A Realistic Mixture of Persistent Organic Pollutants Affects Zebrafish Development, Behavior, and Specifically Eye Formation by Inhibiting the Condensin I Complex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Husbandry and Ethical Considerations
2.2. Chemicals and POP Mixture
2.3. Exposure Tests
2.4. Morphological Observations
2.5. Mitochondrial Toxicity
2.6. Heart Rate
2.7. Behavior
2.8. Injection of Antisense Oligonucleotide Morpholino
2.9. RNA Extraction
2.10. RNAseq
2.11. Data and Statistical Analysis
3. Results and Discussion
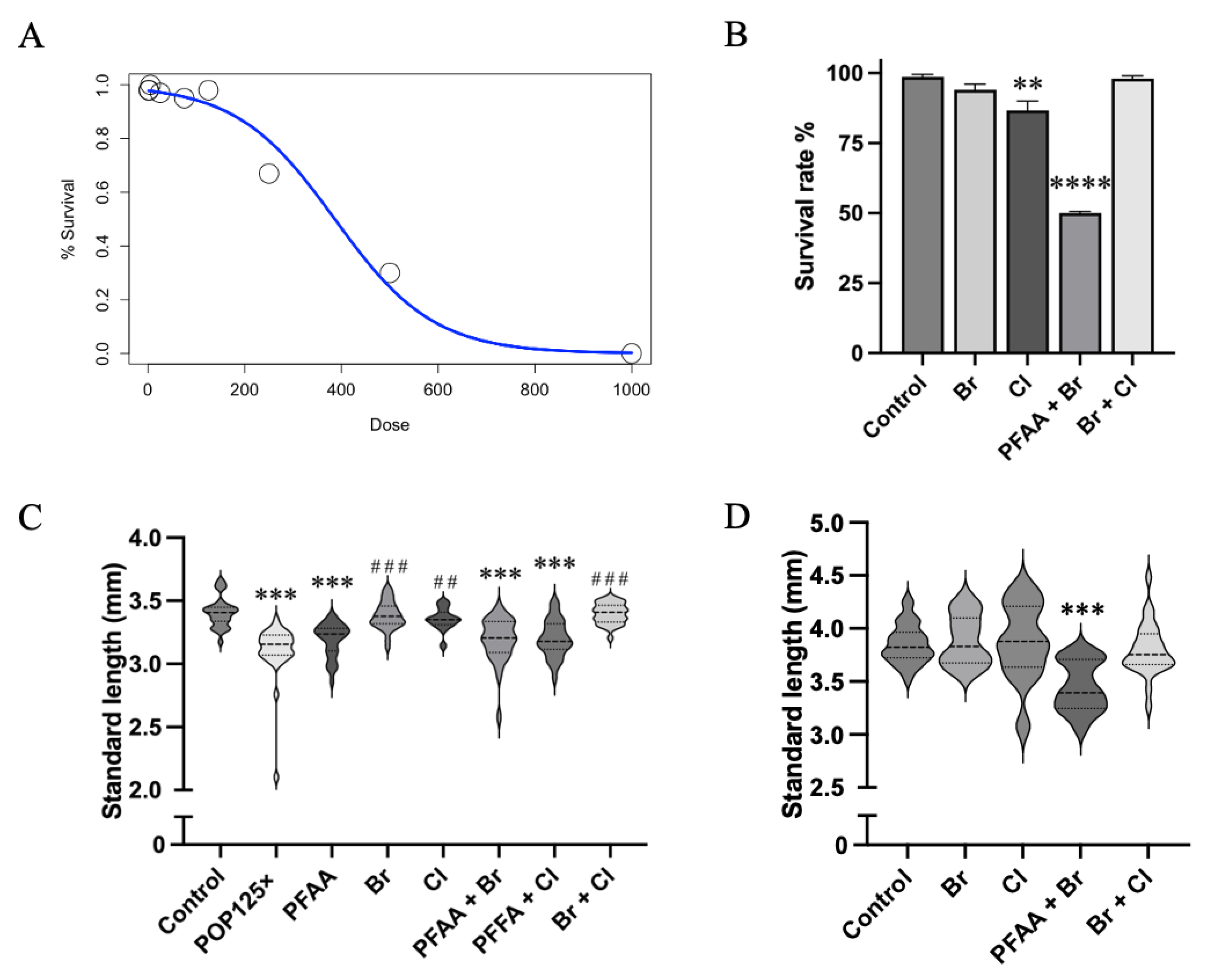
3.1. LC50–Chronic Exposure to Total POP Mix Is Lethal at Relatively High Doses
3.2. The POP Mix Significantly Reduces the Standard Length of Zebrafish Larvae
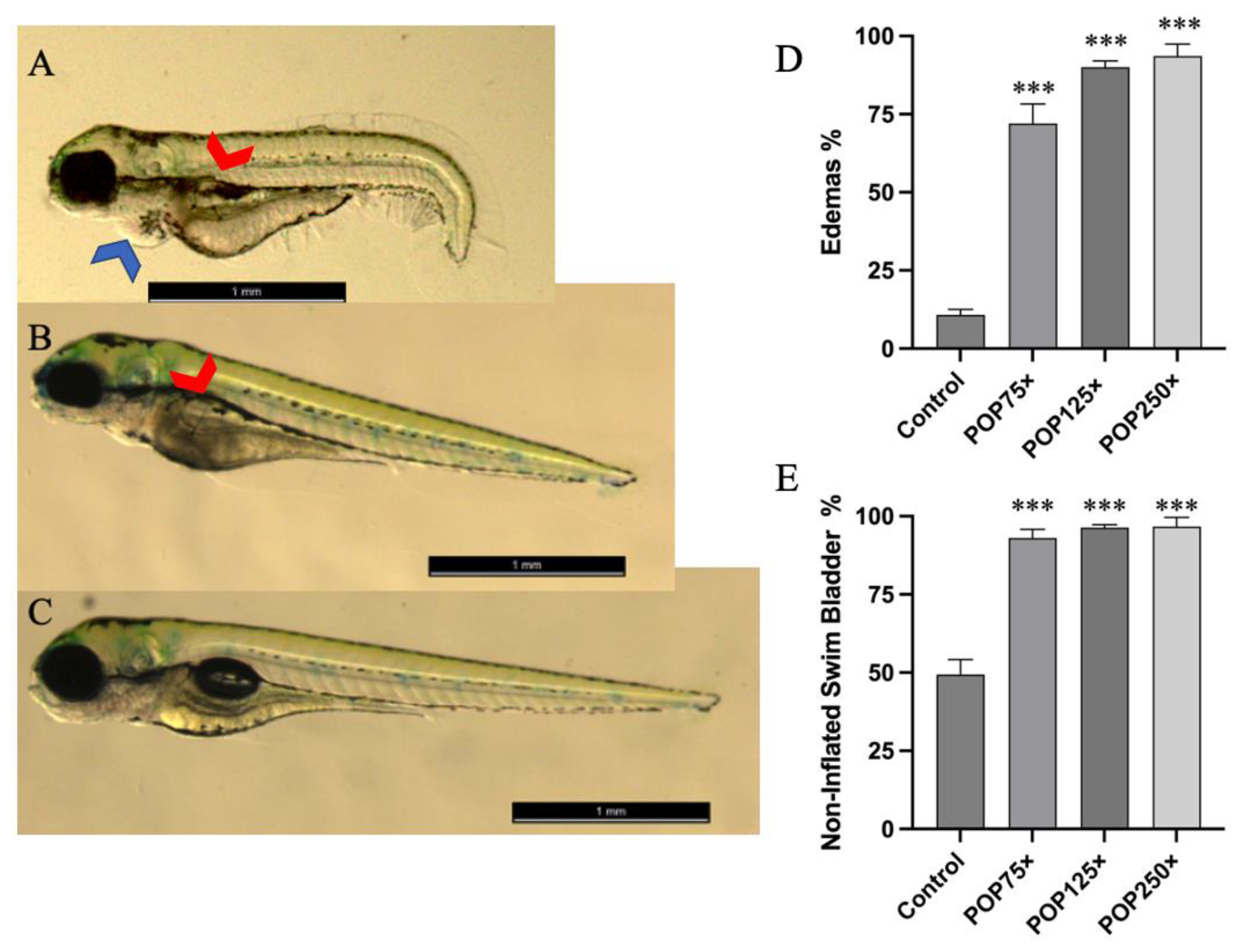
3.3. Common Developmental Toxic Effects Such as Edemas and Non-Inflated Swim Bladder Were Commonly Found following Exposure to POP Mix
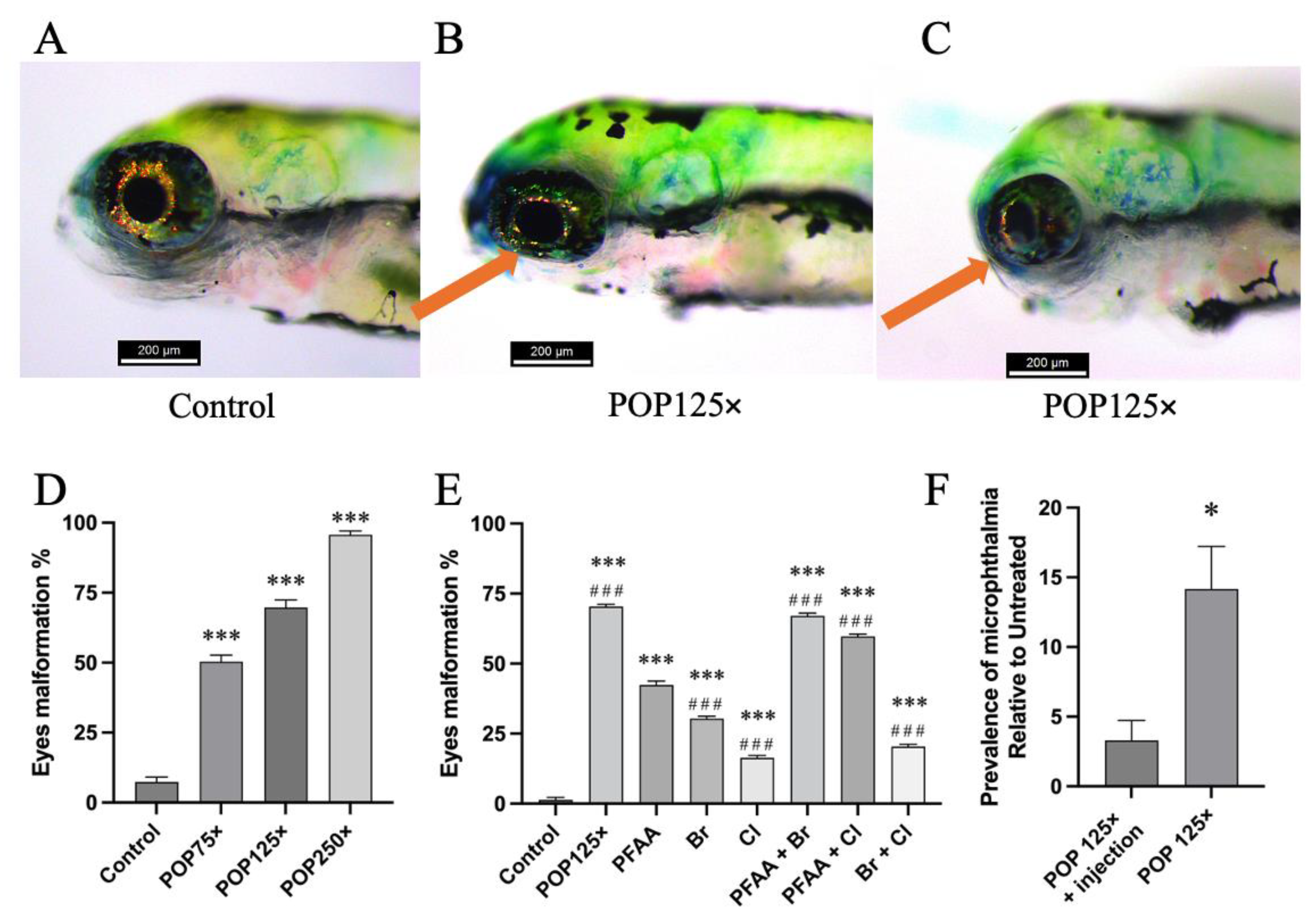
3.4. Striking Eye Malformation in Fish Treated with Any of the POP Mix and Its Sub-Mixes
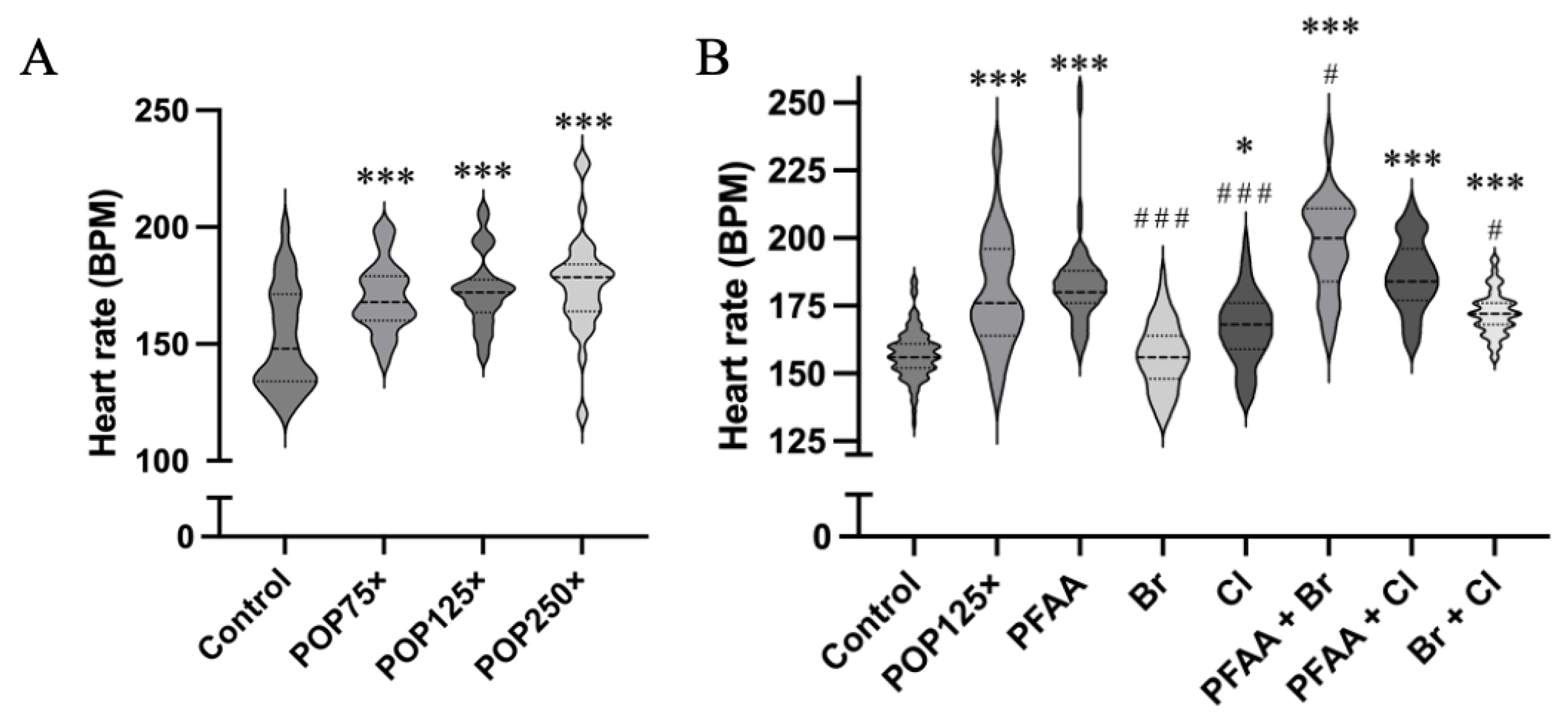
3.5. Heart Rate Is Severely Affected after 96 h of Exposure, Especially When PFAAs Were Present
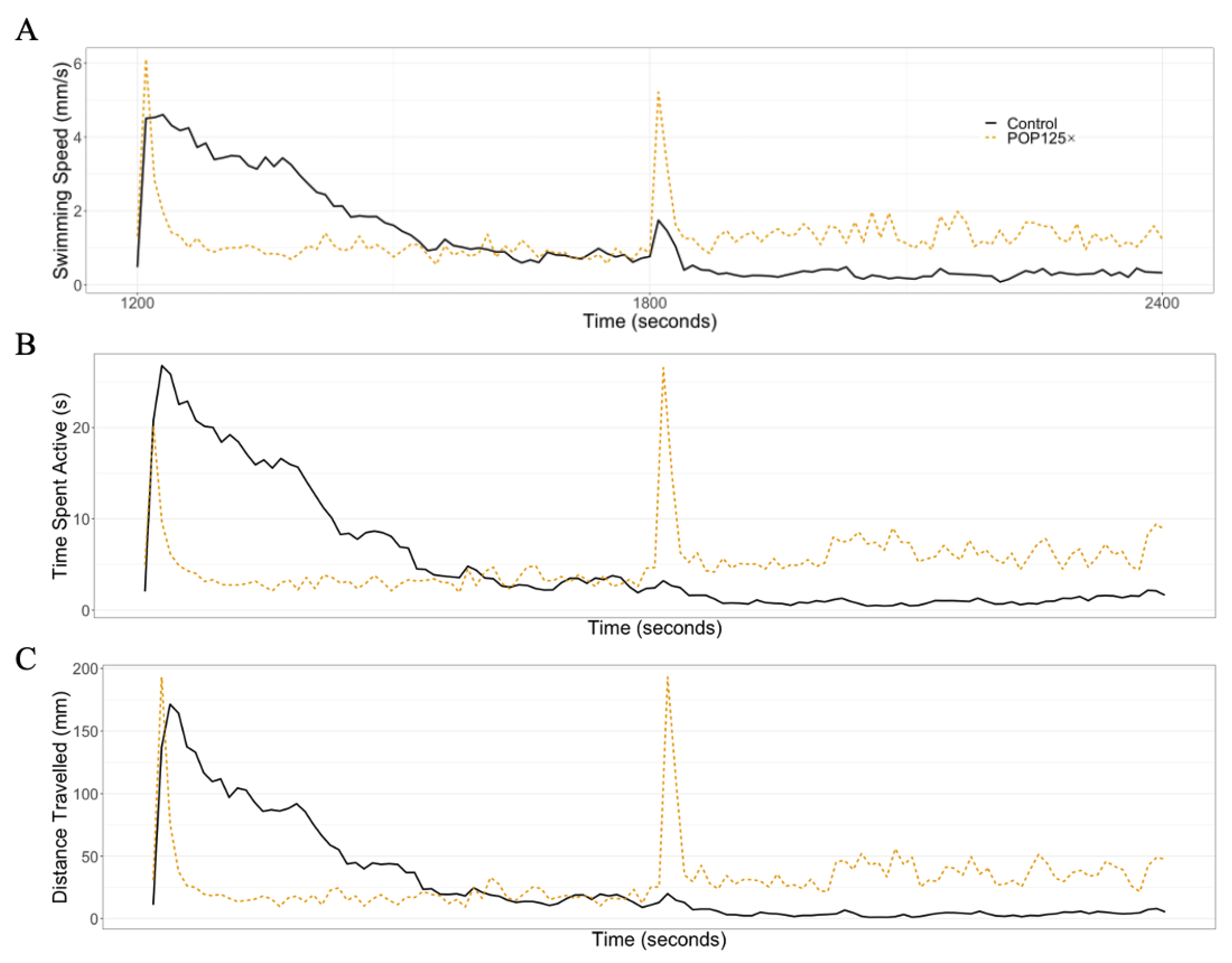
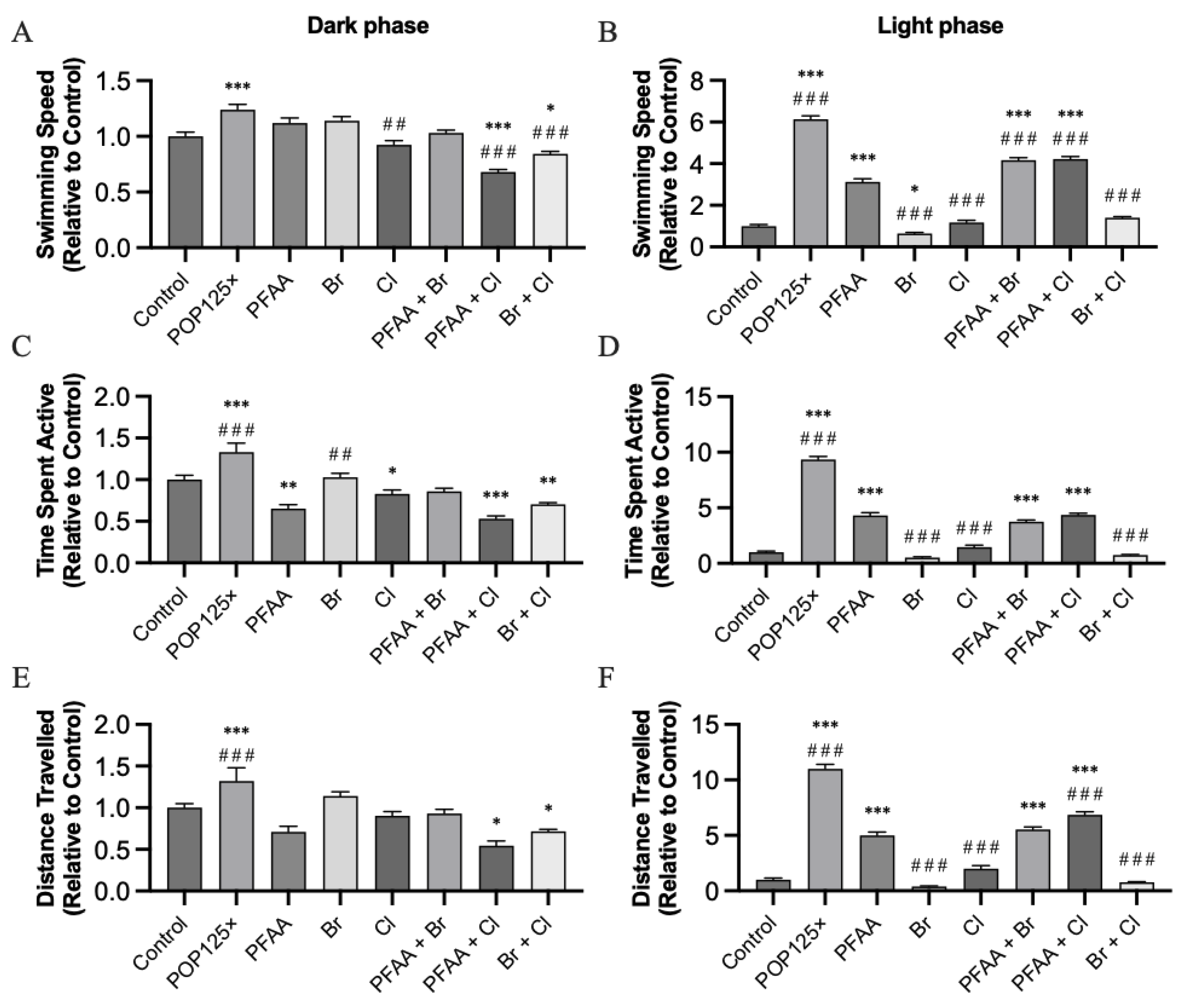
3.6. Fish Were Hyperactive and Responded Notably to Changes in Illumination
3.6.1. Dark–Light Response
3.6.2. Startle Response
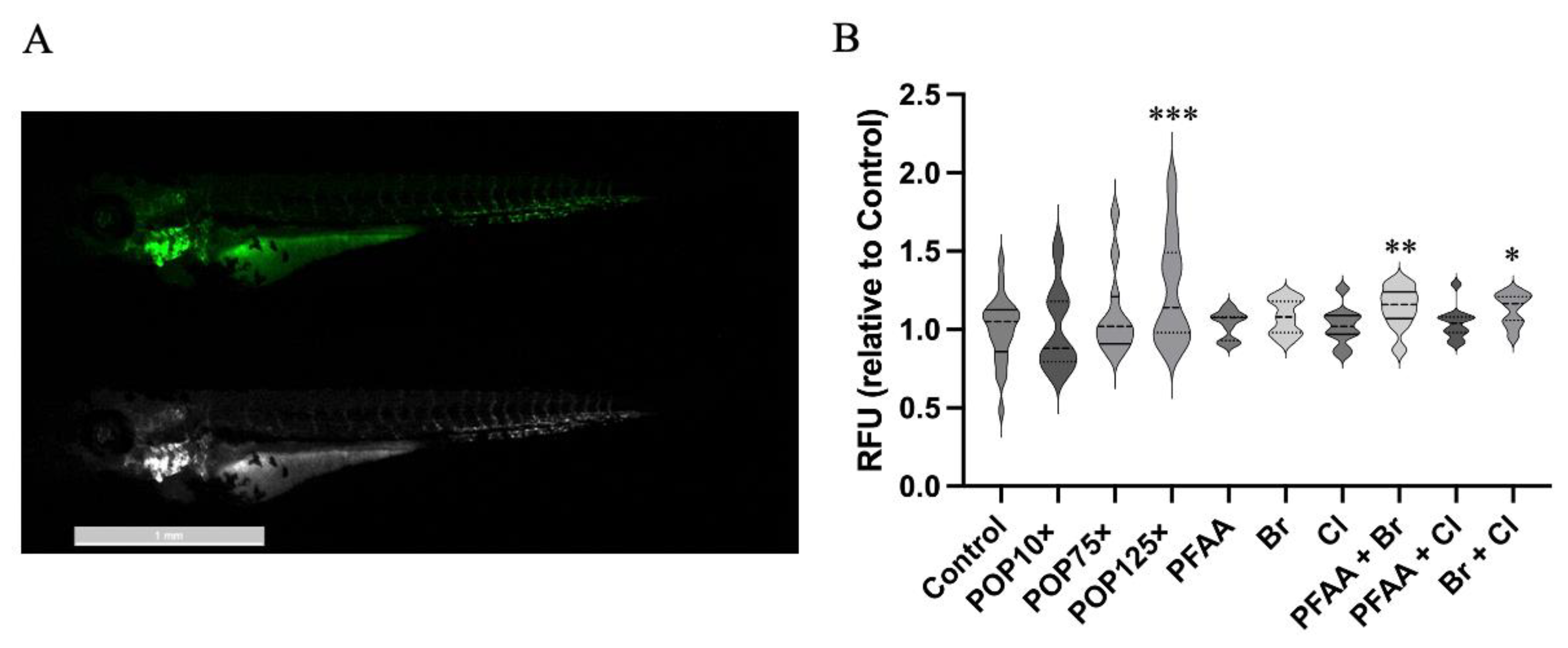
3.7. Mitochondria Responded Notably to POP Mixture
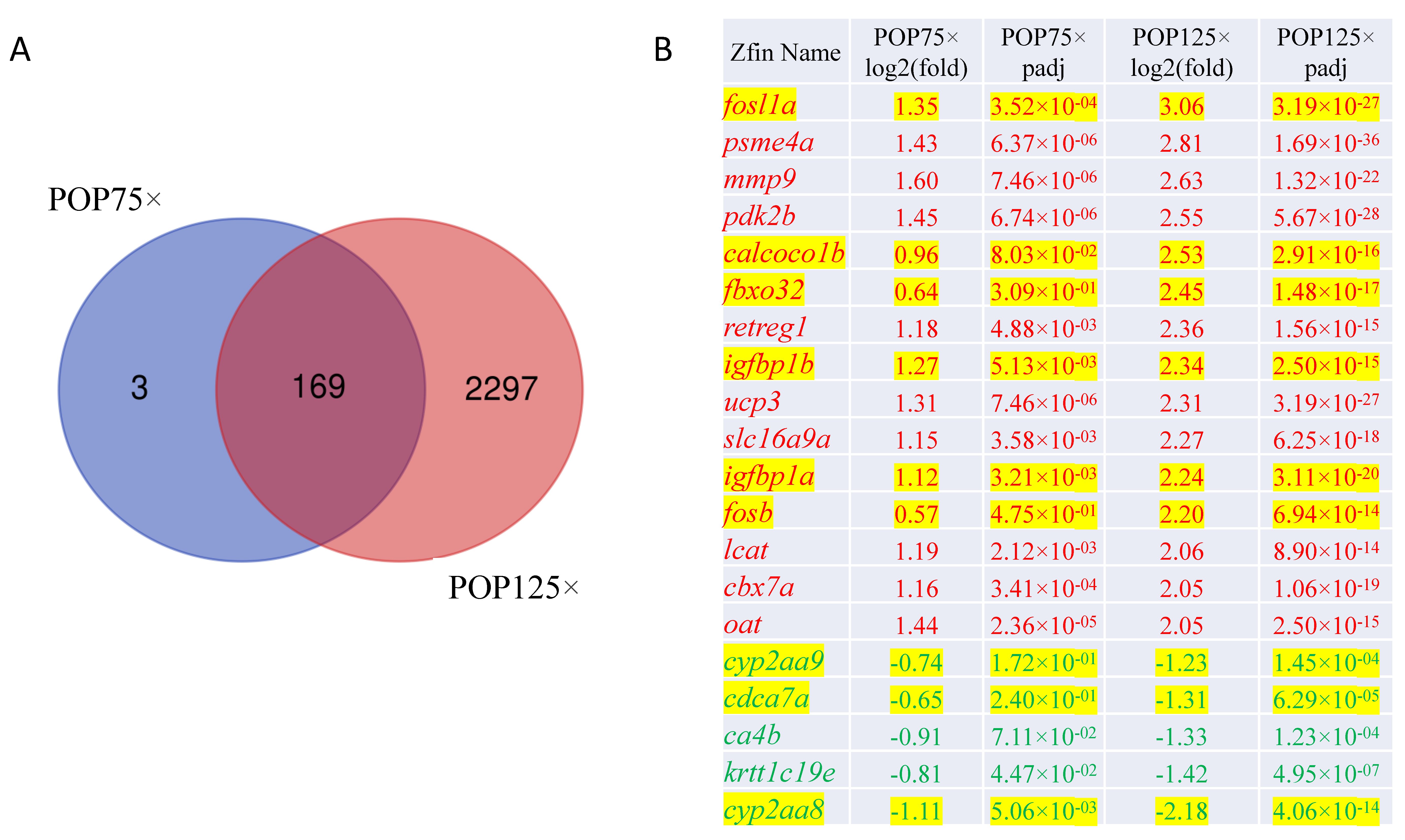
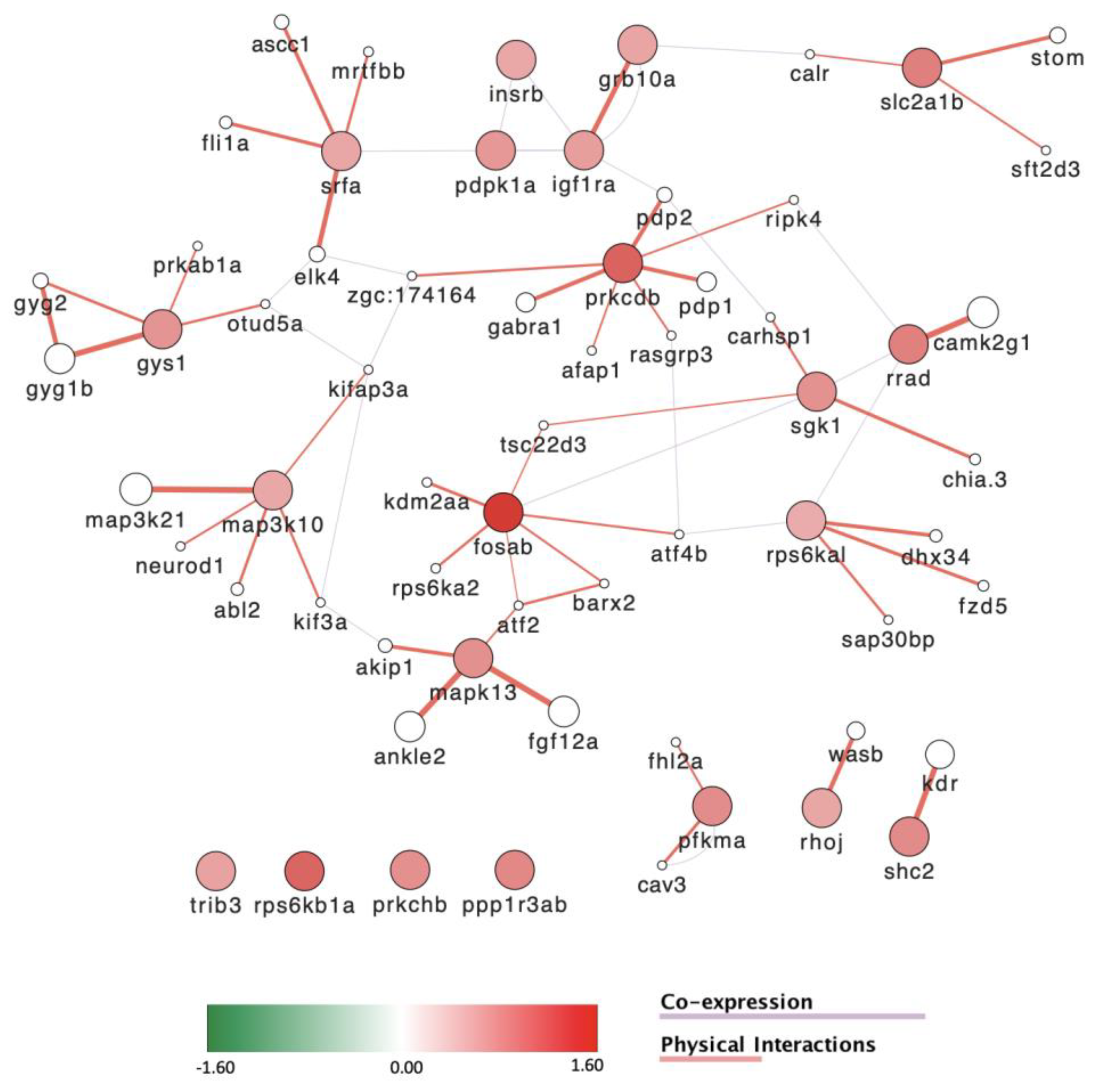
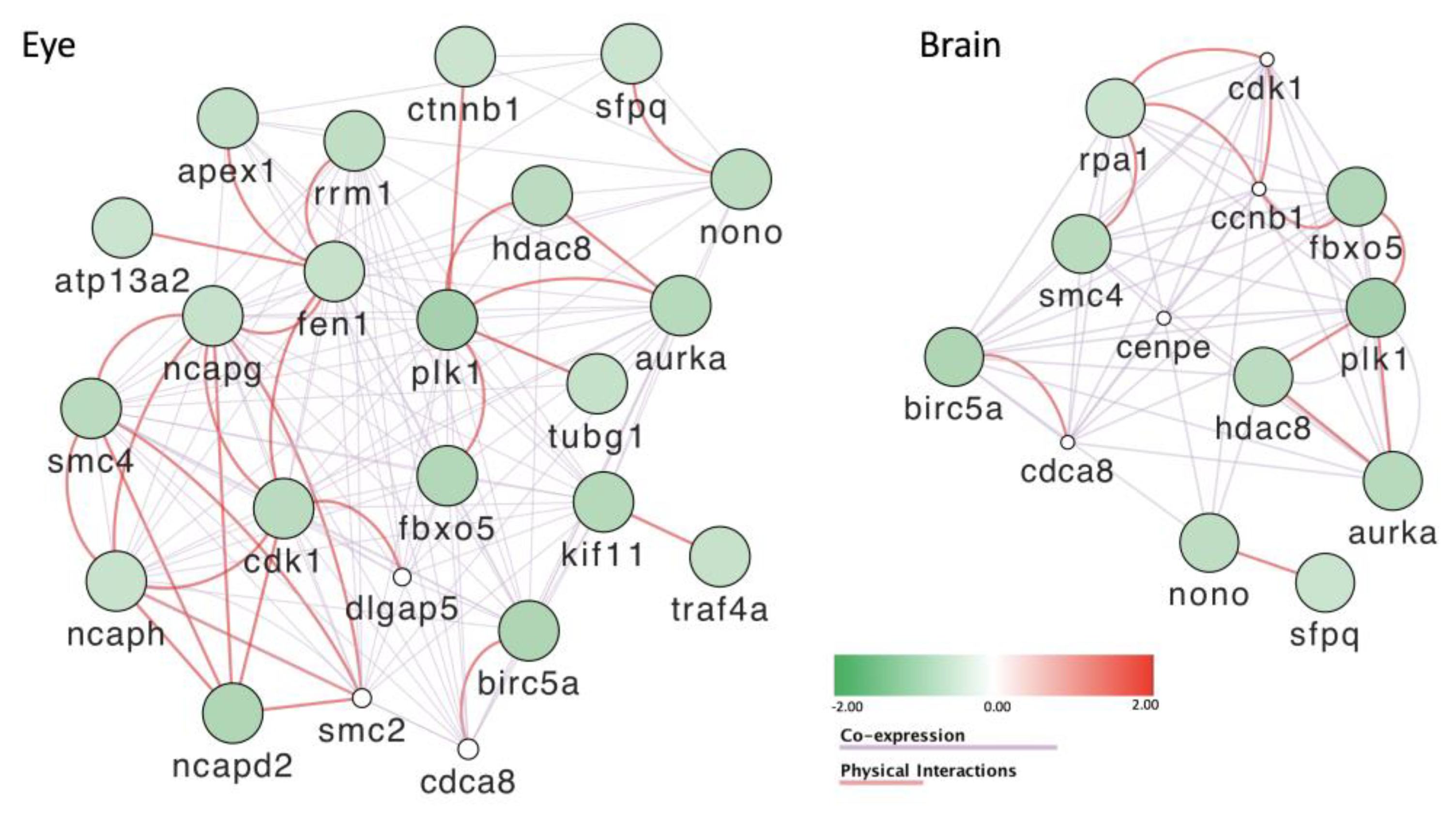
