cadmium: A Major Public Health Concern. Last Updated: 2019-05-01. Available online: https://www.who.int/publications/i/item/WHO-CED-PHE-EPE-19-4-3 (accessed on 17 March 2023).
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Liu, J.; Diwan, B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 2009, 238, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buha, A.; Matovic, V.; Antonijevic, B.; Bulat, Z.; Curcic, M.; Renieri, E.A.; Tsatsakis, A.M.; Schweitzer, A.; Wallace, D. Overview of Cadmium Thyroid Disrupting Effects and Mechanisms. Int. J. Mol. Sci. 2018, 19, 1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milki, A.; Wong, D.; Chan, C.; Sooklal, S.; Kapp, D.S.; Mann, A.K. Increased Urinary Cadmium Levels in Foreign-Born Asian Women-An NHANES Study of 9639 U.S. Participants. Int. J. Environ. Res. Public Health 2022, 19, 1501. [Google Scholar] [CrossRef] [PubMed]
- Urbano, T.; Filippini, T.; Wise, L.A.; Lasagni, D.; De Luca, T.; Sucato, S.; Polledri, E.; Malavolti, M.; Rigon, C.; Santachiara, A.; et al. Associations of urinary and dietary cadmium with urinary 8-oxo-7,8-dihydro-2’-deoxyguanosine and blood biochemical parameters. Environ. Res. 2022, 210, 112912. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, C.; Bian, Y.; Fu, F.; Zhu, B.; Zhao, X.; Zhang, M.; Zhou, C.; Yao, S.; Zhang, Z.; et al. Cadmium exposure promotes thyroid pyroptosis and endocrine dysfunction by inhibiting Nrf2/Keap1 signaling. Ecotoxicol. Environ. Saf. 2023, 249, 114376. [Google Scholar] [CrossRef]
- Kortenkamp, A. Are cadmium and other heavy metal compounds acting as endocrine disrupters? Met. Ions Life Sci. 2011, 8, 305–317. [Google Scholar]
- Nie, X.; Chen, Y.; Chen, Y.; Chen, C.; Han, B.; Li, Q.; Zhu, C.; Xia, F.; Zhai, H.; Wang, N.; et al. Lead and cadmium exposure, higher thyroid antibodies and thyroid dysfunction in Chinese women. Environ. Pollut. 2017, 230, 320–328. [Google Scholar] [CrossRef]
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Aposhian, H.V.; Graziano, J.H.; Thompson, C.; Suk, W.A. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ. Health Perspect 2013, 121, 295–302. [Google Scholar] [CrossRef]
- Sabir, S.; Akash, M.; Fiayyaz, F.; Saleem, U.; Mehmood, M.H.; Rehman, K. Role of cadmium and arsenic as endocrine disruptors in the metabolism of carbohydrates: Inserting the association into perspectives. Biomed. Pharmacother. 2019, 114, 108802. [Google Scholar] [CrossRef]
- Mawia, A.M.; Hui, S.; Zhou, L.; Li, H.; Tabassum, J.; Lai, C.; Wang, J.; Shao, G.; Wei, X.; Tang, S.; et al. Inorganic arsenic toxicity and alleviation strategies in rice. J. Hazard. Mater. 2021, 408, 124751. [Google Scholar] [CrossRef]
- Davey, J.C.; Bodwell, J.E.; Gosse, J.A.; Hamilton, J.W. Arsenic as an endocrine disruptor: Effects of arsenic on estrogen receptor-mediated gene expression in vivo and in cell culture. Toxicol. Sci. 2007, 98, 75–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munoz, A.; Chervona, Y.; Hall, M.; Kluz, T.; Gamble, M.V.; Costa, M. Sex-specific patterns and deregulation of endocrine pathways in the gene expression profiles of Bangladeshi adults exposed to arsenic contaminated drinking water. Toxicol. Appl. Pharmacol. 2015, 284, 330–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.J.; Xiang, P.; Luo, J.; Hong, H.; Lin, H.; Li, H.B.; Ma, L.Q. Mechanisms of arsenic disruption on gonadal, adrenal and thyroid endocrine systems in humans: A review. Environ. Int. 2016, 95, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Guo, H.; Xia, Y. Effects on endocrine system of female rats exposed to chronic arsenic. Wei Sheng Yan Jiu J. Hyg. Res. 2011, 40, 178–179. [Google Scholar]
