Sources, Fate, and Detection of Dust-Associated Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS): A Review
Abstract
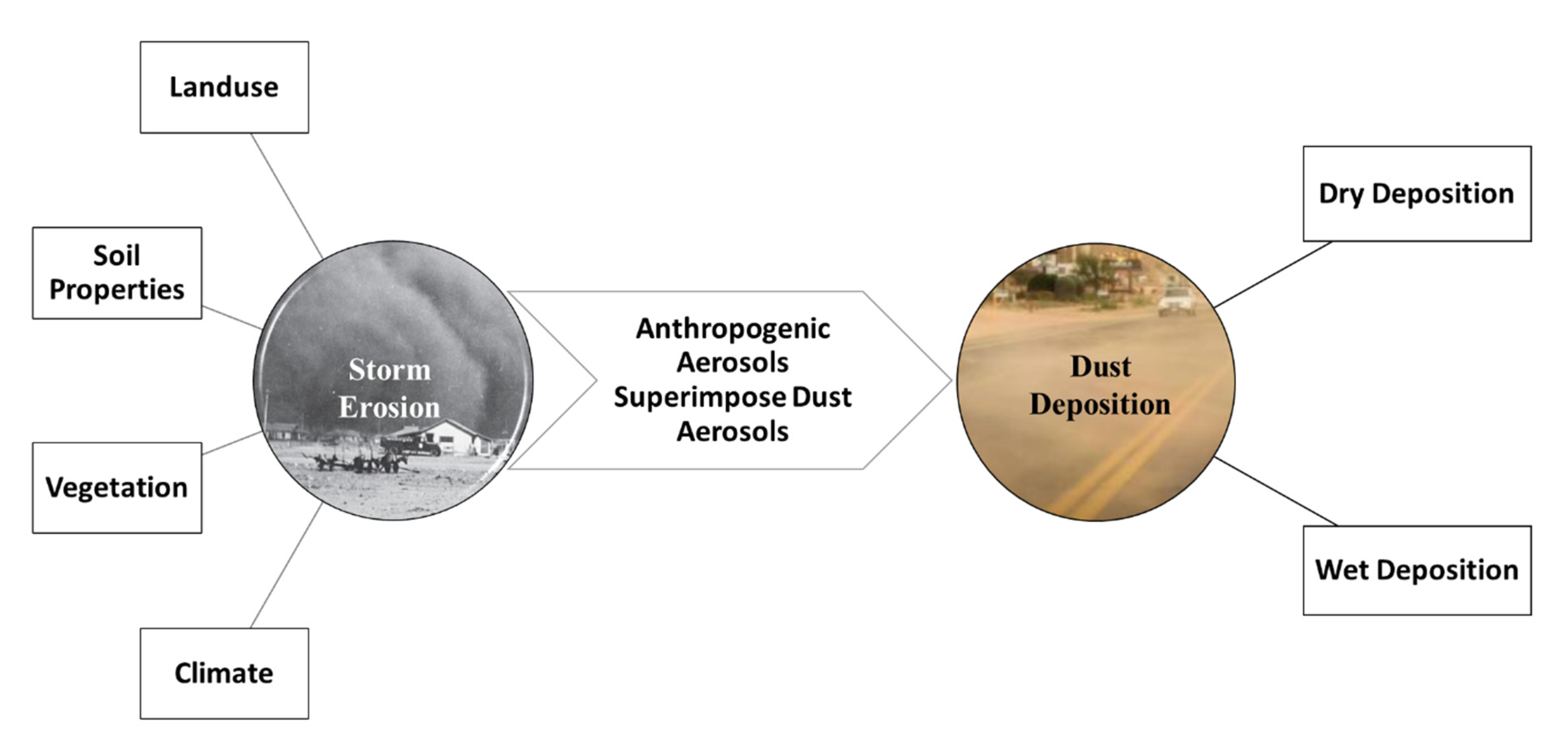
:1. Introduction
2. Per- and Poly-Fluoroalkyl Substances (PFAS) Properties
3. Sources, Exposure Route, and Toxicity of Dust-Associated PFAS
3.1. Sources of Dust-Associated PFAS
3.1.1. Firefighting Stations, Military Bases, and Aviation Sites
3.1.2. Fluorochemical Industry
3.1.3. Indoor Dust and Landfills
3.1.4. Wastewater Treatment Plants
3.1.5. Road Dust
3.2. PFAS’ Exposure Route
3.3. PFAS’ Toxicity
| Class | Species | Location | Tissue | N | Year | PFOS | PFOSA | PFOA | PFHxS | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Mammal | Human | Germany | Breast Milk | 57 | 2006 | 0.028–0.309 [0.119] | – | – | – | [80] |
| Gyor, Hungary | 13 | 1996/1997 | 0.096–0.639 [0.330] | – | – | – | ||||
| USA | Serum | 1432 | 1999–2000 | 27.1–33.9 (30.3) | – | 4.72–5.78 (5.22) | 1.92–2.41 (2.15) | [81] | ||
| 1432 | 1999–2000 | 27.1–33.9 (30.3) | – | 4.72–5.78 (5.22) | 1.42–1.98 (1.67) | |||||
| 2120 | 2005–2006 | 16.0–18.2 (17.1) | – | 3.48–4.42 (3.92) | 1.51–1.82 (1.66) | |||||
| 2233 | 2009–2010 | 8.13–10.7 (9.32) | – | 2.81–3.36 (3.07) | ||||||
| 1954 | 2013–2014 | 4.53–5.54 (5.01) | – | 1.76–2.15 (1.94) | 0.996–1.18 (1.08) | |||||
| 1929 | 2017–2018 | 3.90–4.62 (4.25) | – | 1.33–1.52 (1.42) | ||||||
| California, USA | Serum | 96 | 2016 | 6.51 | <LOD | 1.41 | 0.767 | [82] | ||
| 99 | 2017 | 7.47 | <LOD | 1.69 | 1.29 | |||||
| Melon-Headed Whale | Miyazaki, Japanese coast | Liver | 12 | 1982 | 0.0053–0.0089 (0.0071) | 6.0–12.3 (8.8) | – | – | [76,83,84] | |
| Ibaraki, Japanese coast | 21 | 2001/2002 | 17.8–117 (51.1) | 25.3–111 (65.8) | – | – | ||||
| Indo-Pacific Humpback Dolphin | South China | 10 | 2003–2007 | 26–693 (251) | 9.51–37.6 (18.7) | 0.243–8.32 (1.74) | – | |||
| Finless Porpoises | 10 | 2003–2008 | 51.3–262 (151) | 0.307–7.82 (1.70) | 0.23–0.859 (0.33) | – | ||||
| California Sea Lion | Coastal California | 6 | 2001 | <35–49 | – | – | – | |||
| Polar Bear | Alaska, USA | 17 | 180–680 (350) | – | – | – | ||||
| Bottlenose Dolphin | Mediterranean Sea | 5 | 170–430 (270) | – | – | – | ||||
| Mink | Midwestern USA | 18 | 970–3680 (2630) | – | – | – | ||||
| Ringed Seal | Norwegian Arctic | Plasma | 18 | 5–14 (9) | – | – | – | |||
| Bird | Sea Gull | Rishiri Island, Hokkaido | Liver | 14 | 1998 | 23–89 (53) | – | <19 | – | [76,85] |
| Common Cormorant | Sagami River, Kanagawa Prefecture | 8 | 1999 | 170–650 (385) | – | <19 | – | |||
| Black Tailed Gull | Korea | Liver | 15 | 2001 | 70–500 (170) | – | – | – | ||
| Double-Crested Cormorant | Lake Winnipeg, Canada | Egg yolk | 4 | 130–320 (210) | – | – | – | |||
| Common Cormorant | Italy | Liver | 12 | 33–470 (96) | – | – | – | |||
| Bald Eagle | Midwestern USA | Plasma | 26 | 1–2570 (360) | – | – | – | |||
| Fish | Brown Trout | Michigan waters, USA | Eggs | 3 | 2001 | 49–75 (64) | – | – | – | [76] |
| Blue-Fin Tuna | Mediterranean Sea | Liver | 8 | 21–87 (48) | – | – | – | |||
| Carp | Saginaw Bay, Michigan, USA | Muscle | 10 | 60–300 (120) | – | – | – | |||
| Reptile | Yellow-Blotched Map Turtle | Mississippi, USA | Liver | 6 | 2001 | 39–700 (190) | – | – | – | |
| Amphibian | Green Frogs | Lake St. Clair, Michigan, USA | Plasma | 5 | 2001 | 1–170 (72) | – | – | – | [76] |
4. Analytical Methods for the Detection and Quantification of PFAS
| Matrix | Location | Target Application | Detection Equipment | Detection Limit | Reference |
|---|---|---|---|---|---|
| Dust | |||||
| House and fire station dust | USA and Canada | Presence of PFAS in a fire station and house dust | HPLC-ESI-MS/MS and GC/EI-MS | MDL in ng/g dust for house and fire station dust: 6:2 diPAP = 0.48, 2.54; 8:2 diPAP = 10.63, 9.63; PFCAs = 0.06–15.80, 0.47–48.90; PFSAs = 0.20–22.28, 0.97–8.56; PFPA = 0.14, 1.2. | [38] |
| Fire station dust | Massachusetts, USA | Presence of PFAS in a fire station and house dust | PIGE spectroscopy for total fluorine; for targeted analysis, LC-MS/MS | MDL for total fluorine was 25 µg/g. PFAS MDLS ranged from 0.00242 to 18.1 ng/g | [94] |
| College dust | USA | PFAS in college dust | GC–ECNI/MS and GC–EI/MS or by LC–MS/MS | LOQ in ng/g: PFCAs = 20, PFSAs = 20 | [90] |
| House dust | Belgium, Italy and Spain | PFAS in house dust and human exposure to PFAS | HPLC-MS/MS | LOQ in ng/g: PFCAs = 0.02–0.27; PFSAs = 0.003–0.57. | [96] |
| Indoor dust from urban, industrial, and e-waste dismantling areas | Guangdong Province, China | Detection of PFAS in indoor dust of different indoor facilities | HPLC-MS/MS | LOQ of analytes ranged from 0.02–0.50 ng/mL (liquid extracts were used for detection limit quantification). | [97] |
