Acute Toxicity Assessment and Prediction Models of Four Heavy Metals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Organism
2.2. Experimental Design
2.3. Collection of Toxicity Data
2.4. Model Development and Verification
3. Results
3.1. Acute Toxicity of B. lenok Exposed to Heavy Metals
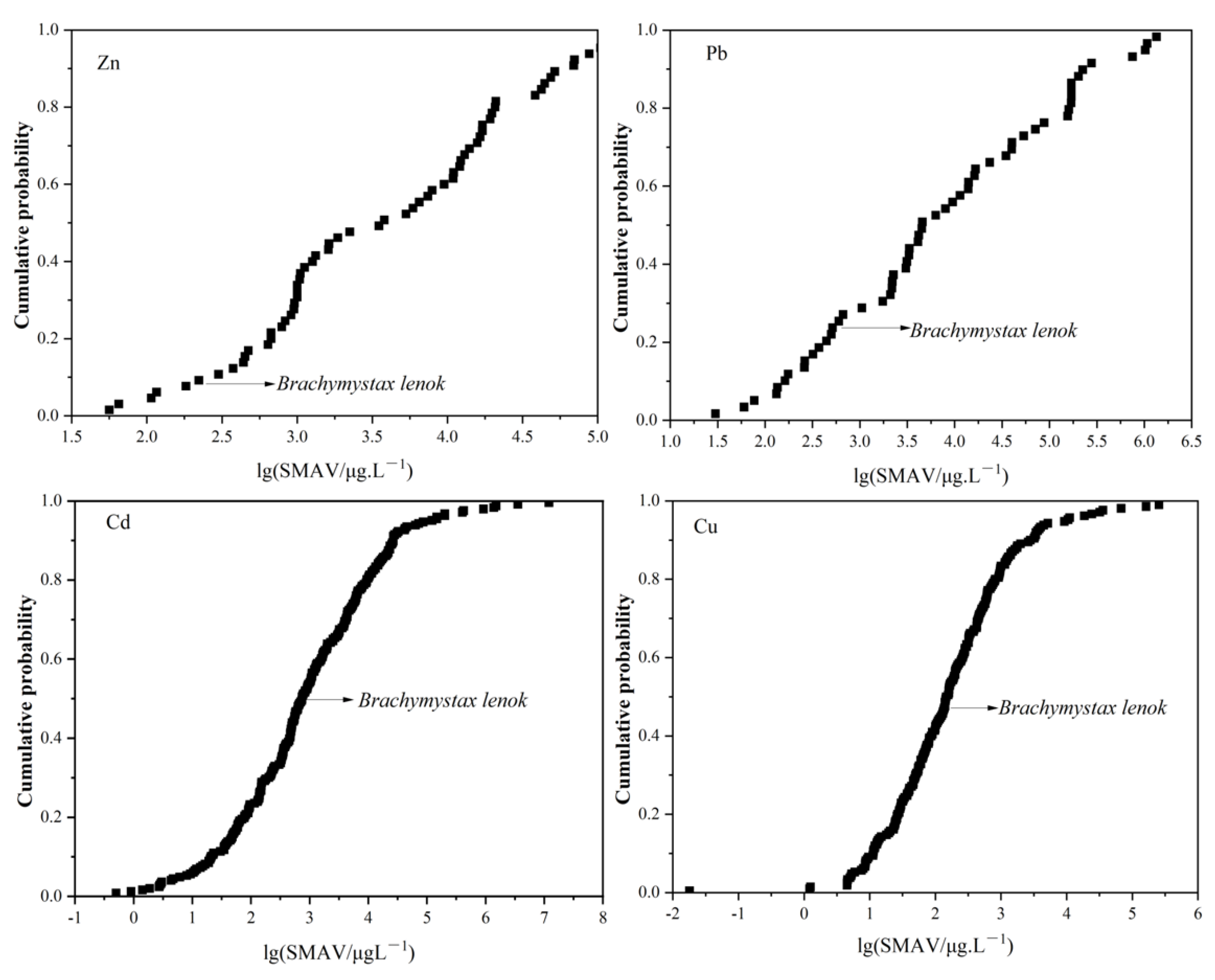
3.2. The Sensitivity of B. lenok among the Freshwater Species
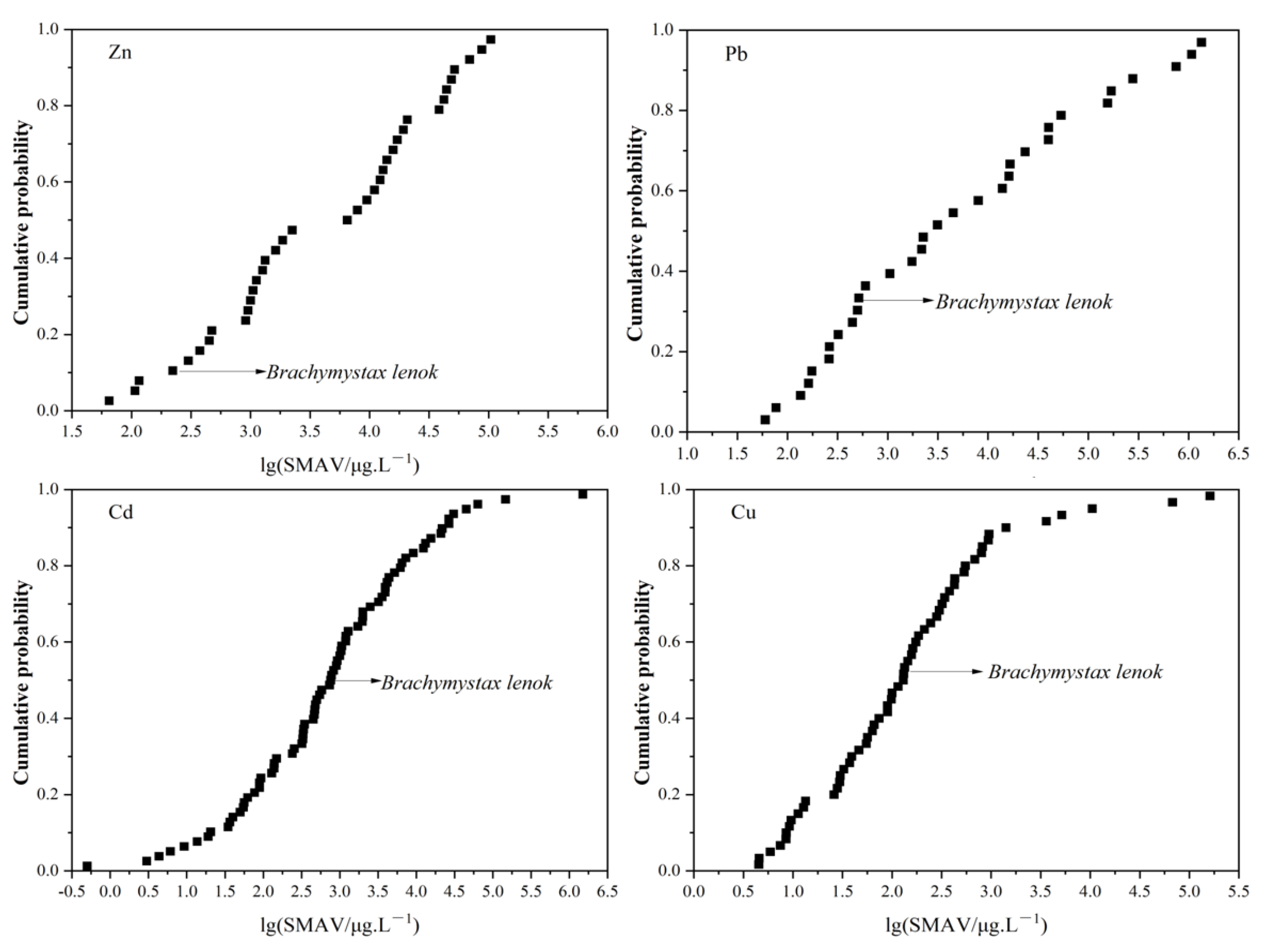
3.3. The Sensitivity of B. lenok among the Native Freshwater Species
3.4. Comparison of Sensitivity of B. lenok and Warm-Water Fish to Four Heavy Metals
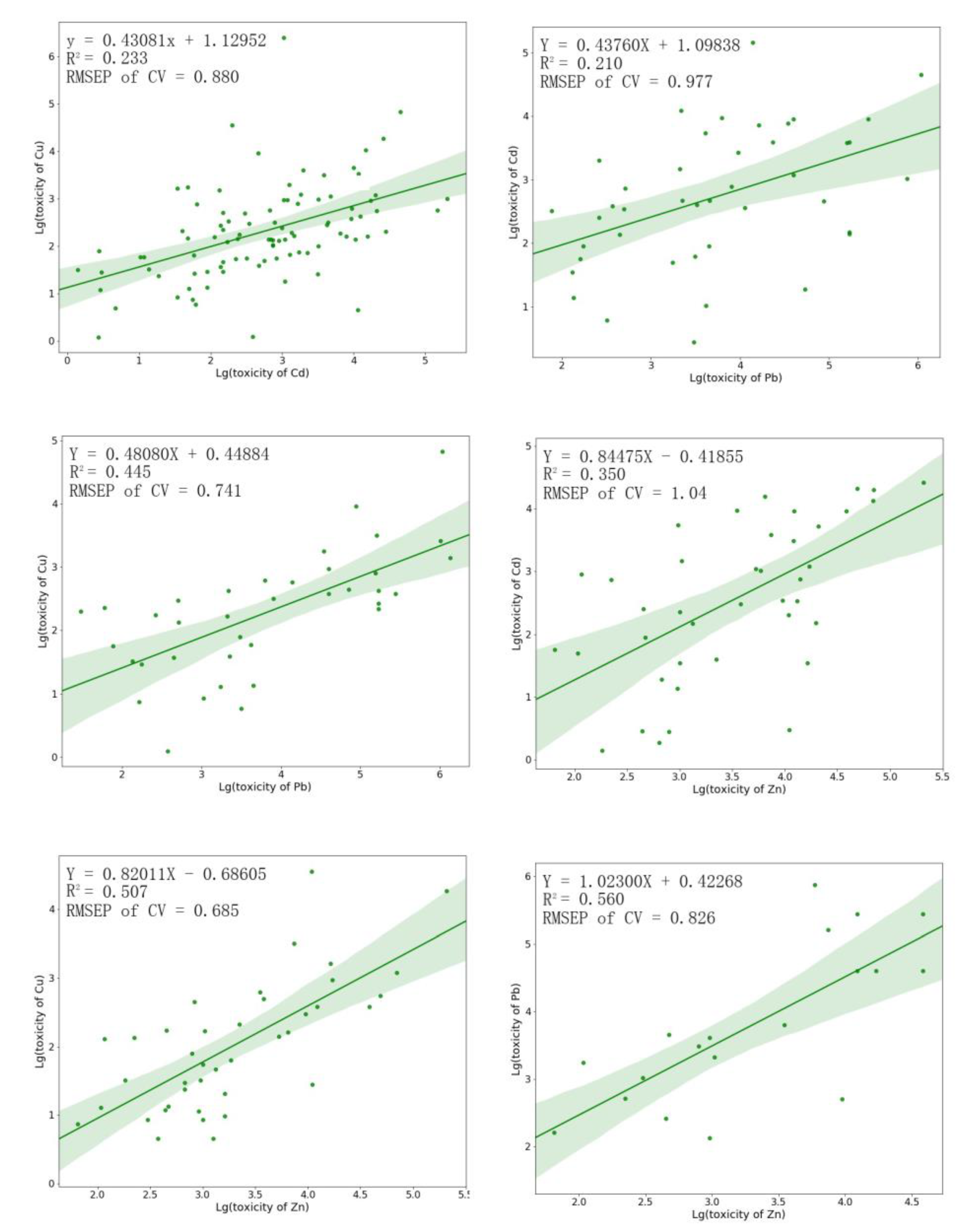
3.5. Development and Verification of the Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, J.; Lü, C.; Fan, Q.; Xue, H.; Bao, J. Distribution of AVS-SEM, transformation mechanism and risk assessment of heavy metals in the Nanhai Lake in China. Environ. Earth Sci. 2011, 64, 2025–2037. [Google Scholar] [CrossRef]
- Li, H.L.; Gao, X.T. Several common heavy metal pollutants and their harm to aquatic organisms. Hebei Fish. 2007, 3, 1–4. [Google Scholar]