3.8. Gene Expression Is Severely Affected by Exposure to POPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- United Nations. Stockholm Convention on Persistent Organic Pollutants. Treaty Ser. 2006, 2256, 119. [Google Scholar]
- European Parliament and Council. Regulation (EC) No 850/2004 of the European Parliament and of the Council of 29 April 2004 on persistent organic pollutants and amending Directive 79/117/EEC. Off. J. Eur. Union 2004, L158/157–149. [Google Scholar]
- Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Zou, W.; Tong, W.; Hong, H. Persistent Organic Pollutants in Food: Contamination Sources, Health Effects and Detection Methods. Int. J. Environ. Res. Public Health 2019, 16, 4361. [Google Scholar] [CrossRef]
- Gregoraszczuk, E.L.; Ptak, A. Endocrine-Disrupting Chemicals: Some Actions of POPs on Female Reproduction. Int. J. Endocrinol. 2013, 2013, 828532. [Google Scholar] [CrossRef] [PubMed]
- Brody, J.G.; Moysich, K.B.; Humblet, O.; Attfield, K.R.; Beehler, G.P.; Rudel, R.A. Environmental pollutants and breast cancer: Epidemiologic studies. Cancer 2007, 109, 2667–2711. [Google Scholar] [CrossRef]
- Sanderson, J.T. The steroid hormone biosynthesis pathway as a target for endocrine-disrupting chemicals. Toxicol. Sci. 2006, 94, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Ljunggren, S.A.; Helmfrid, I.; Salihovic, S.; van Bavel, B.; Wingren, G.; Lindahl, M.; Karlsson, H. Persistent organic pollutants distribution in lipoprotein fractions in relation to cardiovascular disease and cancer. Environ. Int. 2014, 65, 93–99. [Google Scholar] [CrossRef]
