Bismuth Oxide (Bi2O3) Nanoparticles Cause Selective Toxicity in a Human Endothelial (HUVE) Cell Line Compared to Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Electron Microscopy
2.3. Cell Culture and Treatment with BNPs
2.4. Determination of Cell Viability by MTT
2.5. Detection of Intracellular ROS
2.6. Measurement of Intracellular GSH
2.7. Analysis of Intracellular NO
2.8. Analysis of Membrane Damage
2.9. Determination of Mitochondrial Membrane Potential by JC-1
2.10. Protein Estimation
2.11. Statistics
3. Results
3.1. BNPs Killed HUVE Cells at Significantly Lower Concentrations Compared to Cells with Epithelial Origin
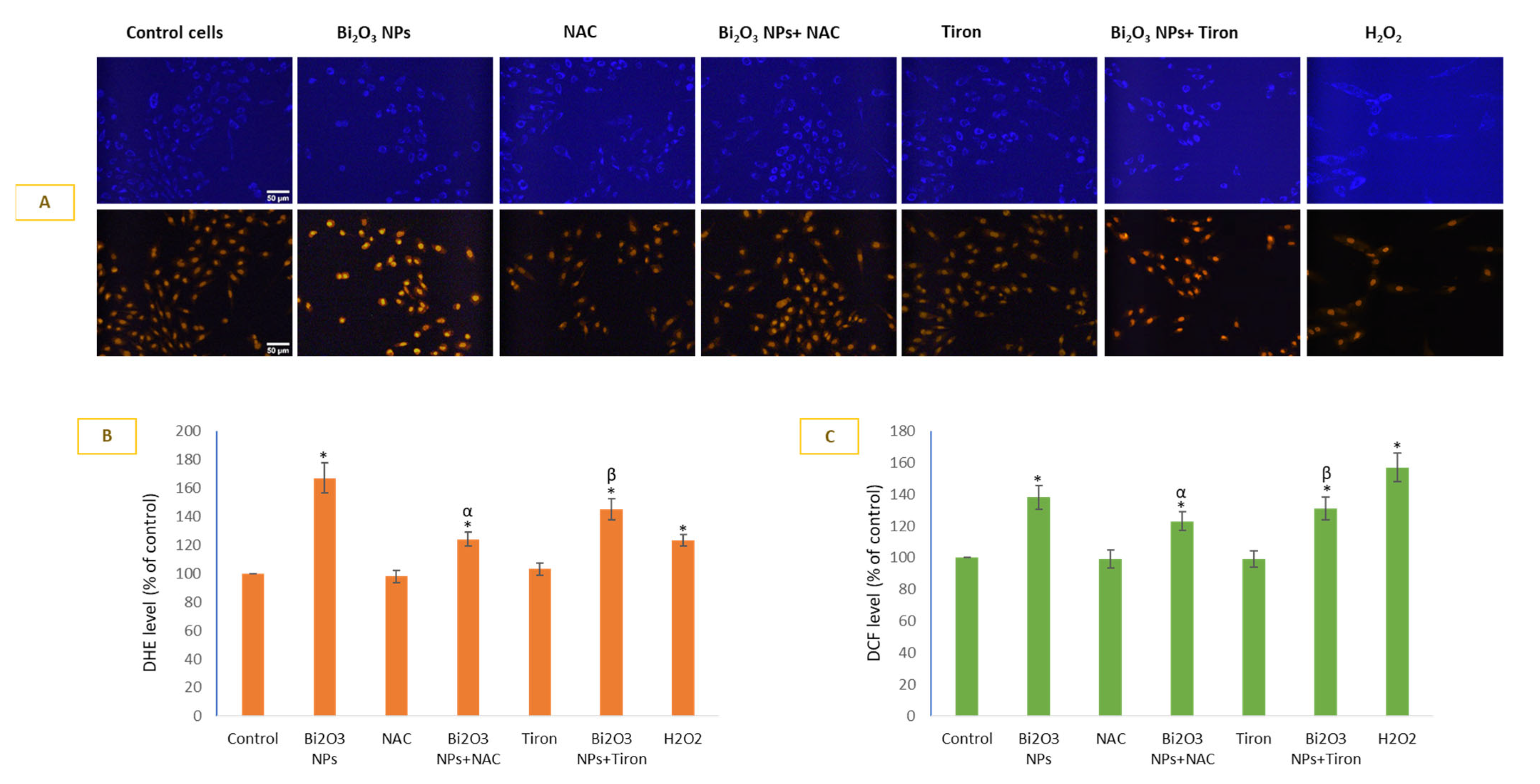
3.2. BNPs Caused Significant O2•− Generation in HUVE Cells
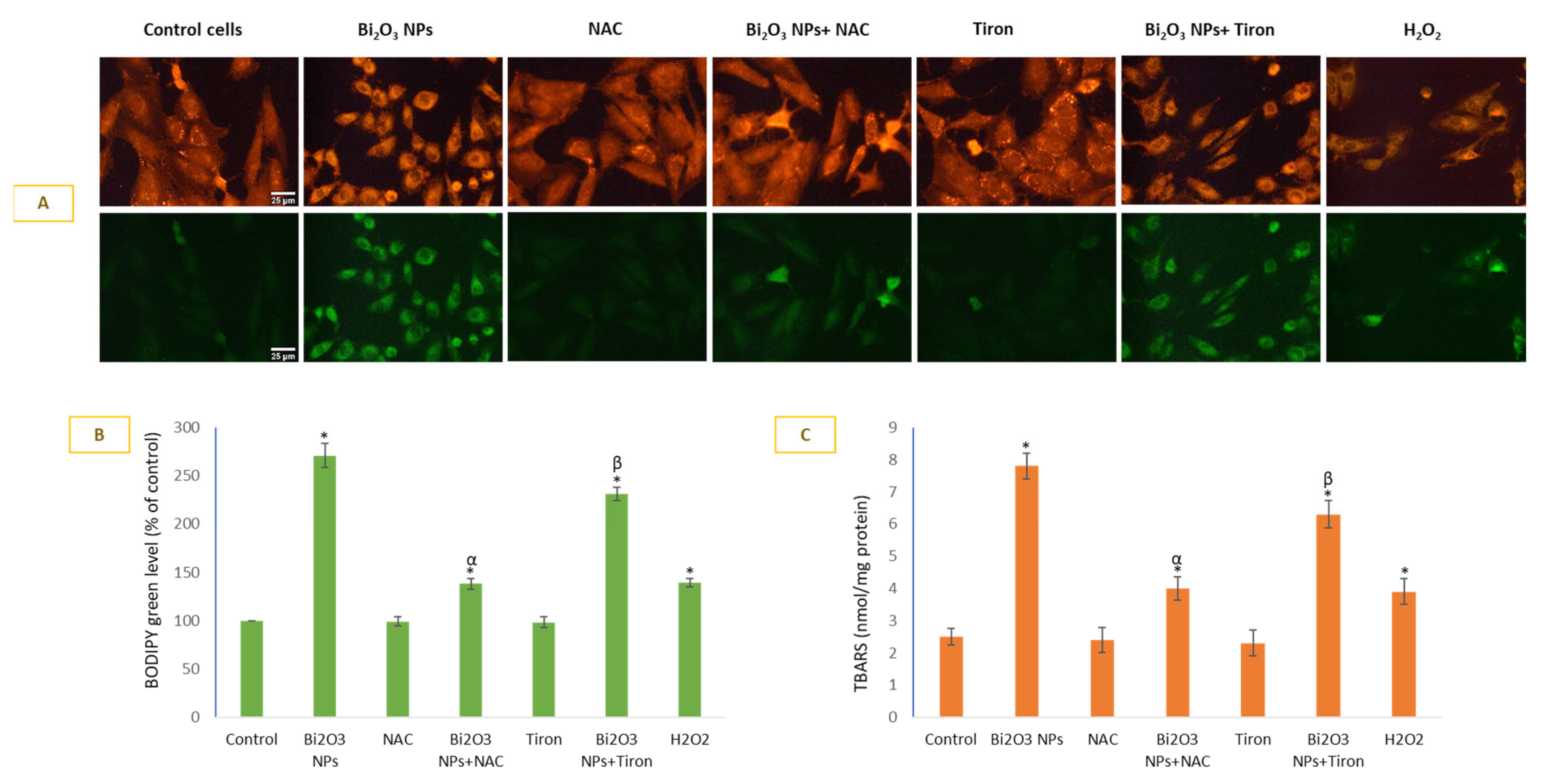
3.3. BNPs Caused Significant Lipid Peroxidation in Membranes of HUVE Cells
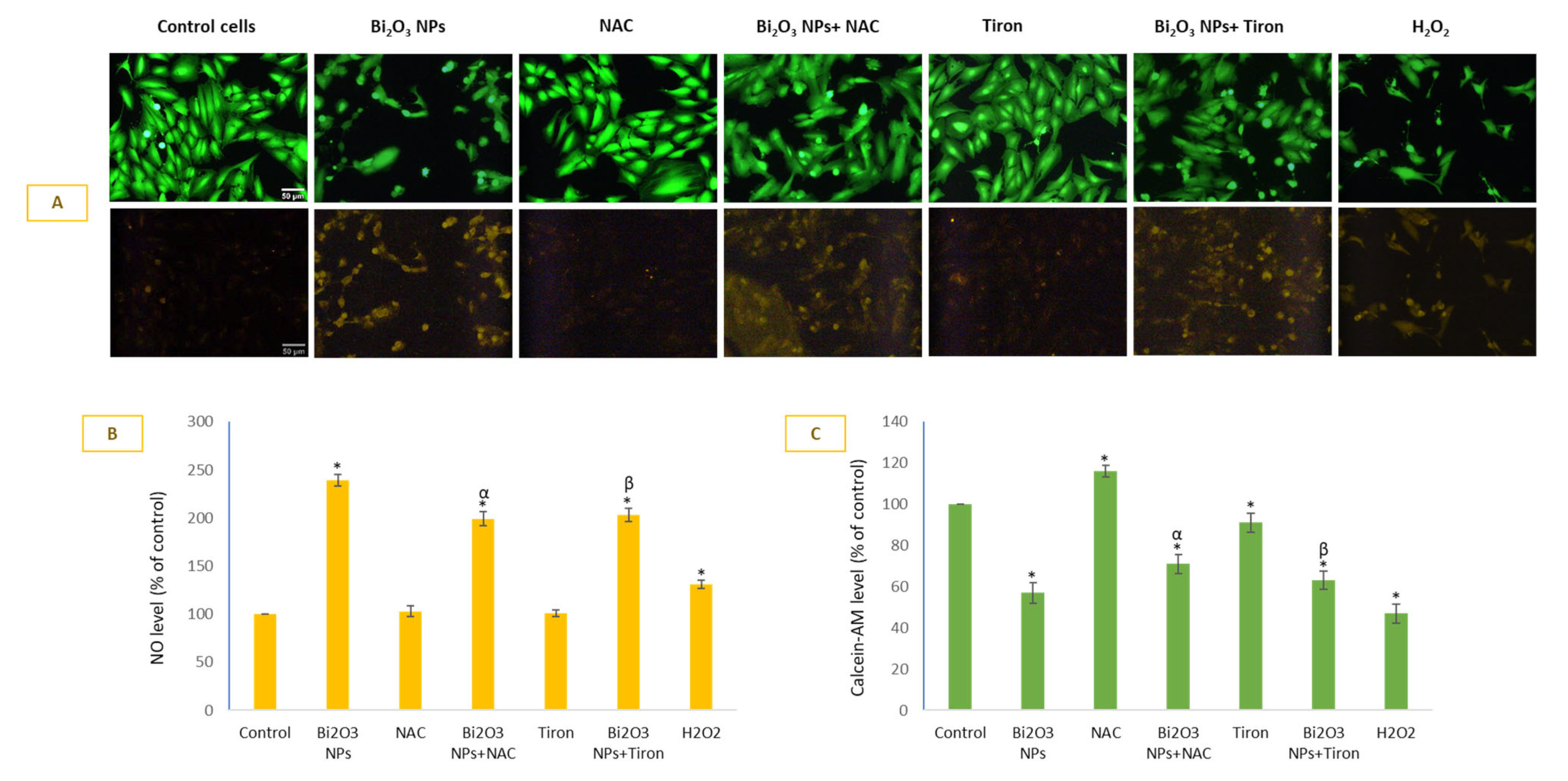
3.4. BNPs Significantly Induced NO Level That Was Least Preventable by Antioxidants in HUVE Cells
3.5. BNPs Caused Significant Loss in MMP and Depletion in Intracellular GSH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahbazi, M.A.; Faghfouri, L.; Ferreira, M.P.A.; Figueiredo, P.; Maleki, H.; Sefat, F.; Hirvonen, J.; Santos, H.A. The Versatile Biomedical Applications of Bismuth-Based Nanoparticles and Composites: Therapeutic, Diagnostic, Biosensing, and Regenerative Properties. Chem. Soc. Rev. 2020, 49, 1253–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Delgadillo, R.; García-Cuéllar, C.M.; Sánchez-Pérez, Y.; Pineda-Aguilar, N.; Martínez-Martínez, M.A.; Rangel-Padilla, E.E.; Nakagoshi-Cepeda, S.E.; Solís-Soto, J.M.; Sánchez-Nájera, R.I.; Nakagoshi-Cepeda, M.A.A.; et al. In Vitro Evaluation of the Antitumor Effect of Bismuth Lipophilic Nanoparticles (BisBAL NPs) on Breast Cancer Cells. Int. J. Nanomed. 