- Xu, S.L.; Wang, D.L.; Ye, J.N.; Jia, C.Y. Joint toxicity effect of four heavy metal ions on Moina macrocopa. J. Biol. 2011, 28, 21–25. [Google Scholar]
- Zhang, Z.Y.; Abuduwaili, J.; Jiang, F.Q. Heavy metal contamination, sources, and pollution assessment of surface water in the Tianshan Mountains of China. Environ. Monit. Assess. 2015, 187, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, M.; Liu, Q.; Wang, Z.; Zhao, L.; Chen, Y. Study of heavy metal pollution, ecological risk and source apportionment in the surface water and sediments of the Jiangsu coastal region, China: A case study of the Sheyang estuary. Mar. Pollut. Bull. 2018, 137, 601–609. [Google Scholar] [CrossRef]
- Besser, J.M.; Mebane, C.A.; Mount, D.R.; Ivey, C.D.; Kunz, J.L.; Greer, I.E.; May, T.W. Ingersoll CG Sensitivity of mottled sculpins (Cottus bairdi) and rainbow trout (Onchorhynchus mykiss) to acute and chronic toxicity of cadmium, copper, and zinc. Environ. Toxicol. Chem. 2007, 26, 1657–1665. [Google Scholar] [CrossRef]
- Karntanut, W.; Pascoe, D. The toxicity of copper, cadmium and zinc to four different hydra (Cnidaria: Hydrozoa). Chemosphere 2002, 47, 1059–1064. [Google Scholar] [CrossRef]
- Cooper, N.L.; Bidwell, J.R.; Kumar, A. Toxicity of Copper, Lead, and Zinc Mixtures to Ceriodaphnia dubia and Daphnia carinata. Ecotoxicol. Environ. Saf. 2009, 72, 1523–1528. [Google Scholar]
- Zhao, T.H.; Zhou, B.H.; Fang, Y.X.; Chen, C.; Wang, Y.; Mu, Y.S.; Takahashi, A. Comparison of Freshwater Biota Species Sensitivity Distributions to Copper in China and the United States. Environ. Sci. Res. 2010, 44, 3923–3929. [Google Scholar]
- Wu, F.; Meng, W.; Zhao, X.; Li, H.; Zhang, R.; Cao, Y.; Liao, H. China embarking on development of its own national water quality criteria system. Environ. Sci. Technol. 2010, 44, 7992–7993. [Google Scholar] [CrossRef]
- HJ 831-2022; Technical Guideline for Deriving Water Quality Criteria for Freshwater Organisms. National Standard of P. R. China; MEP (Ministry of Ecology and Environment of the People’s Republic of China): Beijing, China, 2022.
- Freshwater Aquatic Life Criteria for Cadmium; National Standard of P. R. China. MEEC (Ministry of Ecology and Environment of China): Beijing, China, 2020.
- Freshwater Aquatic Life Criteria for Ammonia; National Standard of P. R. China. MEEC (Ministry of Ecology and Environment of China): Beijing, China, 2020.
- Van der Hoeven, N. Estimating the 5-percentile of the species sensitivity distributions without any assumptions about the distribution. Ecotoxicology. 2001, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.R.; Grist, E.P.; Leung, K.M.; Morritt, D.; Crane, M. Species sensitivity distributions: Data and model choice. Mar. Pollut. Bull. 2002, 45, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Posthuma, L.; Suter, G.W.; Traas, T.P. Species Sensitivity Distributions in Ecotoxicology; Lewis Publishers: Boca Raton, FL, USA, 2002; p. 587. [Google Scholar]
- US EPA. Guidelines for Deriving Numerical National Water Quality Criteria for the Protection of Aquatic Organisms and Their Uses; (PB 85-227049); USEPA: Washington, DC, USA, 1985; pp. 1–104.