| Street sweepings | Across the USA | Detection of PFAS in street sweepings | UHPLC-MS/MS | MDL, MQL in ng/g dust: PFCAs = 0.01–0.02, 0.01–0.69; PFSAs = 0.01–0.13, 0.03–0.42; PFPAs = 0.03–0.12, 0.10–0.41; HFPO-DA = 0.41, 1.37; FHEA = 0.08, 0.26; FOEA = 0.04, 0.13; FOUEA = 0.01, 0.05; FDUEA = 0.01, 0.03; N-6:6 PFPi = 0.01, 0.04; diSAmPAP = 0.04, 0.12; EtFHxSE (SYN 2) = 0.40, 1.33. | [64] |
| Dust from university buildings | USA | To confirm the implementation of “healthier material” manufactured without PFAS in buildings | HPLC-ESI-MS/MS | MQL, MDL in ng/g: PFCAs = 0.05–9.19, 0.02–2.76; PFSAs = 0.05–15.06, 0.02–4.52; NaDONA = 0.10, 0.03. | [98] |
| Soil and Groundwater | |||||
| Artificially contaminated soil | N/A | Soil remediation | LC-TQMS | IDL = 0.0001 mg/L(Soil slurry was analyzed) | [99] |
| Soil sample | Germany | The optimized fast and simple extraction method for PFAS | HR–CS–GFMAS | - | [100] |
| AFFF-impacted soil | USA | New methods for PFAS detection in soil | LC-QTOF-MS | - | [89] |
| Soil samples near industrial areas | Shifang City, China | Determination of PFAS in soil | LC-MS/MS Qtrap with negative ion ESI | LODs, LOQs in ng/g: PFCAs = 0.001–0.006, 0.004–0.018; PFSAs = 0.001–0.01, 0.004–0.034 | [101] |
| Surface soil | New Jersey, USA | Predicting the fate of new generation PFAS | HRUPLC-QtoF MS in –ve ESI for non-targeted analysis; LC-MS/MS for targeted analysis. | - | [92] |
| Soil and groundwater samples | Military bases in Pennsylvania and Michigan impacted by AFFF | Soil and groundwater remediation-electron beam technology | LC-MS/MS | Detection limits ranged from 0.3 ng/g dry weight to 1.2 ng/g dry weight. | [102] |
| Groundwater impacted by AFFF use | Willow Grove, Pennsylvania, USA | Treatment of contaminated groundwater | LC-MS/MS QTOF or QTRAP | LOD between 0.1 To 10 ng/L, MDL between 5000 to 10,000 ng/L | [103] |
| Landfill leachate | Queensland, Australia | Foam-fractionation (water treatment) | HPLC-MS/MS | PFCAs and PFSAs LOD = 0.02–0.05 µg/L, PFCAs and PFSAs LOQ = 0.08–0.17 µg/L, 6:2 FTS LOD = 0.03 µg/L, 6:2 FTS LOQ = 0.1 µg/L; PFECHS LOD = 0.03 µg/L, PFECHS LOQ 0.11 µg/L. | [86] |
| Synthetic soils including clay and sand | N/A | Treatment of contaminated soil pyrolysis and thermal air degradation | UPLC. Thermal desorption−pyrolysis (TD−Pyr) connected to a GC−MS to detect products of PFOA and PFOS | The limits of detection (S/N = 3) are 7 nmol/L for PFBA and PFPeA, 2 nmol/L for PFSAs, 5 nmol/L for other anionic PFAS, and 5 nmol/L for cationic and zwitterionic PFAS (Liquid extracts were used for detection limit quantification). | [104] |
| Soil samples near industrial areas, airports, landfills, and fire stations | Shanghai, China | Detection of PFAS contamination and distribution | LC-MS equipped with C18 column | MDL, MQL in µg/kg (dry weight): PFPrA = 0.05, 0.15; PFCAs = 0.02–0.1, 0.01–0.3; PFSAs = 0.003–0.03, 0.01–0.10; HFPO-DA = 0.003, 0.01. | [105] |
| Biosolid extract and clean extract | USA | New PFAS detection method | 19F-NMR spectroscopy | A detection limit of 50 nM (25 µg/L for PFOS) was achieved in groundwater samples | [95] |
| AFFF impacted groundwater | Colorado, US | Water treatment-NF and UV-sulfite treatment train | LC-QtoF-MS | LOQ of groundwater in ng/L: PFCAs = 0.7; PFSAs = 0.4–0.7; FhxSA = 0.4, 6:2 FTS = 0.7. | [106] |
| Water | |||||
| Water | N/A | Water treatment-electro oxidation | LC-MS/MS | LOD 1.0 ng/L, LOQ 3.0–4.0 ng/L | [107] |
| River water | Pretoria, South Africa | A new extraction method for PFAS in water | HPLC-DAD and UHPLC-MS/MS | LOD and LOQ for HPLC in ng/L = 0.3–0.66, 1.0–2.2; LOD and LOQ for UHPLC in ng/L = 0.011–0.04, 0.037–0.12 | [108] |
| Model and industrial wastewater | N/A | Water treatment-electrochemical oxidation | LC-MS/MS with negative ESI | MRL is 2 ng/L for all samples | [109] |
| Simulated water | N/A | Water treatment-UV photo-catalysis | HPLC-MS equipped with MicroToF MS | PFOA = 1 mg/L, fluoride = 0.1 mg/L, HPLC ToF = 0.01 mg/L | [110] |
| Ultrapure water and river water | Grand River water, Canada | Drinking water treatment-ion-exchange | GC/MS | MDL, LOQ for ultrapure water in ng/L = 11–23, 35–74; MDL, LOQ for river water in ng/L = 16–49, 52–157. | [111] |
| Surface water | Alabama, USA | Spatial distribution of PFAS in surface water | UHPLC-MS/MS equipped with ESI | LOD in ng/L: PFCAs = 0.21–0.72; PFSAs = 0.69–1.27; HFPO-DA = 0.55; ADONA = 0.48; PF4OpeA = 0.91; PF5OhxA = 0.75; 3,6-OPFHpA = 0.69 | [112] |
| Drinking water | China | Drinking water treatment, adsorption | HPLC-TQ MS | PFCAs LOD = 0.01–0.1 ng/L, PFCAs LOQ = 0.05–0.1 ng/L, PFSAs LOD = 0.02–0.05 ng/L, PFSAs LOQ = 0.05–0.12 ng/L | [87] |
| Drinking water, surface water, and wastewater | Thessaloniki WTP, Greece | PFAS analysis technique and workflow | Orbitrap Q ExactiveTM Focus equipped with HESI-II | MQL in ng/L. IDL and IQL in µg/L PFSAs = 0.0011–0.2063, 0.02–0.17, 0.02–0.56; PFCAs = 0.0024–0.2605, 0.02–0.22, 0.05–0.71. | [113] |