2018, 13, 6089–6097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogusz, K.; Tehei, M.; Stewart, C.; McDonald, M.; Cardillo, D.; Lerch, M.; Corde, S.; Rosenfeld, A.; Liu, H.K.; Konstantinov, K. Synthesis of Potential Theranostic System Consisting of Methotrexate-Immobilized (3-Aminopropyl)Trimethoxysilane Coated α-Bi2O3 Nanoparticles for Cancer Treatment. RSC Adv. 2014, 4, 24412–24419. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Delgadillo, R.; Velasco-Arias, D.; Martinez-Sanmiguel, J.J.; Diaz, D.; Zumeta-Dube, I.; Arevalo-Niño, K.; Cabral-Romero, C. Bismuth Oxide Aqueous Colloidal Nanoparticles Inhibit Candida Albicans Growth and Biofilm Formation. Int. J. Nanomed. 2013, 8, 1645–1652. [Google Scholar] [CrossRef] [Green Version]
- Sisin, N.N.T.; Razak, K.A.; Abidin, S.Z.; Mat, N.F.C.; Abdullah, R.; Rashid, R.A.; Anuar, M.A.K.; Rahman, W.N. Synergetic Influence of Bismuth Oxide Nanoparticles, Cisplatin and Baicalein-Rich Fraction on Reactive Oxygen Species Generation and Radiosensitization Effects for Clinical Radiotherapy Beams. Int. J. Nanomed. 2020, 15, 7805–7823. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alrokayan, S.A.; Alhadlaq, H.A. Oxidative Stress Mediated Cytotoxicity and Apoptosis Response of Bismuth Oxide (Bi2O3) Nanoparticles in Human Breast Cancer (MCF-7) Cells. Chemosphere 2019, 216, 823–831. [Google Scholar] [CrossRef]
- Akbarzadeh, F.; Khoshgard, K.; Hosseinzadeh, L.; Arkan, E.; Rezazadeh, D. Investigating the Cytotoxicity of Folate-Conjugated Bismuth Oxide Nanoparticles on KB and A549 Cell Lines. Adv. Pharm. Bull. 2018, 8, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Alamer, A.; Ali, D.; Alarifi, S.; Alkahtane, A.; AL-Zharani, M.; Abdel-Daim, M.M.; Albasher, G.; Almeer, R.; Al-Sultan, N.K.; Almalik, A.; et al. Bismuth Oxide Nanoparticles Induce Oxidative Stress and Apoptosis in Human Breast Cancer Cells. Environ. Sci. Pollut. Res. 2021, 28, 7379–7389. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alhadlaq, H.A. Co-Exposure of Bi2O3 Nanoparticles and Bezo[a]Pyrene-Enhanced in Vitro Cytotoxicity of Mouse Spermatogonia Cells. Environ. Sci. Pollut. Res. 2021, 28, 17109–17118. [Google Scholar] [CrossRef]
- Mohd Zainudin, N.H.; Razak, K.A.; Abidin, S.Z.; Abdullah, R.; Rahman, W.N. Influence of Bismuth Oxide Nanoparticles on Bystander Effects in MCF-7 and HFOB 1.19 Cells under 10 MV Photon Beam Irradiation. Radiat. Phys. Chem. 2020, 177, 109143. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alaizeri, Z.M.; Alhadlaq, H. Facile Synthesis of Zn-Doped Bi2O3 nanoparticles and Their Selective Cytotoxicity toward Cancer Cells. ACS Omega 2021, 6, 17353–17361. [Google Scholar] [CrossRef]
- Cao, Y. The Toxicity of Nanoparticles to Human Endothelial Cells. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2018; Volume 1048, pp. 59–69. [Google Scholar]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H. A Selective Toxicity of Pt-Coated Au Nanoparticles in Cancerous MCF-7 Cells over Non-Cancerous HUVE Cells. J. King Saud Univ.-Sci. 2023, 35, 102583. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H. Anti-Inflammatory CeO2 Nanoparticles Prevented Cytotoxicity Due to Exogenous Nitric Oxide Donors via Induction Rather than Inhibition of Superoxide/Nitric Oxide in Huve Cells. Molecules 2021, 26, 5416. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H. CeO2-Zn Nanocomposite Induced Superoxide, Autophagy and a Non-Apoptotic Mode of Cell Death in Human Umbilical-Vein-Derived Endothelial (HUVE) Cells. Toxics 2022, 10, 250. [Google Scholar] [CrossRef]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an Antioxidant and Disulphide Breaking Agent: The Reasons Why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef] [Green Version]