- Van Oostdam, J.C.; Dewailly, E.; Gilman, A.; Hansen, J.C.; Odland, J.O.; Chashchin, V.; Berner, J.; Butler-Walker, J.; Lagerkvist, B.J.; Olafsdottir, K.; et al. Circumpolar maternal blood contaminant survey, 1994–1997 organochlorine compounds. Sci. Total Environ. 2004, 330, 55–70. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Kvalem, H.E.; Thomsen, C.; Froshaug, M.; Haugen, M.; Becher, G.; Alexander, J.; Meltzer, H.M. Dietary exposure to brominated flame retardants correlates with male blood levels in a selected group of Norwegians with a wide range of seafood consumption. Mol. Nutr. Food Res. 2008, 52, 217–227. [Google Scholar] [CrossRef]
- Polder, A.; Skaare, J.U.; Skjerve, E.; Loken, K.B.; Eggesbo, M. Levels of chlorinated pesticides and polychlorinated biphenyls in Norwegian breast milk (2002–2006), and factors that may predict the level of contamination. Sci. Total Environ. 2009, 407, 4584–4590. [Google Scholar] [CrossRef]
- Haug, L.S.; Salihovic, S.; Jogsten, I.E.; Thomsen, C.; van Bavel, B.; Lindstrom, G.; Becher, G. Levels in food and beverages and daily intake of perfluorinated compounds in Norway. Chemosphere 2010, 80, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Berntsen, H.F.; Berg, V.; Thomsen, C.; Ropstad, E.; Zimmer, K.E. The design of an environmentally relevant mixture of persistent organic pollutants for use in in vivo and in vitro studies. J. Toxicol. Environ. Health Part A 2017, 80, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- McComb, J.; Mills, I.G.; Muller, M.; Berntsen, H.F.; Zimmer, K.E.; Ropstad, E.; Verhaegen, S.; Connolly, L. Human blood-based exposure levels of persistent organic pollutant (POP) mixtures antagonise androgen receptor transactivation and translocation. Environ. Int. 2019, 132, 105083. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.Q.; Berntsen, H.F.; Verhaegen, S.; Ropstad, E.; Connolly, L.; Igout, A.; Muller, M.; Scippo, M.L. A mixture of persistent organic pollutants relevant for human exposure inhibits the transactivation activity of the aryl hydrocarbon receptor in vitro. Environ. Pollut. 