| Drinking water samples | Multiple locations in USA | New extraction method for PFAS in water | HPLC-MS/MS | LOD in ng/L: PFCAs = 0.08–0.30; PFSAs = 0.05–0.30. | [114] |
| Surface water | Netherlands | Determination of PFAS in surface waters and validation of new analytical methods | UHPLC-MS/MS connected to a Sciex Qtrap 5500 | I-LOD = 0.01–0.09 ng/mL; I-LOQ = 0.03–0.30 ng/mL; MDL = 0.02–0.75 ng/L; MQL = 0.07–2.55 ng/L. | [115] |
| Municipal wastewater samples | Northern New Jersey, USA | Rapid analytical method for PFAS quantification | HPLC-TQMS and Nano-ESI-HRMS | LODs in ng/L first for Nano-ESI HRMS and then for HPLC-TQMS: PFSAs = 4.2–25.1, 1.1–135.6; PFCAs = 3.2–36.2, 6.7–87.8; GenX = 6.4, 96.6 | [116] |
| Surface water | Poyang Lake, China | Distribution, partitioning behavior, and flux of PFAS | UPLC TQ in negative ESI | MQL, MDL in pg/L: PFCAs = 0.41–18, 0.81–39; PFSAs = 1.4–33, 2.0–35; 6:2 Cl-PFESA = 1.8, 15, 8:2 Cl-PFESA = 1.0, 3.6; OBS = 2.0, 92; PF4OpeA = 6.8, 3.1; PF5OhxA = 3.0, 2.7; NaDONA = 3.0, 4.6; PFEESA = 0.94, n.d; HFPO-DA 6.9, 7.0, HFPO-TA = 11, 71 | [117] |
| AFFF-impacted stormwater | USA | Stormwater treatment-photocatalysis | LC-QtoF-MS. ICS-90 for fluoride; UHPLC for the analyte | MDL in ng/L for column: PFCAs = 400–950; PFSAs = 100–250. | [118] |
| Surface and tap waters | Biscayne Bay, USA | Presence of PFAS in water | LC-MS/MS system equipped with AJS ESI source | MDL, IQL in ng/L: PFCAs = 0.01–1.99, 0.26–205; PFSAs = 0.01–0.45, 0.51–97.3; Adona = 0.02, 0.98; GenX = 0.02, 5.90; PFOUDS = 0.36, 66.00. | [119] |
| Wastewater treatment plant influent | South-East Queensland, Australia | The trend of PFAS in WWTP influent | HPLC-MS/MS using TurboIonSpray® probe | PFCAs = 0.27–8.7; PFSAs = 0.54–32.0; ADONA = 1.0, PFECHS = 0.47, 6:2FTAB = 5.4, 8-Cl-PFOS = 1.0, PFOPA = 1.8. | [120] |
| Drinking water source | Tianjin City, China | Detection of PFAS in lakes. | HPLC-MS/MS in negative ESI mode | LOD, LOQ in ng/L: PFCAs = 0.02–0.14, 0.05–0.47; PFSAs = 0.00–0.03, 0.01–0.11; 8:2Cl-PFESA = 0.00, 0.01; 6:2Cl-PFESA = 0.00, 0.01; PFECHS = 0.00, 0.01; HFPO-DA = 0.08, 0.28. | [121] |
| Spiked synthetic tap water | University of Notre Dame, USA | A new method for PFAS screening | PIGE spectroscopy | LOD in ppt F, LOD in ppt analyte, HFPO-DA = 48.8, 77.1; PFSAs = 31.5–64.7, 34.6–59.4; PFCAs = 31.6–40, 45.9–58.8. | [93] |
| Air | |||||
| Air samples and carpets | Southern Rhode Island, USA | To study the partitioning of volatile PFAS between air, dust, and carpet | GC-MSD operating in positive chemical ionization mode using selected ion monitoring | LOQ in (ng/uL): PFCAs = 0.01–0.07; PFSAs = 0.002–0.20; 8:2 FTAcr = 0.01, 10:2 FTAcr = 0.02. | [91] |
| Air matrix | 42 developing countries | Monitoring of air quality | LC–MS/MS with negative ESI mode | LOQ was the lowest point in the calibration curve since target compounds were not detected. | [122] |
| Fine airborne particulate matter (PM2.5) | Dublin and Enniscorthy, Ireland | Screening of atmospheric PM | On-line SPE LC-HRMS | LOD, LOQ in pg/mL: PFCAs = 0.17–0.51, 0.58–1.69; PFSAs = 0.08–0.17, 0.26–0.58. | [123] |
| Atmosphere | Japan | Detection of PFAS in the atmosphere | Combustion ion chromatography (CIC), HPIC, ILC-TQ MS with negative ESI and GCMS | IDL in pg, LOQ in pg, LOQ in pg/m3: PFSAs = 0.003–3.67, 1.77–500, 0.033–9.43; PFCAs = 0.001–5.88, 1.89–500, 0.036–18.9; TFA = 0.836,200, 3.77; FTIs = 0.039–4.72, 50–1000, 0.943–18.9; PFDeI = 1.20, 250, 4.72, PFDoI = 1.72, 250, 4.72 PFBuDil = 0.103, 50.0, 0.943, PFHxDil = 0.246, 50.0, 0.943, PFODil = 1.07, 250, 4.7; 6:2 Cl-PFESA = 0.005, 1.86, 0.035, HFPO-DA = 0.007, 2.00, 0.038 | [124] |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Middleton, N.J. REVIEW Dust Storms in the Middle East. J. Arid Environ. 1986, 10, 83–96. [Google Scholar] [CrossRef]
- Cao, H.; Amiraslani, F.; Liu, J.; Zhou, N. Identification of Dust Storm Source Areas in West Asia Using Multiple Environmental Datasets. Sci. Total. Environ. 2015, 502, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Dong, C.H. A Review on East Asian Dust Storm Climate, Modelling and Monitoring. Glob. Planet. Chang. 2006, 52, 1–22. [Google Scholar] [CrossRef]
- Al-Dousari, A.; Doronzo, D.; Ahmed, M. Types, Indications and Impact Evaluation of Sand and Dust Storms Trajectories in the Arabian Gulf. Sustainability 2017, 9, 1526. [Google Scholar] [CrossRef]
- Chang, C.-C.; Lee, I.-M.; Tsai, S.-S.; Yang, C.-Y. Correlation of Asian Dust Storm Events with Daily Clinic Visits for Allergic Rhinitis in Taipei, Taiwan. J. Toxicol. Environ. Health A 2006, 69, 229–235. [Google Scholar] [CrossRef]
- Goudie, A.S. Desert Dust and Human Health Disorders. Environ. Int. 2014, 63, 101–113. [Google Scholar] [CrossRef]
- APDIM. Sand and Dust Storms Risk Assessment in Asia and the Pacific. Tehran. 2021. Available online: https://apdim.unescap.org/knowledge-hub/sand-and-dust-storms-risk-assessment-asia-and-pacific (accessed on 4 March 2023).