- Zhang, C.; Tang, H.L. Effects of arsenic on reproductive and endocrine system of female rats. Wei Sheng Yan Jiu J. Hyg. Res. 2005, 34, 537–538. [Google Scholar]
- Meakin, C.J.; Szilagyi, J.T.; Avula, V.; Fry, R.C. Inorganic arsenic and its methylated metabolites as endocrine disruptors in the placenta: Mechanisms underpinning glucocorticoid receptor (GR) pathway perturbations. Toxicol. Appl. Pharmacol. 2020, 409, 115305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, L.; Fan, Q.; Wu, X.; Wang, F.; Wang, R.; Ma, Z.; Yang, J.; Lu, S.H. Arsenic trioxide re-sensitizes ERalpha-negative breast cancer cells to endocrine therapy by restoring ERalpha expression in vitro and in vivo. Oncol. Rep. 2011, 26, 621–628. [Google Scholar]
- Cegolon, L.; Petranich, E.; Pavoni, E.; Floreani, F.; Barago, N.; Papassissa, E.; Larese, F.F.; Covelli, S. Concentration of mercury in human hair and associated factors in residents of the Gulf of Trieste (North-Eastern Italy). Environ. Sci. Pollut. Res. Int. 2022, 30, 21425–21437. [Google Scholar] [CrossRef]
- He, T.; Mao, X.; Lin, H.; Hassan, M.M.; Zhu, S.; Lu, Q.; Qin, J.; Su, S. Methylmercury bioaccumulation in water flea Daphnia carinata by AIEgen. Ecotoxicol. Environ. Saf. 2022, 248, 114271. [Google Scholar] [CrossRef]
- Kendricks, D.R.; Boomhower, S.R.; Newland, M.C. Adolescence as a sensitive period for neurotoxicity: Lifespan developmental effects of methylmercury. Pharmacol. Biochem. Behav. 2022, 217, 173389. [Google Scholar] [CrossRef]
- Esparza, I.; Elliott, K.H.; Choy, E.S.; Braune, B.M.; Letcher, R.J.; Patterson, A.; Fernie, K.J. Mercury, legacy and emerging POPs, and endocrine-behavioural linkages: Implications of Arctic change in a diving seabird. Environ. Res. 2022, 212, 113190. [Google Scholar] [CrossRef]
- Cediel-Ulloa, A.; Yu, X.; Hinojosa, M.; Johansson, Y.; Forsby, A.; Broberg, K.; Ruegg, J. Methylmercury-induced DNA methylation-From epidemiological observations to experimental evidence. Front. Genet. 2022, 13, 993387. [Google Scholar] [CrossRef] [PubMed]
- Ke, T.; Tinkov, A.A.; Skalny, A.V.; Santamaria, A.; Rocha, J.; Bowman, A.B.; Chen, W.; Aschner, M. Epigenetics and Methylmercury-Induced Neurotoxicity, Evidence from Experimental Studies. Toxics 2023, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Usuki, F. Cellular Conditions Responsible for Methylmercury-Mediated Neurotoxicity. Int. J. Mol. Sci. 2022, 23, 7218. [Google Scholar] [CrossRef] [PubMed]
- Sarzo, B.; Ballester, F.; Soler-Blasco, R.; Lopez-Espinosa, M.J.; Lozano, M.; Iriarte, G.; Beneito, A.; Riutort-Mayol, G.; Murcia, M.; Llop, S. Pre and postnatal exposure to mercury and sexual development in 9-year-old children in Spain: The role of brain-derived neurotrophic factor. Environ. Res. 2022, 213, 113620. [Google Scholar] [CrossRef]