2019, 254, 113098. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Amber, M.; Zosen, D.; Labba, N.A.; Huiberts, E.H.W.; Samulin Erdem, J.; Haugen, F.; Berntsen, H.F.; Zienolddiny, S.; Paulsen, R.E.; et al. A human relevant mixture of persistent organic pollutants (POPs) and perfluorooctane sulfonic acid (PFOS) enhance nerve growth factor (NGF)-induced neurite outgrowth in PC12 cells. Toxicol. Lett. 2021, 338, 85–96. [Google Scholar] [CrossRef]
- Amber, M.; Xie, Y.; Berntsen, H.F.; Zimmer, K.E.; Ropstad, E.; Verhaegen, S.; Connolly, L. Effects of Defined Mixtures of Persistent Organic Pollutants (POPs) on Pre-lethal Cytotoxicity in the Human A-498 Kidney Cell Line In Vitro. Expo. Health 2021, 13, 465–475. [Google Scholar] [CrossRef]
- Segner, H. Zebrafish (Danio rerio) as a model organism for investigating endocrine disruption. Comp. Biochem. Physiol. C Toxicol. Pharm. 2009, 149, 187–195. [Google Scholar] [CrossRef]
- Herkenne, S.; Ek, O.; Zamberlan, M.; Pellattiero, A.; Chergova, M.; Chivite, I.; Novotna, E.; Rigoni, G.; Fonseca, T.B.; Samardzic, D.; et al. Developmental and Tumor Angiogenesis Requires the Mitochondria-Shaping Protein Opa1. Cell Metab. 2020, 31, 987–1003.e1008. [Google Scholar] [CrossRef]
- Lammer, E.; Carr, G.J.; Wendler, K.; Rawlings, J.M.; Belanger, S.E.; Braunbeck, T. Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 149, 196–209. [Google Scholar] [CrossRef]
- Westerfield, M. A guide for the laboratory use of zebrafish (Danio rerio). In The Zebrafish Book, 5th ed.; University of Oregon Press: Eugene, OR, USA, 2007. [Google Scholar]