- Prospero, J.M.; Mayol-Bracero, O.L. Understanding the Transport and Impact of African Dust on the Caribbean Basin. Bull. Am. Meteorol. Soc. 2013, 94, 1329–1337. [Google Scholar] [CrossRef]
- Abbasi, S.; Rezaei, M.; Ahmadi, F.; Turner, A. Atmospheric Transport of Microplastics during a Dust Storm. Chemosphere 2022, 292, 133456. [Google Scholar] [CrossRef]
- Holmes, C.W.; Miller, R. Atmospherically Transported Elements and Deposition in the Southeastern United States: Local or Transoceanic? Appl. Geochem. 2004, 19, 1189–1200. [Google Scholar] [CrossRef]
- Alharbi, B.H.; Pasha, M.J.; Alotaibi, M.D.; Alduwais, A.K.; Al-Shamsi, M.A.S. Contamination and Risk Levels of Metals Associated with Urban Street Dust in Riyadh, Saudi Arabia. Environ. Sci. Pollut. Res. 2020, 27, 18475–18487. [Google Scholar] [CrossRef]
- Alghamdi, A.G.; EL-Saeid, M.H.; Alzahrani, A.J.; Ibrahim, H.M. Heavy Metal Pollution and Associated Health Risk Assessment of Urban Dust in Riyadh, Saudi Arabia. PLoS ONE 2022, 17, e0261957. [Google Scholar] [CrossRef]
- Khoder, M.I.; al Ghamdi, M.A.; Shiboob, M.H. Heavy Metal Distribution in Street Dust of Urban and Industrial Areas in Jeddah, Saudi Arabia. JKAU Met. Env. Arid Land Agric. Sci. 2012, 23, 55–75. [Google Scholar] [CrossRef]
- Idris, A.M.; Said, T.O.; Brima, E.I.; Sahlabji, T.; Alghamdi, M.M.; El-Zahhar, A.A.; Arshad, M.; el Nemr, A.M. Assessment of Contents of Selected Heavy Metals in Street Dust from Khamees-Mushait City, Saudi Arabia, Using Multivariate Statistical Analysis, GIS Mapping, Geochemical Indices and Health Risk. Fresenius Environ. Bull. 2019, 28, 6059–6069. [Google Scholar]
- Suleman, N.M.; Mohammad, I.A.; Almesned, S.S.; Aljaghwani, A.A. Measurements of Some Trace Elements in Street Dust in Zilfi Province at Saudi Arabia Using Inductively Coupled Plasma-Mass Spectrometer. World Environ. 2012, 2, 135–139. [Google Scholar] [CrossRef]
- Suleman, N.M.; Mohammad, I.A.; Almesned, S.S.; Aljaghwani, A.A. Some Trace Elements in Zilfi Streets Dust. Am. J. Chem. 2013, 2013, 10–13. [Google Scholar] [CrossRef]
- Ahmed, K.O.; Ai-Swaidan, H.M. Lead and Cadmium in Urban Dust of Riyadh, Saudi Arabia. Sci. Total. Environ. 1993, 136, 205–210. [Google Scholar]
- Ahmed, K.O.; Ai-Swaidan, H.M.; Davies, B.E. Simultaneous Elemental Analysis in Dust of the City of Riyadh, Saudi Arabia by Inductively Coupled Plasma Mass Spectrometry (ICP/MS). Sci. Total Environ. 1993, 138, 207–212. [Google Scholar] [CrossRef]
- Modaihsh, A.S.; Mahjou, M.O. Falling Dust Characteristics in Riyadh City, Saudi Arabia During Winter Months. APCBEE Procedia 2013, 5, 50–58. [Google Scholar] [CrossRef]
- Borthakur, A.; Leonard, J.; Koutnik, V.S.; Ravi, S.; Mohanty, S.K. Inhalation Risks of Wind-Blown Dust from Biosolid-Applied Agricultural Lands: Are They Enriched with Microplastics and PFAS? Curr. Opin. Environ. Sci. Health 2022, 25, 100309. [Google Scholar]
- Wang, Z.; Dewitt, J.C.; Higgins, C.P.; Cousins, I.T. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ. Sci. Technol. 2017, 51, 2508–2518. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for Perfluoroalkyls. 2021. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf (accessed on 4 March 2023).
- Kim, M.; Li, L.Y.; Grace, J.R.; Yue, C. Selecting Reliable Physicochemical Properties of Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) Based on Molecular Descriptors. Environ. Pollut. 2015, 196, 462–472. [Google Scholar] [PubMed]
- Zhang, M.; Yamada, K.; Bourguet, S.; Guelfo, J.; Suuberg, E.M. Vapor Pressure of Nine Perfluoroalkyl Substances (PFASs) Determined Using the Knudsen Effusion Method. J. Chem. Eng. Data 2020, 65, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Krusic, P.J.; Marchione, A.A.; Davidson, F.; Kaiser, M.A.; Kao, C.P.C.; Richardson, R.E.; Botelho, M.; Waterland, R.L.; Buck, R.C. Vapor Pressure and Intramolecular Hydrogen Bonding in Fluorotelomer Alcohols. J. Phys. Chem. A 2005, 109, 6232–6241. [Google Scholar] [CrossRef] [PubMed]
- USEPA CompTox Chemicals Dashboard|US EPA. Available online: https://www.epa.gov/chemical-research/comptox-chemicals-dashboard (accessed on 9 February 2023).
- Wang, Z.; MacLeod, M.; Cousins, I.T.; Scheringer, M.; Hungerbühler, K. Using COSMOtherm to Predict Physicochemical Properties of Poly- and Perfluorinated Alkyl Substances (PFASs). Environ. Chem. 2011, 8, 389–398. [Google Scholar] [CrossRef]
- Borg, D.; Håkansson, H.; Institutet, K. Environmental and Health Risk Assessment of Perfluoroalkylated and Polyfluoroalkylated Substances (PFASs) in Sweden. 2012. Available online: https://www.diva-portal.org/smash/get/diva2:770762/FULLTEXT01.pdf (accessed on 4 March 2023).
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P.J. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr. Environ. Assess Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Bergström, S. Transport of Per- and Polyfluoroalkyl Substances in Soil and Groundwater in Uppsala, Sweden. 2014. Available online: https://stud.epsilon.slu.se/7392/18/bergstrom_s_141008.pdf (accessed on 4 March 2023).
- Shoeib, T.; Hassan, Y.; Rauert, C.; Harner, T. Poly- and Perfluoroalkyl Substances (PFASs) in Indoor Dust and Food Packaging Materials in Egypt: Trends in Developed and Developing Countries. Chemosphere 2016, 144, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- D’Eon, J.C.; Hurley, M.D.; Wallington, T.J.; Mabury, S.A. Atmospheric Chemistry of N-Methyl Perfluorobutane Sulfonamidoethanol, C4F9SO2N(CH3)CH2CH 2OH: Kinetics and Mechanism of Reaction with OH. Environ. Sci. Technol. 2006, 40, 1862–1868. [Google Scholar] [CrossRef]
- Kitahara, K.I.; Nakata, H. Plastic Additives as Tracers of Microplastic Sources in Japanese Road Dusts. Sci. Total Environ. 2020, 736, 139694. [Google Scholar] [CrossRef] [PubMed]
- Monira, S.; Bhuiyan, M.A.; Haque, N.; Shah, K.; Roychand, R.; Hai, F.I.; Pramanik, B.K. Understanding the Fate and Control of Road Dust-Associated Microplastics in Stormwater. Process. Saf. Environ. Prot. 2021, 152, 47–57. [Google Scholar]
- O’Brien, S.; Okoffo, E.D.; Rauert, C.; O’Brien, J.W.; Ribeiro, F.; Burrows, S.D.; Toapanta, T.; Wang, X.; Thomas, K.V. Quantification of Selected Microplastics in Australian Urban Road Dust. J. Hazard. Mater. 2021, 416, 125811. [Google Scholar] [CrossRef]
- Salvatore, D.; Mok, K.; Garrett, K.K.; Poudrier, G.; Brown, P.; Birnbaum, L.S.; Goldenman, G.; Miller, M.F.; Patton, S.; Poehlein, M.; et al. Presumptive Contamination: A New Approach to PFAS Contamination Based on Likely Sources. Environ. Sci. Technol. Lett. 2022, 9, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Rotander, A.; Toms, L.M.L.; Aylward, L.; Kay, M.; Mueller, J.F. Elevated Levels of PFOS and PFHxS in Firefighters Exposed to Aqueous Film Forming Foam (AFFF). Environ. Int. 2015, 82, 28–34. [Google Scholar] [CrossRef]
- Hall, S.M.; Patton, S.; Petreas, M.; Zhang, S.; Phillips, A.L.; Hoffman, K.; Stapleton, H.M. Per- And Polyfluoroalkyl Substances in Dust Collected from Residential Homes and Fire Stations in North America. Environ. Sci. Technol. 2020, 54, 14558–14567. [Google Scholar] [CrossRef] [PubMed]
- Mark Hewitt, L.; Roy, J.W.; Rowland, S.J.; Bickerton, G.; DeSilva, A.V.; Headley, J.B.; Milestone, C.G.; Scarlett, A.; Brown, S.; Spencer, C.; et al. Advances in Distinguishing Groundwater Influenced by Oil Sands Process-Affected Water (OSPW) from Natural Bitumen-Influenced Groundwaters. Environ. Sci. Technol. 2020, 54, 1522–1532. [Google Scholar] [CrossRef]
- Khan, M.F.; Elnakar, H. Treatment of Oil Sands’ Mature Fine Tailings Using Advanced Wet Air Oxidation (WAO) and Wet Air Peroxide Oxidation (WAPO). Catalysts 2022, 12, 1518. [Google Scholar] [CrossRef]