- Cobbina, S.J.; Xu, H.; Zhao, T.; Mao, G.; Zhou, Z.; Wu, X.; Liu, H.; Zou, Y.; Wu, X.; Yang, L. A multivariate assessment of innate immune-related gene expressions due to exposure to low concentration individual and mixtures of four kinds of heavy metals on zebrafish (Danio rerio) embryos. Fish. Shellfish. Immunol. 2015, 47, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Hayashi, T.I.; Kamo, M. Comparison of population-level effects of heavy metals on fathead minnow (Pimephales promelas). Ecotoxicol. Environ. Saf. 2010, 73, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ingersoll, C.G.; Dorman, R.A.; Brumbaugh, W.G.; Mebane, C.A.; Kunz, J.L. Chronic sensitivity of white sturgeon (Acipenser transmontanus) and rainbow trout (Oncorhynchus mykiss) to cadmium, copper, lead, or zinc in laborator water-only exposures. Environ. Toxicol. Chem. 2014, 33, 2246–2258. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.L.; Jiang, H.F.; Bai, S.Y. Determination of 28 trace elements in three farmed cyprinid fish species from Northeast China. Food Control 2015, 50, 1–8. [Google Scholar] [CrossRef]
- Besser, J.M.; Dorman, R.; Ivey, C.D.; Cleveland, D.; Steevens, J.A. Sensitivity of Warm-Water Fishes and Rainbow Trout to Selected Contaminants. Bull. Environ. Contam. Toxicol. 2020, 104, 321–326. [Google Scholar] [CrossRef]
- Ma, B.; Yin, J.S.; Li, J.P. Morphological comparison and taxonomic status of two Brachymystax in Heilongjiang. Zoological. Syst. 2005, 30, 257–260. [Google Scholar]
- Lee, S.; Yoshizaki, G. Successful cryopreservation of spermatogonia in critically endangered Manchurian trout (Brachymystax lenok). Cryobiology 2016, 72, 165–168. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.Y.; Xu, J.B. The response of larvae Brachymystax lenok during continous and single pulses exposure to copper, zinc, lead and cadmium. Ecotoxicol. Environ. Saf. 2018, 165, 153–159. [Google Scholar] [CrossRef]
- OECD. Test No. 203: Fish, Acute Toxicity Test. In Guidelines for the Testing of Chemicals, Sect. 2: Effects on Biotic Systems; Organization for Economic Co-Operation and Development: Paris, France, 1992. [Google Scholar]
- GB/T 27861-2011; Chemicals-Fish Acute Toxicity Text; National Standard of P. R. China. Administration of Quality Supervision, Inspection and Quarantine, Standardization Administration of China: Beijing, China, 2011.
- Stephan, C.E. Methods for calculating an LC50. In Aquatic Toxicology and Hazard Evaluation; Mayer, F.I., Hamelink, J.L., Eds.; U.S. Environmental Protection Agency: Washington, DC, USA, 1977; Volume 634, pp. 65–84. [Google Scholar]
- Yan, Z.G.; Wang, W.L.; Zhou, J.L.; Yi, X.L.; Zhang, J.; Wang, X.N.; Liu, Z.T. Screening of high phytotoxicity priority pollutants and their ecological risk assessment in china’s surface waters. Chemosphere 2015, 128, 28–35. [Google Scholar] [CrossRef]
- Zheng, X.; Zang, W.C.; Yan, Z.G.; Hong, Y.G.; Liu, Z.T.; Yi, X.L.; Wang, X.N.; Liu, T.T.; Zhou, L.M. Species sensitivity analysis of heavy metals to freshwater organisms. Ecotoxicology 2015, 24, 1621–1631. [Google Scholar]
- Wang, W.L.; Yan, Z.G.; Liu, Z.T. Screening of native annelids and aquatic insects for deriving aquatic life criteria. Res. Environ. Sci. 2014, 27, 365–372. [Google Scholar]