- Pruvot, B.; Quiroz, Y.; Voncken, A.; Jeanray, N.; Piot, A.; Martial, J.A.; Muller, M. A panel of biological tests reveals developmental effects of pharmaceutical pollutants on late stage zebrafish embryos. Reprod. Toxicol. 2012, 34, 568–583. [Google Scholar] [CrossRef]
- Philip, A.M.; Wang, Y.; Mauro, A.; El-Rass, S.; Marshall, J.C.; Lee, W.L.; Slutsky, A.S.; dosSantos, C.C.; Wen, X.Y. Development of a zebrafish sepsis model for high-throughput drug discovery. Mol. Med. 2017, 23, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Dalcq, J.; Pasque, V.; Ghaye, A.; Larbuisson, A.; Motte, P.; Martial, J.A.; Muller, M. Runx3, Egr1 and Sox9b form a regulatory cascade required to modulate BMP-signaling during cranial cartilage development in zebrafish. PLoS ONE 2012, 7, e50140. [Google Scholar] [CrossRef]
- Larbuisson, A.; Dalcq, J.; Martial, J.A.; Muller, M. Fgf receptors Fgfr1a and Fgfr2 control the function of pharyngeal endoderm in late cranial cartilage development. Differentiation 2013, 86, 192–206. [Google Scholar] [CrossRef]
- Ewels, P.A.; Peltzer, A.; Fillinger, S.; Patel, H.; Alneberg, J.; Wilm, A.; Garcia, M.U.; Di Tommaso, P.; Nahnsen, S. The nf-core framework for community-curated bioinformatics pipelines. Nat. Biotechnol. 2020, 38, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M. KEGG mapping tools for uncovering hidden features in biological data. Protein Sci. 2022, 31, 47–53. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing (4.0.2); R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.r-project.org/index.html (accessed on 9 March 2023).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Pinheiro, J.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; SAGE Publications Inc.: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 9 March 2023).