- Anderson, R.H.; Long, G.C.; Porter, R.C.; Anderson, J.K. Occurrence of Select Perfluoroalkyl Substances at U.S. Air Force Aqueous Film-Forming Foam Release Sites Other than Fire-Training Areas: Field-Validation of Critical Fate and Transport Properties. Chemosphere 2016, 150, 678–685. [Google Scholar] [CrossRef]
- de Silva, A.O.; Armitage, J.M.; Bruton, T.A.; Dassuncao, C.; Heiger-Bernays, W.; Hu, X.C.; Kärrman, A.; Kelly, B.; Ng, C.; Robuck, A.; et al. PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding. Environ. Toxicol. Chem. 2021, 40, 631–657. [Google Scholar] [PubMed]
- Hu, X.C.; Andrews, D.Q.; Lindstrom, A.B.; Bruton, T.A.; Schaider, L.A.; Grandjean, P.; Lohmann, R.; Carignan, C.C.; Blum, A.; Balan, S.A.; et al. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. [Google Scholar] [CrossRef]
- Prevedouros, K.; Cousins, I.T.; Buck, R.C.; Korzeniowski, S.H. Sources, Fate and Transport of Perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar]
- Zabaleta, I.; Bizkarguenaga, E.; Bilbao, D.; Etxebarria, N.; Prieto, A.; Zuloaga, O. Fast and Simple Determination of Perfluorinated Compounds and Their Potential Precursors in Different Packaging Materials. Talanta 2016, 152, 353–363. [Google Scholar] [CrossRef]
- Herzke, D.; Olsson, E.; Posner, S. Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) in Consumer Products in Norway—A Pilot Study. Chemosphere 2012, 88, 980–987. [Google Scholar] [CrossRef]
- Yuan, G.; Peng, H.; Huang, C.; Hu, J. Ubiquitous Occurrence of Fluorotelomer Alcohols in Eco-Friendly Paper-Made Food-Contact Materials and Their Implication for Human Exposure. Environ. Sci. Technol. 2016, 50, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar]
- Savvaides, T.; Koelmel, J.P.; Zhou, Y.; Lin, E.Z.; Stelben, P.; Aristizabal-Henao, J.J.; Bowden, J.A.; Godri Pollitt, K.J. Prevalence and Implications of Per- and Polyfluoroalkyl Substances (PFAS) in Settled Dust. Curr. Environ. Health Rep. 2021, 8, 323–335. [Google Scholar]
- Chen, J.; Tang, L.; Tang, L.; Chen, W.Q.; Chen, W.Q.; Peaslee, G.F.; Jiang, D. Flows, Stock, and Emissions of Poly-and Perfluoroalkyl Substances in California Carpet in 2000–2030 under Different Scenarios. Environ. Sci. Technol. 2020, 54, 6908–6918. [Google Scholar] [CrossRef] [PubMed]
- Elnakar, H. Disinfection and Antimicrobial Processes. Water Environ. Res. 2020, 92, 1625–1628. [Google Scholar] [CrossRef]
- Elnakar, H.; Buchanan, I. Treatment of Bypass Wastewater Using Novel Integrated Potassium Ferrate(VI) and Iron Electrocoagulation System. J. Environ. Eng. 2020, 146, 04020075. [Google Scholar] [CrossRef]
- Elnakar, H.; Buchanan, I. Treatment of Bypass Wastewater Using Potassium Ferrate(VI): Assessing the Role of Mixing. Environ. Technol. 2019, 41, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Elnakar, H.; Buchanan, I. Soluble Chemical Oxygen Demand Removal from Bypass Wastewater Using Iron Electrocoagulation. Sci. Total. Environ. 2020, 706, 136076. [Google Scholar] [CrossRef] [PubMed]
- Shigei, M.; Ahren, L.; Hazaymeh, A.; Dalahmeh, S.S. Per- and Polyfluoroalkyl Substances in Water and Soil in Wastewater-Irrigated Farmland in Jordan. Sci. Total. Environ. 2020, 716, 137057. [Google Scholar] [CrossRef] [PubMed]
- Dalahmeh, S.; Tirgani, S.; Komakech, A.J.; Niwagaba, C.B.; Ahrens, L. Per- and Polyfluoroalkyl Substances (PFASs) in Water, Soil and Plants in Wetlands and Agricultural Areas in Kampala, Uganda. Sci. Total. Environ. 2018, 631–632, 660–667. [Google Scholar] [CrossRef]
- Armstrong, D.L.; Lozano, N.; Rice, C.P.; Ramirez, M.; Torrents, A. Temporal Trends of Perfluoroalkyl Substances in Limed Biosolids from a Large Municipal Water Resource Recovery Facility. J Environ Manag. 2016, 165, 88–95. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Egami, R.T.; Frazier, C.A.; Lu, Z.; Goodrich, A.; Bird, A. Evaluation of Regenerative-Air Vacuum Street Sweeping on Geological Contributions to Pm10. J. Air Waste Manag. Assoc. 1990, 40, 1134–1142. [Google Scholar] [CrossRef]
- Sabin, L.D.; Hee Lim, J.; Teresa Venezia, M.; Winer, A.M.; Schiff, K.C.; Stolzenbach, K.D. Dry Deposition and Resuspension of Particle-Associated Metals near a Freeway in Los Angeles. Atmos. Environ. 2006, 40, 7528–7538. [Google Scholar] [CrossRef]
- Azah, E.; Kim, H.; Townsend, T. Assessment of Direct Exposure and Leaching Risk from PAHs in Roadway and Stormwater System Residuals. Sci. Total. Environ. 2017, 609, 58–67. [Google Scholar] [CrossRef]
- Chrysikou, L.P.; Gemenetzis, P.G.; Samara, C.A. Wintertime Size Distribution of Polycyclic Aromatic Hydrocarbons (PAHs), Polychlorinated Biphenyls (PCBs) and Organochlorine Pesticides (OCPs) in the Urban Environment: Street- vs. Rooftop-Level Measurements. Atmos Environ 2009, 43, 290–300. [Google Scholar] [CrossRef]
- Shabbaj, I.I.; Alghamdi, M.A.; Shamy, M.; Hassan, S.K.; Alsharif, M.M.; Khoder, M.I. Risk Assessment and Implication of Human Exposure to Road Dust Heavy Metals in Jeddah, Saudi Arabia. Int. J. Environ. Res. Public Health 2017, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Harb, M.K.; Ebqa’ai, M.; Al-rashidi, A.; Alaziqi, B.H.; Al Rashdi, M.S.; Ibrahim, B. Investigation of Selected Heavy Metals in Street and House Dust from Al-Qunfudah, Kingdom of Saudi Arabia. Environ. Earth Sci. 2015, 74, 1755–1763. [Google Scholar] [CrossRef]
- Ahmadireskety, A.; da Silva, B.F.; Robey, N.M.; Douglas, T.E.; Aufmuth, J.; Solo-Gabriele, H.M.; Yost, R.A.; Townsend, T.G.; Bowden, J.A. Per- And Polyfluoroalkyl Substances (PFAS) in Street Sweepings. Environ. Sci. Technol. 2021, 56, 6069–6077. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Roychand, R.; Monira, S.; Bhuiyan, M.; Jegatheesan, V. Fate of Road-Dust Associated Microplastics and per- and Polyfluorinated Substances in Stormwater. Process. Saf. Environ. Prot. 2020, 144, 236–241. [Google Scholar] [CrossRef]
- Murakami, M.; Takada, H. Perfluorinated Surfactants (PFSs) in Size-Fractionated Street Dust in Tokyo. Chemosphere 2008, 73, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- McGoldrick, D.J.; Murphy, E.W. Concentration and Distribution of Contaminants in Lake Trout and Walleye from the Laurentian Great Lakes (2008–2012). Environ. Pollut. 2016, 217, 85–96. [Google Scholar] [CrossRef]
- Franko, J.; Meade, B.J.; Frasch, H.F.; Barbero, A.M.; Anderson, S.E. Dermal Penetration Potential of Perfluorooctanoic Acid (PFOA) in Human and Mouse Skin. J. Toxicol. Environ. Health A 2012, 75, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Ghisi, R.; Vamerali, T.; Manzetti, S. Accumulation of Perfluorinated Alkyl Substances (PFAS) in Agricultural Plants: A Review. Environ. Res. 2019, 169, 326–341. [Google Scholar] [CrossRef]
- Trudel, D.; Horowitz, L.; Wormuth, M.; Scheringer, M.; Cousins, I.T.; Hungerbühler, K. Estimating Consumer Exposure to PFOS and PFOA. Risk Anal. 2008, 28, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Tittlemier, S.A.; Völkel, W.; Wilhelm, M.; Twardella, D. Perfluorinated Compounds—Exposure Assessment for the General Population in Western Countries. Int. J. Hyg. Environ. Health 2009, 212, 239–270. [Google Scholar] [CrossRef]
- Yao, Y.; Sun, H.; Gan, Z.; Hu, H.; Zhao, Y.; Chang, S.; Zhou, Q. Nationwide Distribution of Per- and Polyfluoroalkyl Substances in Outdoor Dust in Mainland China from Eastern to Western Areas. Environ. Sci. Technol. 2016, 50, 3676–3685. [Google Scholar] [CrossRef]
- Lewis, R.C.; Johns, L.E.; Meeker, J.D. Serum Biomarkers of Exposure to Perfluoroalkyl Substances in Relation to Serum Testosterone and Measures of Thyroid Function among Adults and Adolescents from NHANES 2011–2012. Int. J. Environ. Res. Public Health 2015, 12, 6098–6114. [Google Scholar] [CrossRef]
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals. 2015. Available online: https://www.cdc.gov/biomonitoring/pdf/fourthreport_updatedtables_feb2015.pdf (accessed on 4 March 2023).