- Yamada, J.; Yoshimura, S.; Yamakawa, H.; Sawada, M.; Nakagawa, M.; Hara, S.; Kaku, Y.; Iwama, T.; Naganawa, T.; Banno, Y.; et al. Cell Permeable ROS Scavengers, Tiron and Tempol, Rescue PC12 Cell Death Caused by Pyrogallol or Hypoxia/Reoxygenation. Neurosci. Res. 2003, 45, 1–8. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying Cellular Oxidative Stress by Dichlorofluorescein Assay Using Microplate Reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A Fluorometric Method for Determination of Oxidized and Reduced Glutathione in Tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Von Bohlen und Halbach, O. Nitric Oxide Imaging in Living Neuronal Tissues Using Fluorescent Probes. Nitric Oxide-Biol. Chem. 2003, 9, 217–228. [Google Scholar] [CrossRef]
- Kojima, H.; Hirotani, M.; Nakatsubo, N.; Kikuchi, K.; Urano, Y.; Higuchi, T.; Hirata, Y.; Nagano, T. Bioimaging of Nitric Oxide with Fluorescent Indicators Based on the Rhodamine Chromophore. Anal. Chem. 2001, 73, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wan, A. Fluorescent Probes for Real-Time Measurement of Nitric Oxide in Living Cells. Analyst 2015, 140, 7129–7141. [Google Scholar] [CrossRef] [PubMed]
- Welder, A.A. A Primary Culture System of Adult Rat Heart Cells for the Evaluation of Cocaine Toxicity. Toxicology 1992, 72, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Sayes, C.M.; Gobin, A.M.; Ausman, K.D.; Mendez, J.; West, J.L.; Colvin, V.L. Nano-C60 Cytotoxicity Is Due to Lipid Peroxidation. Biomaterials 2005, 26, 7587–7595. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Kumar, S.; Alrokayan, S.A. Mitochondrial Dysfunction, Autophagy Stimulation and Non-Apoptotic Cell Death Caused by Nitric Oxide-Inducing Pt-Coated Au Nanoparticle in Human Lung Carcinoma Cells. Biochim. Biophys. Acta-Gen. Subj. 2020, 1864, 129452. [Google Scholar] [CrossRef]