- Lenth, R.V. Emmeans: Estimated Marginal Means, aka Least-Squares Means. In R Package Version 1.7.5; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://CRAN.R-project.org/package=emmeans (accessed on 9 March 2023).
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Pelka, K.E.; Henn, K.; Keck, A.; Sapel, B.; Braunbeck, T. Size does matter–Determination of the critical molecular size for the uptake of chemicals across the chorion of zebrafish (Danio rerio) embryos. Aquat. Toxicol. 2017, 185, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khezri, A.; Fraser, T.W.; Nourizadeh-Lillabadi, R.; Kamstra, J.H.; Berg, V.; Zimmer, K.E.; Ropstad, E. A Mixture of Persistent Organic Pollutants and Perfluorooctanesulfonic Acid Induces Similar Behavioural Responses, but Different Gene Expression Profiles in Zebrafish Larvae. Int. J. Mol. Sci. 2017, 18, 291. [Google Scholar] [CrossRef] [PubMed]
- Christou, M.; Fraser, T.W.K.; Berg, V.; Ropstad, E.; Kamstra, J.H. Calcium signaling as a possible mechanism behind increased locomotor response in zebrafish larvae exposed to a human relevant persistent organic pollutant mixture or PFOS. Environ. Res. 2020, 187, 109702. [Google Scholar] [CrossRef]
- Fox, K.; Zauke, G.P.; Butte, W. Kinetics of bioconcentration and clearance of 28 polychlorinated biphenyl congeners in zebrafish (Brachydanio rerio). Ecotoxicol. Env. Saf. 1994, 28, 99–109. [Google Scholar] [CrossRef] [PubMed]
- US-EPA. Emerging Contaminants Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA). In Emerging Contaminants Fact Sheet; Report 505F14001; 2014. Available online: https://nepis.epa.gov (accessed on 8 April 2023).