- Calafat, A.M.; Wong, L.Y.; Kuklenyik, Z.; Reidy, J.A.; Needham, L.L. Polyfluoroalkyl Chemicals in the U.S. Population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and Comparisons with NHANES 1999–2000. Environ. Health Perspect. 2007, 115, 1596–1602. [Google Scholar] [CrossRef]
- Giesy, J.P.; Kannan, K. Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. [Google Scholar] [CrossRef]
- USEPA EPA’s Per- and Polyfluoroalkyl Substances (PFAS) Action Plan. 2019. Available online: https://www.epa.gov/sites/default/files/2019-02/documents/pfas_action_plan_021319_508compliant_1.pdf (accessed on 4 March 2023).
- USEPA Drinking Water Health Advisory for Perfluorooctane Sulfonate (PFOS). 2016. Available online: https://www.epa.gov/sites/default/files/2016-05/documents/pfos_health_advisory_final-plain.pdf (accessed on 4 March 2023).
- USEPA Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA). 2016. Available online: https://www.epa.gov/sites/default/files/2016-05/documents/pfoa_health_advisory_final-plain.pdf (accessed on 4 March 2023).
- Völkel, W.; Genzel-Boroviczény, O.; Demmelmair, H.; Gebauer, C.; Koletzko, B.; Twardella, D.; Raab, U.; Fromme, H. Perfluorooctane Sulphonate (PFOS) and Perfluorooctanoic Acid (PFOA) in Human Breast Milk: Results of a Pilot Study. Int. J. Hyg. Environ. Health 2008, 211, 440–446. [Google Scholar] [CrossRef]
- ATSDR National Report on Human Exposure to Environmental Chemicals Perfluoroalkyl and Polyfluoroalkyl Substances: Surfactants. 2017. Available online: https://www.cdc.gov/biomonitoring/pdf/fourthreport_updatedtables_volume1_jan2017.pdf (accessed on 4 March 2023).
- Biomonitoring California Results for Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs)|Measuring Chemicals in Californians. Available online: https://biomonitoring.ca.gov/results/chemical/2183 (accessed on 21 March 2023).
- Hart, K.; Kannan, K.; Isobe, T.; Takahashi, S.; Yamada, T.K.; Miyazaki, N.; Tanabe, S. Time Trends and Transplacental Transfer of Perfluorinated Compounds in Melon-Headed Whales Stranded along the Japanese Coast in 1982, 2001/2002, and 2006. Environ. Sci. Technol. 2008, 42, 7132–7137. [Google Scholar] [CrossRef] [PubMed]
- Yeung, L.W.Y.; Miyake, Y.; Wang, Y.; Taniyasu, S.; Yamashita, N.; Lam, P.K.S. Total Fluorine, Extractable Organic Fluorine, Perfluorooctane Sulfonate and Other Related Fluorochemicals in Liver of Indo-Pacific Humpback Dolphins (Sousa Chinensis) and Finless Porpoises (Neophocaena Phocaenoides) from South China. Environ. Pollut. 2009, 157, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Choi, J.-W.; Iseki, N.; Senthilkumar, K.; Kim, D.H.; Masunaga, S.; Giesy, J.P. Concentrations of Perfluorinated Acids in Livers of Birds from Japan and Korea. Chemosphere 2002, 49, 225–231. [Google Scholar] [CrossRef]
- Vo, P.H.N.; Buckley, T.; Xu, X.; Nguyen, T.M.H.; Rudolph, V.; Shukla, P. Foam Fractionation of Per- and Polyfluoroalkyl Substances (PFASs) in Landfill Leachate Using Different Cosurfactants. Chemosphere 2023, 310, 136869. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Huang, X.; Li, G.; Yu, Y.; Shi, B. Performance of In-Service Granular Activated Carbon for Perfluoroalkyl Substances Removal under Changing Water Quality Conditions. Sci. Total. Environ. 2022, 848, 157723. [Google Scholar] [CrossRef] [PubMed]
- Dixit, F.; Munoz, G.; Mirzaei, M.; Barbeau, B.; Liu, J.; Duy, S.V.; Sauvé, S.; Kandasubramanian, B.; Mohseni, M. Removal of Zwitterionic PFAS by MXenes: Comparisons with Anionic, Nonionic, and PFAS-Specific Resins. Environ. Sci. Technol. 2021, 56, 6212–6222. [Google Scholar] [CrossRef]
- Shojaei, M.; Kumar, N.; Guelfo, J.L. An Integrated Approach for Determination of Total Per- and Polyfluoroalkyl Substances (PFAS). Environ. Sci. Technol. 2022, 56, 14517–14527. [Google Scholar] [CrossRef]
- Schildroth, S.; Rodgers, K.M.; Strynar, M.; McCord, J.; Poma, G.; Covaci, A.; Dodson, R.E. Per-and Polyfluoroalkyl Substances (PFAS) and Persistent Chemical Mixtures in Dust from U.S. Colleges. Environ. Res. 2022, 206, 112530. [Google Scholar] [CrossRef]
- Morales-Mcdevitt, M.E.; Becanova, J.; Blum, A.; Bruton, T.A.; Vojta, S.; Woodward, M.; Lohmann, R. The Air That We Breathe: Neutral and Volatile PFAS in Indoor Air. Environ. Sci. Technol. Lett. 2021, 8, 897–902. [Google Scholar] [CrossRef]
- Evich, M.G.; Davis, M.; Weber, E.J.; Tebes-Stevens, C.; Acrey, B.; Henderson, W.M.; Goodrow, S.; Bergman, E.; Washington, J.W. Environmental Fate of Cl-PFPECAs: Predicting the Formation of PFAS Transformation Products in New Jersey Soils. Environ. Sci. Technol. 2022, 56, 7779–7788. [Google Scholar] [CrossRef]
- Peaslee, G.F.; Tighe, M.; Jin, Y.; Whitehead, H.D.; Hayes, K.; Lieberman, M.; Pannu, M.; Plumlee, M.H. Screening for Per- and Polyfluoroalkyl Substances in Water with Particle Induced Gamma-Ray Emission Spectroscopy. ACS ES&T Water 2021, 1, 2477–2484. [Google Scholar] [CrossRef]
- Young, A.S.; Sparer-Fine, E.H.; Pickard, H.M.; Sunderland, E.M.; Peaslee, G.F.; Allen, J.G. Per- and Polyfluoroalkyl Substances (PFAS) and Total Fluorine in Fire Station Dust. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Camdzic, D.; Dickman, R.A.; Aga, D.S. Total and Class-Specific Analysis of per- and Polyfluoroalkyl Substances in Environmental Samples Using Nuclear Magnetic Resonance Spectroscopy. J. Hazard. Mater. Lett. 2021, 2, 100023. [Google Scholar] [CrossRef]