- Shi, Q.; Sun, N.; Kou, H.; Wang, H.; Zhao, H. Chronic effects of mercury on Bufo gargarizans larvae: Thyroid disruption, liver damage, oxidative stress and lipid metabolism disorder. Ecotoxicol. Environ. Saf. 2018, 164, 500–509. [Google Scholar] [CrossRef]
- Li, S.; Han, B.; Wu, P.; Yang, Q.; Wang, X.; Li, J.; Liao, Y.; Deng, N.; Jiang, H.; Zhang, Z. Effect of inorganic mercury exposure on reproductive system of male mice: Immunosuppression and fibrosis in testis. Environ. Toxicol. 2022, 37, 69–78. [Google Scholar] [CrossRef]
- Meyer, E.; Eagles-Smith, C.A.; Sparling, D.; Blumenshine, S. Mercury exposure associated with altered plasma thyroid hormones in the declining western pond turtle (Emys marmorata) from California mountain streams. Environ. Sci. Technol. 2014, 48, 2989–2996. [Google Scholar] [CrossRef]
- Iavicoli, I.; Fontana, L.; Bergamaschi, A. The effects of metals as endocrine disruptors. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 206–223. [Google Scholar] [CrossRef]
- Rana, S.V. Perspectives in endocrine toxicity of heavy metals—A review. Biol. Trace Elem. Res. 2014, 160, 1–14. [Google Scholar] [CrossRef]
- Rahman, A.; Kumarathasan, P.; Gomes, J. Infant and mother related outcomes from exposure to metals with endocrine disrupting properties during pregnancy. Sci. Total Environ. 2016, 569–570, 1022–1031. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, L.; Zhao, J.; Ren, M.; Li, Z.; Wang, J.; Wang, S.; Liu, Y.; An, H.; Li, Y.; et al. Associations between endocrine-disrupting heavy metals in maternal hair and gestational diabetes mellitus: A nested case-control study in China. Environ. Int. 2021, 157, 106770. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, B.; Georgescu, C.; Dărăban, S.; Bouaru, A.; Paşcalău, S. Heavy Metals Acting as Endocrine Disrupters. Sci. Pap. Anim. Sci. Biotechnol. 2011, 44, 89–93. [Google Scholar]
- Yu, M.; Zhang, J. Serum and Hair Nickel Levels and Breast Cancer: Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2017, 179, 32–37. [Google Scholar] [CrossRef]
- Cao, J.; Wang, G.; Wang, T.; Chen, J.; Wenjing, G.; Wu, P.; He, X.; Xie, L. Copper caused reproductive endocrine disruption in zebrafish (Danio rerio). Aquat. Toxicol. 2019, 211, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Correia, T.G.; Vieira, V.; de Moraes, N.A.; Zampieri, R.A.; Floeter-Winter, L.M.; Moreira, R.G. Endocrine disruption caused by the aquatic exposure to aluminum and manganese in Astyanax altiparanae (Teleostei: Characidae) females during the final ovarian maturation. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 249, 109132. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Shi, Q.; Liu, C.; Sun, Q.; Zeng, X. Effects of Endocrine-Disrupting Heavy Metals on Human Health. Toxics 2023, 11, 322. https://doi.org/10.3390/toxics11040322
Liu D, Shi Q, Liu C, Sun Q, Zeng X. Effects of Endocrine-Disrupting Heavy Metals on Human Health. Toxics. 2023; 11(4):322. https://doi.org/10.3390/toxics11040322
Chicago/Turabian StyleLiu, Dongling, Qianhan Shi, Cuiqing Liu, Qinghua Sun, and Xiang Zeng. 2023. "Effects of Endocrine-Disrupting Heavy Metals on Human Health" Toxics 11, no. 4: 322. https://doi.org/10.3390/toxics11040322
APA StyleLiu, D., Shi, Q., Liu, C., Sun, Q., & Zeng, X. (2023). Effects of Endocrine-Disrupting Heavy Metals on Human Health. Toxics, 11(4), 322. https://doi.org/10.3390/toxics11040322