- Wang, X.N.; Zheng, X.; Yan, Z.G. Screening of native fishes for deriving aquatic life criteria. Res. Environ. Sci. 2014, 27, 341–348. [Google Scholar]
- Dyer, S.D.; Belanger, S.E.; Carr, G.J. An initial evaluation of the use of Euro/North American fish species for tropical effects assessments. Chemosphere 1997, 35, 2767–2781. [Google Scholar] [CrossRef]
- Mayer, F.L.; Ellersieck, M.R. Manual of Acute Toxicity: Interpretation and Data Base for 410 Chemicals and 66 Species of Freshwater Animals; Resource. Pub. 160; U.S. Fish and Wildlife Serv.: Washington, DC, USA, 1986; p. 506. [Google Scholar]
- Yan, Z.G.; Zhang, Z.S.; Wang, H.; Liang, F.; Li, J.; Liu, H.L.; Sun, C.; Liang, L.J.; Liu, Z.T. Development of aquatic life criteria for nitrobenzene in China. Environ. Pollut. 2012, 162, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Yan, Z.G.; Liu, Z.T.; Liu, J.D.; Liang, F.; Wang, X.N.; Wang, W.L. Development of water quality criteria for phenanthrene and comparison of the sensitivity between native and non-native species. Environ. Pollut. 2015, 196, 141–146. [Google Scholar] [CrossRef]
- Gravenmier, J.J.; Johnston, D.W.; Santore, R.C.; Arnold, W.R. Acute toxicity of copper to the three spine stickleback, Gasterosteus aculeatus. Environ. Toxicol. 2005, 20, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Mineau, P.; Barron, M.G. Estimation of Chemical Toxicity to Wildlife Species Using Interspecies Correlation Models. Environ. Sci. Technol. 2007, 41, 5888–5894. [Google Scholar] [CrossRef]
- Fan, J.T.; Yan, Z.G.; Zheng, X.; Wu, J.; Wang, S.P.; Wang, P.Y.; Zhang, Q.Y. Development of Interspecies Correlation Estimation (ICE) models to predict the reproduction toxicity of EDCs to aquatic species. Chemosphere 2019, 224, 833–839. [Google Scholar] [CrossRef]
- Fan, J.T.; Huang, G.X.; Chi, M.H.; Shi, Y.; Jiang, J.Y.; Feng, C.Y.; Yan, Z.H.; Xu, Z.X. Prediction of chemical reproductive toxicity to aquatic species using a machine learning model: An application in an ecological risk assessment of the Yangtze River, China. Sci. Total Environ. 2021, 796, 148901. [Google Scholar] [CrossRef]
- Xu, J.Y.; Heng, L.; Yan, Z.G.; Feng, L.L.; Ling, J.H. Effective extrapolation models for ecotoxicity of benzene, toluene, ethylbenzene, and xylene (BTEX). Chemosphere 2020, 240, 124906. [Google Scholar] [CrossRef]
- Xi, B.D.; Huo, S.L.; Chen, Q.; Chen, Y.Q.; Zan, F.Y.; Xia, X.F. U.S Water Quality Standard System and Its Revelation for China. Environ. Sci. Technol. 2011, 34, 100–103. [Google Scholar]
- Shi, W.L. Fish faunal complex theory and its evaluation. Fishery. Sci. 1985, 2, 42–45. (In Chinese) [Google Scholar]
- Goofyear, C.D.; Edsall, T.A.; Ormsby, D. Atlas of the Spawning and Nursery Areas of Great Lakes Fishes; Lake Superior; U.S. Fish and Wildlife Service: Washington, DC, USA, 1982; Volume 2, 4, 5, 9, 11. [Google Scholar]
- Cai, J.; Yan, Z.G.; He, L. Screening of native amphibians for deriving aquatic life criteria. Res. Environ. Sci. 2014, 27, 349–355. [Google Scholar]
- Zheng, X.; Yan, Z.G.; Wang, X.N. Screening of native crustaceans for deriving aquatic life criteria. Res. Environ. Sci. 2014, 27, 356–364. [Google Scholar]
- US EPA. National Recommended Water Quality Criteria; Office of Water, Office of Science and Technology: Washington DC, USA, 2009.