- de la Torre, A.; Navarro, I.; Sanz, P.; Mártinez, M. de los Á. Occurrence and Human Exposure Assessment of Perfluorinated Substances in House Dust from Three European Countries. Sci. Total Environ. 2019, 685, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; He, Y.; Huang, Y.; Hong, D.; Yao, Y.; Wang, L.; Sun, W.; Yang, B.; Huang, X.; Song, S.; et al. Novel and Legacy Poly- and Perfluoroalkyl Substances (PFASs) in Indoor Dust from Urban, Industrial, and e-Waste Dismantling Areas: The Emergence of PFAS Alternatives in China. Environ. Pollut. 2020, 263, 114461. [Google Scholar] [CrossRef] [PubMed]
- Young, A.S.; Pickard, H.M.; Sunderland, E.M.; Allen, J.G. Organic Fluorine as an Indicator of Per- and Polyfluoroalkyl Substances in Dust from Buildings with Healthier versus Conventional Materials. Environ. Sci. Technol. 2022, 56, 17090–17099. [Google Scholar] [CrossRef]
- Kewalramani, J.A.; Wang, B.; Marsh, R.W.; Meegoda, J.N.; Rodriguez Freire, L. Coupled High and Low-Frequency Ultrasound Remediation of PFAS-Contaminated Soils. Ultrason. Sonochem 2022, 88, 106063. [Google Scholar] [CrossRef]
- Simon, F.; Gehrenkemper, L.; von der Au, M.; Wittwer, P.; Roesch, P.; Pfeifer, J.; Cossmer, A.; Meermann, B. A Fast and Simple PFAS Extraction Method Utilizing HR–CS–GFMAS for Soil Samples. Chemosphere 2022, 295, 133922. [Google Scholar] [CrossRef] [PubMed]
- dan Gan, C.; Peng, M.Y.; Liu, H.B.; Yang, J.Y. Concentration and Distribution of Metals, Total Fluorine, per- and Poly-Fluoroalkyl Substances (PFAS) in Vertical Soil Profiles in Industrialized Areas. Chemosphere 2022, 302, 134855. [Google Scholar] [CrossRef] [PubMed]
- Lassalle, J.; Gao, R.; Rodi, R.; Kowald, C.; Feng, M.; Sharma, V.K.; Hoelen, T.; Bireta, P.; Houtz, E.F.; Staack, D.; et al. Degradation of PFOS and PFOA in Soil and Groundwater Samples by High Dose Electron Beam Technology. Radiat. Phys. Chem. 2021, 189, 109705. [Google Scholar] [CrossRef]
- Ellis, A.C.; Liu, C.J.; Fang, Y.; Boyer, T.H.; Schaefer, C.E.; Higgins, C.P.; Strathmann, T.J. Pilot Study Comparison of Regenerable and Emerging Single-Use Anion Exchange Resins for Treatment of Groundwater Contaminated by per- and Polyfluoroalkyl Substances (PFASs). Water Res. 2022, 223, 119019. [Google Scholar] [CrossRef]
- Alinezhad, A.; Challa Sasi, P.; Zhang, P.; Yao, B.; Kubátová, A.; Golovko, S.A.; Golovko, M.Y.; Xiao, F. An Investigation of Thermal Air Degradation and Pyrolysis of Per- and Polyfluoroalkyl Substances and Aqueous Film-Forming Foams in Soil. ACS ES&T Eng. 2022, 2, 198–209. [Google Scholar] [CrossRef]
- Zhu, Q.; Qian, J.; Huang, S.; Li, Q.; Guo, L.; Zeng, J.; Zhang, W.; Cao, X.; Yang, J. Occurrence, Distribution, and Input Pathways of per- and Polyfluoroalkyl Substances in Soils near Different Sources in Shanghai. Environ. Pollut. 2022, 308, 119620. [Google Scholar] [CrossRef]
- Liu, C.J.; McKay, G.; Jiang, D.; Tenorio, R.; Cath, J.T.; Amador, C.; Murray, C.C.; Brown, J.B.; Wright, H.B.; Schaefer, C.; et al. Pilot-Scale Field Demonstration of a Hybrid Nanofiltration and UV-Sulfite Treatment Train for Groundwater Contaminated by per- and Polyfluoroalkyl Substances (PFASs). Water Res. 2021, 205, 117677. [Google Scholar] [CrossRef]
- Hwang, J.H.; Li Sip, Y.Y.; Kim, K.T.; Han, G.; Rodriguez, K.L.; Fox, D.W.; Afrin, S.; Burnstine-Townley, A.; Zhai, L.; Lee, W.H. Nanoparticle-Embedded Hydrogel Synthesized Electrodes for Electrochemical Oxidation of Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS). Chemosphere 2022, 296, 134001. [Google Scholar] [CrossRef] [PubMed]
- Selahle, S.K.; Mpupa, A.; Nomngongo, P.N. Liquid Chromatographic Determination of Per- and Polyfluoroalkyl Substances in Environmental River Water Samples. Arab. J. Chem. 2022, 15, 103960. [Google Scholar] [CrossRef]
- Nienhauser, A.B.; Ersan, M.S.; Lin, Z.; Perreault, F.; Westerhoff, P.; Garcia-Segura, S. Boron-Doped Diamond Electrodes Degrade Short- and Long-Chain per- and Polyfluorinated Alkyl Substances in Real Industrial Wastewaters. J. Environ. Chem. Eng. 2022, 10, 107192. [Google Scholar] [CrossRef]
- Duan, L.; Wang, B.; Heck, K.N.; Clark, C.A.; Wei, J.; Wang, M.; Metz, J.; Wu, G.; Tsai, A.L.; Guo, S.; et al. Titanium Oxide Improves Boron Nitride Photocatalytic Degradation of Perfluorooctanoic Acid. Chem. Eng. J. 2022, 448, 137735. [Google Scholar] [CrossRef]
- Rahman, M.F.; Anderson, W.B.; Peldszus, S.; Huck, P.M. Ion-Exchange Treatment of Perfluorinated Carboxylic Acids in Water: Comparison of Polystyrenic and Polyacrylic Resin Structures and Impact of Sulfate on Their Performance. ACS ES&T Water 2022, 2, 1195–1205. [Google Scholar] [CrossRef]
- Viticoski, R.L.; Wang, D.; Feltman, M.A.; Mulabagal, V.; Rogers, S.R.; Blersch, D.M.; Hayworth, J.S. Spatial Distribution and Mass Transport of Perfluoroalkyl Substances (PFAS) in Surface Water: A Statewide Evaluation of PFAS Occurrence and Fate in Alabama. Sci. Total. Environ. 2022, 836, 155524. [Google Scholar] [CrossRef]
- Koronaiou, L.A.; Nannou, C.; Xanthopoulou, N.; Seretoudi, G.; Bikiaris, D.; Lambropoulou, D.A. High-Resolution Mass Spectrometry-Based Strategies for the Target Analysis and Suspect Screening of per- and Polyfluoroalkyl Substances in Aqueous Matrices. Microchem. J. 2022, 179, 107457. [Google Scholar] [CrossRef]
- Skaggs, C.S.; Logue, B.A. Ultratrace Analysis of Per- and Polyfluoroalkyl Substances in Drinking Water Using Ice Concentration Linked with Extractive Stirrer and High Performance Liquid Chromatography—Tandem Mass Spectrometry. J. Chromatogr. A 2021, 1659, 462493. [Google Scholar] [CrossRef]
- Awchi, M.; Gebbink, W.A.; Berendsen, B.J.A.; Benskin, J.P.; van Leeuwen, S.P.J. Development, Validation, and Application of a New Method for the Quantitative Determination of Monohydrogen-Substituted Perfluoroalkyl Carboxylic Acids (H–PFCAs) in Surface Water. Chemosphere 2022, 287, 132143. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, Q.; Chen, H.; Li, M. Rapid Quantitative Analysis and Suspect Screening of Per-and Polyfluorinated Alkyl Substances (PFASs) in Aqueous Film-Forming Foams (AFFFs) and Municipal Wastewater Samples by Nano-ESI-HRMS. Water Res. 2022, 219, 118542. [Google Scholar] [CrossRef]