- De Silva, A.O.; Armitage, J.M.; Bruton, T.A.; Dassuncao, C.; Heiger-Bernays, W.; Hu, X.C.; Karrman, A.; Kelly, B.; Ng, C.; Robuck, A.; et al. PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding. Environ. Toxicol. Chem. 2021, 40, 631–657. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Sinclair, G.M.; Long, S.M.; Jones, O.A.H. What are the effects of PFAS exposure at environmentally relevant concentrations? Chemosphere 2020, 258, 127340. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Ye, X.; Calafat, A.M. PFASs in the General Population. In Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances; De Witt, J.C., Ed.; Series Molecular and Integrative Toxicology; Nunes, C., Ed. Humana Press: Totowa, NJ, USA, 2015; pp. 51–76. [Google Scholar] [CrossRef]
- Wasel, O.; Thompson, K.M.; Freeman, J.L. Assessment of unique behavioral, morphological, and molecular alterations in the comparative developmental toxicity profiles of PFOA, PFHxA, and PFBA using the zebrafish model system. Environ. Int. 2022, 170, 107642. [Google Scholar] [CrossRef]
- Jantzen, C.E.; Annunziato, K.A.; Bugel, S.M.; Cooper, K.R. PFOS, PFNA, and PFOA sub-lethal exposure to embryonic zebrafish have different toxicity profiles in terms of morphometrics, behavior and gene expression. Aquat. Toxicol. 2016, 175, 160–170. [Google Scholar] [CrossRef]
- Usenko, C.Y.; Robinson, E.M.; Usenko, S.; Brooks, B.W.; Bruce, E.D. PBDE developmental effects on embryonic zebrafish. Environ. Toxicol. Chem. 2011, 30, 1865–1872. [Google Scholar] [CrossRef]
- Singleman, C.; Zimmerman, A.; Harrison, E.; Roy, N.K.; Wirgin, I.; Holtzman, N.G. Toxic Effects of Polychlorinated Biphenyl Congeners and Aroclors on Embryonic Growth and Development. Environ. Toxicol. Chem. 2021, 40, 187–201. [Google Scholar] [CrossRef]
- OECD Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Publishing: Paris, France, 2013.
- Abe, F.R.; de Oliveira, A.A.S.; Marino, R.V.; Rialto, T.C.R.; Oliveira, D.P.; Dorta, D.J. A comparison of developmental toxicity of brominated and halogen-free flame retardant on zebrafish. Ecotoxicol. Environ. Saf. 2021, 208, 111745. [Google Scholar] [CrossRef]
- Godfrey, A.; Hooser, B.; Abdelmoneim, A.; Horzmann, K.A.; Freemanc, J.L.; Sepulveda, M.S. Thyroid disrupting effects of halogenated and next generation chemicals on the swim bladder development of zebrafish. Aquat. Toxicol. 2017, 193, 228–235. [Google Scholar] [CrossRef]
- Raldua, D.; Babin, P.J. Simple, rapid zebrafish larva bioassay for assessing the potential of chemical pollutants and drugs to disrupt thyroid gland function. Environ. Sci. Technol. 2009, 43, 6844–6850. [Google Scholar] [CrossRef] [PubMed]
- Loosli, F.; Staub, W.; Finger-Baier, K.C.; Ober, E.A.; Verkade, H.; Wittbrodt, J.; Baier, H. Loss of eyes in zebrafish caused by mutation of chokh/rx3. EMBO Rep. 2003, 4, 894–899. [Google Scholar] [CrossRef]
- Pavlou, S.; Astell, K.; Kasioulis, I.; Gakovic, M.; Baldock, R.; van Heyningen, V.; Coutinho, P. Pleiotropic effects of Sox2 during the development of the zebrafish epithalamus. PLoS ONE 2014, 9, e87546. [Google Scholar] [CrossRef]
- Chassaing, N.; Sorrentino, S.; Davis, E.E.; Martin-Coignard, D.; Iacovelli, A.; Paznekas, W.; Webb, B.D.; Faye-Petersen, O.; Encha-Razavi, F.; Lequeux, L.; et al. OTX2 mutations contribute to the otocephaly-dysgnathia complex. J. Med. Genet. 2012, 49, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, M.; Stegmaier, J.; Kobitski, A.Y.; Schott, B.; Weger, B.D.; Margariti, D.; Cereceda Delgado, A.R.; Gourain, V.; Scherr, T.; Yang, L.; et al. Pax6 organizes the anterior eye segment by guiding two distinct neural crest waves. PLoS Genet. 2020, 16, e1008774. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ptak, D.; Zhang, L.; Walls, E.K.; Zhong, W.; Leung, Y.F. Phenylthiourea specifically reduces zebrafish eye size. PLoS ONE 2012, 7, e40132. [Google Scholar] [CrossRef]
- Kim, K.T.; Zaikova, T.; Hutchison, J.E.; Tanguay, R.L. Gold nanoparticles disrupt zebrafish eye development and pigmentation. Toxicol. Sci. 2013, 133, 275–288. [Google Scholar] [CrossRef]
- Barbagallo, S.; Baldauf, C.; Orosco, E.; Roy, N.M. Di-butyl phthalate (DBP) induces defects during embryonic eye development in zebrafish. Ecotoxicology 2022, 31, 178–185. [Google Scholar] [CrossRef]
- Wang, Y.P.; Hong, Q.; Qin, D.N.; Kou, C.Z.; Zhang, C.M.; Guo, M.; Guo, X.R.; Chi, X.; Tong, M.L. Effects of embryonic exposure to polychlorinated biphenyls on zebrafish (Danio rerio) retinal development. J. Appl. Toxicol. 2012, 32, 186–193. [Google Scholar] [CrossRef]
- Li, M.; Yang, T.; Gao, L.; Xu, H. An inadvertent issue of human retina exposure to endocrine disrupting chemicals: A safety assessment. Chemosphere 2021, 264, 128484. [Google Scholar] [CrossRef] [PubMed]
- Seipold, S.; Priller, F.C.; Goldsmith, P.; Harris, W.A.; Baier, H.; Abdelilah-Seyfried, S. Non-SMC condensin I complex proteins control chromosome segregation and survival of proliferating cells in the zebrafish neural retina. BMC Dev. Biol. 2009, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Hagenaars, A.; Vergauwen, L.; De Coen, W.; Knapen, D. Structure-activity relationship assessment of four perfluorinated chemicals using a prolonged zebrafish early life stage test. Chemosphere 2011, 82, 764–772. [Google Scholar] [CrossRef]
- Gong, H.; Du, J.; Xu, J.; Yang, Y.; Lu, H.; Xiao, H. Perfluorononanoate and Perfluorobutane Sulfonate Induce Cardiotoxic Effects in Zebrafish. Environ. Toxicol. Chem. 2022, 41, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Singleman, C.; Holtzman, N.G. PCB and TCDD derived embryonic cardiac defects result from a novel AhR pathway. Aquat. Toxicol. 2021, 233, 105794. [Google Scholar] [CrossRef]
- Everett, C.J.; Mainous, A.G., 3rd; Frithsen, I.L.; Player, M.S.; Matheson, E.M. Association of polychlorinated biphenyls with hypertension in the 1999-2002 National Health and Nutrition Examination Survey. Environ. Res. 2008, 108, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zuo, Z.; Li, B.; Huang, L.; Chen, M.; Wang, C. Effects of low-level hexabromocyclododecane (HBCD) exposure on cardiac development in zebrafish embryos. Ecotoxicology 2013, 22, 1200–1207. [Google Scholar] [CrossRef]
- El-Nahhal, Y.; El-Nahhal, I. Cardiotoxicity of some pesticides and their amelioration. Environ. Sci. Pollut. Res. Int. 2021, 28, 44726–44754. [Google Scholar] [CrossRef]