- Mountouris, A.; Voutsas, E.; Tassios, D. Bioconcentration of heavy metals in aquatic environments: The importance of bioavailability. Mar. Pollut. Bull. 2002, 44, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Penttinen, S.; Malk, V.; Vaisanen, A.; Penttinen, O.P. Using the critical body residue approach to determine the acute toxicity of cadmium at varying levels of water hardness and dissolved organic carbon concentrations. Ecotoxicol. Environ. Saf. 2011, 74, 1151–1155. [Google Scholar] [CrossRef]
- Li, X.F.; Wang, P.F.; Feng, C.L.; Liu, D.Q.; Chen, J.K.; Wu, F.C. Acute toxicity and hazardous concentrations of zinc to native freshwater organisms under different pH values in China. Bull. Environ. Contam. Toxicol. 2018, 103, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Niyogi, S. Wood CM.Biotic ligand model, a flexible tool for developing site-specific water quality guidelines for metals. Environ. Sci. Technol. 2004, 38, 6177–6192. [Google Scholar] [CrossRef]
- Apte, S.C.; Batley, G.E.; Bowles, K.C.; Brown, N.; Creighton, L.T.; Hales, R.V.; Hyne, M.; Julli, S.J.; Markich, F.P. A comparison of copper speciation measurements with the toxic responses of three sensitive freshwater organisms. Environ. Chem. 2005, 2, 320. [Google Scholar] [CrossRef]
| Pollutant | Measured Concentration (μg L−1) | Fitted Equation | R2 | 96 h-LC50 (μg L−1) | 95% Confidence Intervals (μg L−1) |
|---|---|---|---|---|---|
| Cu | 90, 145, 235, 365, 575 | y = 2.9723x − 1.3217 | 0.9806 | 134 | 106–212 |
| Zn | 105, 160, 250, 370, 585 | y = 4.4423x − 5.4226 | 0.9228 | 222 | 176–296 |
| Pb | 360, 575, 900, 1540, 2440 | y = 2.8342x − 2.6829 | 0.9424 | 514 | 386–874 |
| Cd | 420, 610, 990, 1570, 2425 | y = 3.7899x − 5.8602 | 0.9956 | 734 | 597–1142 |
| Pollutants | Total Number of Species | More Sensitive Warm-Water Fish | Order | Cumulative Probability |
|---|---|---|---|---|
| Cu | 209 | Megalobrama terminalis | 89 | 42.38% |
| Misgurnus mizolepis | 93 | 44.29% | ||
| Gambusia affinis | 96 | 45.17% | ||
| Hypophthalmichthys molitrix | 97 | 46.19% | ||
| Brachymystax lenok | 98 | 46.67% | ||
| Zn | 64 | Brachymystax lenok | 6 | 9.23% |
| Pb | 58 | Cyprinus carpio | 9 | 15.25% |
| Brachymystax lenok | 14 | 23.73% | ||
| Cd | 245 | Oreochromis mossambicus | 31 | 12.60% |
| Cichlasoma facetum | 57 | 23.17% | ||
| Oryzias latipes | 64 | 26.02% | ||
| Fundulus diaphanus | 71 | 28.86% | ||
| Cyprinus carpio | 81 | 32.93% | ||
| Acrossocheilus paradoxus | 85 | 34.35% | ||
| Brachymystax lenok | 122 | 49.59% |
| Pollutant | Order | Total Number of Species | Cumulative Probability |
|---|---|---|---|
| Cu | 32 | 59 | 53.33% |
| Zn | 4 | 37 | 10.53% |
| Pb | 11 | 32 | 33.33% |
| Cd | 38 | 77 | 48.72% |
| Surrogate Metals | Observed Value (μg/L) | Predicted Metals | Observed Value (μg/L) | Predicted Value (μg/L) | Relative Error |
|---|---|---|---|---|---|
| Zn | 222 | Pb | 514 | 665 | 29% |
| Cu | 134 | 17 | 87% | ||
| Cd | 734 | 37 | 95% | ||
| Pb | 514 | Cu | 134 | 57 | 58% |
| Cd | 734 | 193 | 74% | ||
| Cd | 734 | Cu | 134 | 231 | 73% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Wei, C.; Fan, J.; Liu, X.; Hou, Y.; Ling, J.; Wei, J.; Liu, P. Acute Toxicity Assessment and Prediction Models of Four Heavy Metals. Toxics 2023, 11, 346. https://doi.org/10.3390/toxics11040346
Zheng X, Wei C, Fan J, Liu X, Hou Y, Ling J, Wei J, Liu P. Acute Toxicity Assessment and Prediction Models of Four Heavy Metals. Toxics. 2023; 11(4):346. https://doi.org/10.3390/toxics11040346
Chicago/Turabian StyleZheng, Xin, Chao Wei, Juntao Fan, Xinyu Liu, Yin Hou, Jianan Ling, Jian Wei, and Peiyuan Liu. 2023. "Acute Toxicity Assessment and Prediction Models of Four Heavy Metals" Toxics 11, no. 4: 346. https://doi.org/10.3390/toxics11040346
APA StyleZheng, X., Wei, C., Fan, J., Liu, X., Hou, Y., Ling, J., Wei, J., & Liu, P. (2023). Acute Toxicity Assessment and Prediction Models of Four Heavy Metals. Toxics, 11(4), 346. https://doi.org/10.3390/toxics11040346