- Tang, A.; Zhang, X.; Li, R.; Tu, W.; Guo, H.; Zhang, Y.; Li, Z.; Liu, Y.; Mai, B. Spatiotemporal Distribution, Partitioning Behavior and Flux of per- and Polyfluoroalkyl Substances in Surface Water and Sediment from Poyang Lake, China. Chemosphere 2022, 295, 133855. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, H.; Minda, V.; Hawley, E.; Deeb, R.; Hart, M. Coupled Photocatalytic Alkaline Media as a Destructive Technology for Per- and Polyfluoroalkyl Substances in Aqueous Film-Forming Foam Impacted Stormwater. Chemosphere 2022, 291, 132790. [Google Scholar] [CrossRef]
- Li, X.; Fatowe, M.; Cui, D.; Quinete, N. Assessment of Per- and Polyfluoroalkyl Substances in Biscayne Bay Surface Waters and Tap Waters from South Florida. Sci. Total. Environ. 2022, 806, 150393. [Google Scholar] [CrossRef]
- Gallen, C.; Bignert, A.; Taucare, G.; O’Brien, J.; Braeunig, J.; Reeks, T.; Thompson, J.; Mueller, J.F. Temporal Trends of Perfluoroalkyl Substances in an Australian Wastewater Treatment Plant: A Ten-Year Retrospective Investigation. Sci. Total. Environ. 2022, 804, 150211. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Niu, Z.; Zhang, Y. Occurrence of Legacy and Emerging Poly- and Perfluoroalkyl Substances in Water: A Case Study in Tianjin (China). Chemosphere 2022, 287, 132409. [Google Scholar] [CrossRef] [PubMed]
- Camoiras González, P.; Sadia, M.; Baabish, A.; Sobhanei, S.; Fiedler, H. Air Monitoring with Passive Samplers for Perfluoroalkane Substances in Developing Countries (2017–2019). Chemosphere 2021, 282, 131069. [Google Scholar] [CrossRef] [PubMed]
- Kourtchev, I.; Hellebust, S.; Heffernan, E.; Wenger, J.; Towers, S.; Diapouli, E.; Eleftheriadis, K. A New On-Line SPE LC-HRMS Method for the Analysis of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) in PM2.5 and Its Application for Screening Atmospheric Particulates from Dublin and Enniscorthy, Ireland. Sci. Total. Environ. 2022, 835, 155496. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Taniyasu, S.; Yamazaki, E.; Wu, R.; Lam, P.K.S.; Eun, H.; Yamashita, N. Fluorine Mass Balance Analysis and Per- and Polyfluoroalkyl Substances in the Atmosphere. J. Hazard. Mater. 2022, 435, 129025. [Google Scholar] [CrossRef] [PubMed]
| PFAS Name | Acronym | Molecular Formula, Wt (g/mol) | Solubility (mg/L) (25 °C) | Melting Point (°C) | Boiling Point (°C) | Density (g/cm3) | Vapor Pressure (Pa) | References | |
|---|---|---|---|---|---|---|---|---|---|
| Perfluoroalkyl Substances | Perfluorocarboxylic Acids (PFCAs) | ||||||||
| Perfluorobutanoic acid | PFBA | C3F7COOH, 214.0 | 49,000 | −17.5 | 121 | 1.651 (20 °C) | 251 (25 °C) | [22,23] | |
| Perfluoropentanoic acid | PFPeA | C4F9COOH, 264.1 | 9812 | −13.2–−5.9 | – | 1.713 (25 °C) | 151 (25 °C) | [23,24] | |
| Perfluorohexanoic acid | PFHxA | C5F11COOH, 314.1 | 1893 | 7.8–14.8 | – | 1.759 (20 °C) | 13.2 (25 °C) | ||
| Perfluoroheptanoic acid | PFHpA | C6F13COOH, 364.1 | 356 | 19.1–32.8 | – | – | 55 (25 °C) | ||
| Perfluorooctanoic acid | PFOA | C7F15COOH, 414.1 | 4340 (24.1 °C) | 44.8–52.3 | – | 1.8 | 2.0 (25 °C) | [24,25] | |
| Perfluorosulfonic Acids (PFSAs) | |||||||||
| Perfluorobutane sulfonic acid | PFBS | C4F9SO3H, 300.1 | 6875 | 20.4–70.4 | 80–211 | 1.81–1.85 | 132 (25 °C) | [23,26] | |
| Perfluorohexane sulfonic acid | PFHxS | C6F13SO3H, 400.1 | 236 | 190 | 95–452 | 1.84 | 47.9 (25 °C) | [23,24] | |
| Perfluorooctane sulfonic acid | PFOS | C8F17SO3H, 500.1 | 7.7 | 15.2–185 | 133–249 | 1.84–1.85 | 16.98 (25 °C) | ||
| Polyfluoroalkyl Substances | Fluorotelomer Carboxylic Acids (FTCAs) | ||||||||
| 6:2 Fluorotelomer carboxylic acid | 6:2 FTCA | C6F13CH2COOH, 378.1 | 559 | 55 | 175–193 | 1.64–1.67 | 5.8 | [26,27] | |
| Fluorotelomer Sulfonic Acids (FTSAs) | |||||||||
| 4:2 Fluorotelomer sulfonic acid | 4:2 FTSA | C4F9(CH2)2SO3H, 328.2 | 28000 | 107 | 216 | 1.68 | 0.33 | [26,27] | |
| 6:2 Fluorotelomer sulfonic acid | 6:2 FTSA | C6F13(CH2)2SO3H, 528.2 | 1323 | 18.7–80.7 | 219–272 | 1.64–1.71 | 0.01 | ||
| 8:2 Fluorotelomer sulfonic acid | 8:2 FTSA | C8F17(CH2)2SO3H, 528.2 | 58 | 16.8–91.1 | 224–293 | 1.69 | 0.01 | ||
| Fluorotelomer Alcohols (FTOHs) | |||||||||
| 4:2 Fluorotelomer alcohol | 4:2 FTOH | C4F9(CH2)2OH, 264.1 | 2703 | – | 137.5 | – | 105 (25 °C) | [23,25] | |
| 6:2 Fluorotelomer alcohol | 6:2 FTOH | C6F13(CH2)2OH, 364.1 | 98 | – | 171.5–173.5 | – | 38 (25 °C) | [26,27] | |
| 8:2 Fluorotelomer alcohol | 8:2 FTOH | C8F17(CH2)2OH, 464.1 | 3.2 | 44.75–46.95 | 201.3–202.0 | – | 13.5 (25 °C) | [24,26,27] | |
| 10:2 Fluorotelomer alcohol | 10:2 FTOH | C10F21(CH2)2OH, 564.1 | 0.10 | 89.75–92.35 | 228.4 | – | 4.90 (25 °C) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, U.M.; Elnakar, H.; Khan, M.F. Sources, Fate, and Detection of Dust-Associated Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS): A Review. Toxics 2023, 11, 335. https://doi.org/10.3390/toxics11040335
Ismail UM, Elnakar H, Khan MF. Sources, Fate, and Detection of Dust-Associated Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS): A Review. Toxics. 2023; 11(4):335. https://doi.org/10.3390/toxics11040335
Chicago/Turabian StyleIsmail, Usman M., Haitham Elnakar, and Muhammad Faizan Khan. 2023. "Sources, Fate, and Detection of Dust-Associated Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS): A Review" Toxics 11, no. 4: 335. https://doi.org/10.3390/toxics11040335