- Raudsepp, P.; Brüggemann, D.A.; Andersen, M.L. Detection of Radicals in Single Droplets of Oil-in-Water Emulsions with the Lipophilic Fluorescent Probe BODIPY665/676 and Confocal Laser Scanning Microscopy. Free Radic. Biol. Med. 2014, 70, 233–240. [Google Scholar] [CrossRef]
- Smiley, S.T.; Reers, M.; Mottola-Hartshorn, C.; Lin, M.; Chen, A.; Smith, T.W.; Steele, G.D.; Chen, L.B. Intracellular Heterogeneity in Mitochondrial Membrane Potentials Revealed by a J-Aggregate-Forming Lipophilic Cation JC-1. Proc. Natl. Acad. Sci. USA 1991, 88, 3671–3675. [Google Scholar] [CrossRef] [Green Version]
- Abudayyak, M.; Öztaş, E.; Arici, M.; Özhan, G. Investigation of the Toxicity of Bismuth Oxide Nanoparticles in Various Cell Lines. Chemosphere 2017, 169, 117–123. [Google Scholar] [CrossRef]
- Du, F.; Lou, J.; Jiang, R.; Fang, Z.; Zhao, X.; Niu, Y.; Zou, S.; Zhang, M.; Gong, A.; Wu, C. Hyaluronic Acid-Functionalized Bismuth Oxide Nanoparticles for Computed Tomography Imaging-Guided Radiotherapy of Tumor. Int. J. Nanomed. 2017, 12, 5973–5992. [Google Scholar] [CrossRef] [Green Version]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting Cancer Cells by ROS-Mediated Mechanisms: A Radical Therapeutic Approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.L.; Liu, H.X. The Double-Edged Roles of ROS in Cancer Prevention and Therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef]
- Fukuto, J.M.; Carrington, S.J.; Tantillo, D.J.; Harrison, J.G.; Ignarro, L.J.; Freeman, B.A.; Chen, A.; Wink, D.A. Small Molecule Signaling Agents: The Integrated Chemistry and Biochemistry of Nitrogen Oxides, Oxides of Carbon, Dioxygen, Hydrogen Sulfide, and Their Derived Species. Chem. Res. Toxicol. 2012, 25, 769–793. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alshamsan, A. Mechanism of ROS Scavenging and Antioxidant Signalling by Redox Metallic and Fullerene Nanomaterials: Potential Implications in ROS Associated Degenerative Disorders. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 802–813. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Alhadlaq, H.A.; Alrokayan, S.A. Assessment of the Lung Toxicity of Copper Oxide Nanoparticles: Current Status. Nanomedicine 2015, 10, 2365–2377. [Google Scholar] [CrossRef]
- Skonieczna, M.; Hejmo, T.; Poterala-Hejmo, A.; Cieslar-Pobuda, A.; Buldak, R.J. NADPH Oxidases (NOX): Insights into Selected Functions and Mechanisms of Action in Cancer and Stem Cells. Oxid. Med. Cell. Longev. 2017, 2017, 9420539. [Google Scholar] [CrossRef] [Green Version]
- Radi, R. Oxygen Radicals, Nitric Oxide, and Peroxynitrite: Redox Pathways in Molecular Medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [Green Version]
- Rota, C.; Bergamini, S.; Daneri, F.; Tomasi, A.; Virgili, F.; Iannone, A. N-Acetylcysteine Negatively Modulates Nitric Oxide Production in Endotoxin-Treated Rats through Inhibition of NF-ĸB Activation. Antioxid. Redox Signal. 2002, 4, 221–226. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, M.J.; Ahamed, M.; Alhadlaq, H. Bismuth Oxide (Bi2O3) Nanoparticles Cause Selective Toxicity in a Human Endothelial (HUVE) Cell Line Compared to Epithelial Cells. Toxics 2023, 11, 343. https://doi.org/10.3390/toxics11040343
Akhtar MJ, Ahamed M, Alhadlaq H. Bismuth Oxide (Bi2O3) Nanoparticles Cause Selective Toxicity in a Human Endothelial (HUVE) Cell Line Compared to Epithelial Cells. Toxics. 2023; 11(4):343. https://doi.org/10.3390/toxics11040343
Chicago/Turabian StyleAkhtar, Mohd Javed, Maqusood Ahamed, and Hisham Alhadlaq. 2023. "Bismuth Oxide (Bi2O3) Nanoparticles Cause Selective Toxicity in a Human Endothelial (HUVE) Cell Line Compared to Epithelial Cells" Toxics 11, no. 4: 343. https://doi.org/10.3390/toxics11040343