- Loerracher, A.K.; Braunbeck, T. Cytochrome P450-dependent biotransformation capacities in embryonic, juvenile and adult stages of zebrafish (Danio rerio)-a state-of-the-art review. Arch. Toxicol. 2021, 95, 2299–2334. [Google Scholar] [CrossRef]
- Schmidt, K.; Steinberg, C.E.W.; Pflugmacher, S.; Staaks, G.B.O. Xenobiotic substances such as PCB mixtures (Aroclor 1254) and TBT can influence swimming behavior and biotransformation activity (GST) of carp (Cyprinus carpio). Environ. Toxicol. 2004, 19, 460–470. [Google Scholar] [CrossRef]
- Schmidt, K.; Staaks, G.B.O.; Pflugmacher, S.; Steinberg, C.E.W. Impact of PCB mixture (Aroclor 1254) and TBT and a mixture of both on swimming behavior, body growth and enzymatic biotransformation activities (GST) of young carp (Cyprinus carpio). Aquat. Toxicol. 2005, 71, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-T.; Hsiao, Y.-C.; Ko, F.-C.; Cheng, J.-O.; Cheng, Y.-M.; Chen, T.-H. Chronic exposure of 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47) alters locomotion behavior in juvenile zebrafish (Danio rerio). Aquat. Toxicol. 2010, 98, 388–395. [Google Scholar] [CrossRef]
- Haimbaugh, A.; Wu, C.C.; Akemann, C.; Meyer, D.N.; Connell, M.; Abdi, M.; Khalaf, A.; Johnson, D.; Baker, T.R. Multi- and Transgenerational Effects of Developmental Exposure to Environmental Levels of PFAS and PFAS Mixture in Zebrafish (Danio rerio). Toxics 2022, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Fraser, T.W.K.; Khezri, A.; Lewandowska-Sabat, A.M.; Henry, T.; Ropstad, E. Endocrine disruptors affect larval zebrafish behavior: Testing potential mechanisms and comparisons of behavioral sensitivity to alternative biomarkers. Aquat. Toxicol. 2017, 193, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Yaghoobi, B.; Miller, G.W.; Holland, E.B.; Li, X.; Harvey, D.; Li, S.; Lehmler, H.J.; Pessah, I.N.; Lein, P.J. Ryanodine receptor-active non-dioxin-like polychlorinated biphenyls cause neurobehavioral deficits in larval zebrafish. Front. Toxicol. 2022, 4, 947795. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ko, E.; Lee, H.; Kim, K.T.; Choi, M.; Shin, S. Mixed Exposure of Persistent Organic Pollutants Alters Oxidative Stress Markers and Mitochondrial Function in the Tail of Zebrafish Depending on Sex. Int. J. Environ. Res. Public Health 2021, 18, 9539. [Google Scholar] [CrossRef]
- Ko, E.; Kim, D.; Kim, K.; Choi, M.; Shin, S. The action of low doses of persistent organic pollutants (POPs) on mitochondrial function in zebrafish eyes and comparison with hyperglycemia to identify a link between POPs and diabetes. Toxicol. Mech. Methods 2020, 30, 275–283. [Google Scholar] [CrossRef]
- Jochum, W.; Passegue, E.; Wagner, E.F. AP-1 in mouse development and tumorigenesis. Oncogene 2001, 20, 2401–2412. [Google Scholar] [CrossRef]
- Knuth, M.M.; Mahapatra, D.; Jima, D.; Wan, D.; Hammock, B.D.; Law, M.; Kullman, S.W. Vitamin D deficiency serves as a precursor to stunted growth and central adiposity in zebrafish. Sci. Rep. 2020, 10, 16032. [Google Scholar] [CrossRef]
- Aceto, J.; Nourizadeh-Lillabadi, R.; Maree, R.; Dardenne, N.; Jeanray, N.; Wehenkel, L.; Alestrom, P.; van Loon, J.J.; Muller, M. Zebrafish bone and general physiology are differently affected by hormones or changes in gravity. PLoS ONE 2015, 10, e0126928. [Google Scholar] [CrossRef]
- Su, T.; Ding, X. Regulation of the cytochrome P450 2A genes. Toxicol. Appl. Pharmacol. 2004, 199, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, J.V.; McArthur, A.G.; Kubota, A.; Zanette, J.; Parente, T.; Jonsson, M.E.; Nelson, D.R.; Stegeman, J.J. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genom. 2010, 11, 643. [Google Scholar] [CrossRef] [PubMed]
- Kubota, A.; Bainy, A.C.; Woodin, B.R.; Goldstone, J.V.; Stegeman, J.J. The cytochrome P450 2AA gene cluster in zebrafish (Danio rerio): Expression of CYP2AA1 and CYP2AA2 and response to phenobarbital-type inducers. Toxicol. Appl. Pharm. 2013, 272, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Hartig, E.I.; Zhu, S.; King, B.L.; Coffman, J.A. Cortisol-treated zebrafish embryos develop into pro-inflammatory adults with aberrant immune gene regulation. Biol. Open 2016, 5, 1134–1141. [Google Scholar] [CrossRef]
- Delcourt, N.; Quevedo, C.; Nonne, C.; Fons, P.; O’Brien, D.; Loyaux, D.; Diez, M.; Autelitano, F.; Guillemot, J.C.; Ferrara, P.; et al. Targeted identification of sialoglycoproteins in hypoxic endothelial cells and validation in zebrafish reveal roles for proteins in angiogenesis. J. Biol. Chem. 2015, 290, 3405–3417. [Google Scholar] [CrossRef]
- Hao, R.; Bondesson, M.; Singh, A.V.; Riu, A.; McCollum, C.W.; Knudsen, T.B.; Gorelick, D.A.; Gustafsson, J.A. Identification of estrogen target genes during zebrafish embryonic development through transcriptomic analysis. PLoS ONE 2013, 8, e79020. [Google Scholar] [CrossRef]
- Haggard, D.E.; Noyes, P.D.; Waters, K.M.; Tanguay, R.L. Transcriptomic and phenotypic profiling in developing zebrafish exposed to thyroid hormone receptor agonists. Reprod. Toxicol. 2018, 77, 80–93. [Google Scholar] [CrossRef]
- European Chemical Agency. Per- and Polyfluoroalkyl Substances (PFASs). 2023. Available online: https://echa.europa.eu/hot-topics/perfluoroalkyl-chemicals-pfas (accessed on 3 March 2023).
- US-EPA. Per- and Polyfluoroalkyl Substances (PFAS). 2023. Available online: https://www.epa.gov/pfas (accessed on 6 March 2023).
- Focus features. Dark Waters, YouTube 2019. Available online: https://www.youtube.com/watch?v=RvAOuhyunhY (accessed on 6 March 2023).
- Rich, N. The Lawyer Who Became DuPont’s Worst Nightmare. The New York Times. 2016. Available online: https://www.nytimes.com/2016/2001/2010/magazine/the-lawyer-who-became-duponts-worst-nightmare.html (accessed on 16 March 2023).
- Salvidge, R.; Hosea, L. What Are PFAS, How Toxic Are They and How Do You Become Exposed? Everything You Need to Know about ‘Forever Chemicals’ Detected in Air, Water, Soils, Sediments and Rain. The Guardian. 2023. Available online: https://www.theguardian.com/environment/2023/feb/2023/what-are-pfas-forever-chemicals-how-toxic-are-they-and-how-do-you-become-exposed (accessed on 16 March 2023).
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Limón, G.; Nivelle, R.; Bich-Ngoc, N.; Duy-Thanh, D.; Muller, M. A Realistic Mixture of Persistent Organic Pollutants Affects Zebrafish Development, Behavior, and Specifically Eye Formation by Inhibiting the Condensin I Complex. Toxics 2023, 11, 357. https://doi.org/10.3390/toxics11040357
Guerrero-Limón G, Nivelle R, Bich-Ngoc N, Duy-Thanh D, Muller M. A Realistic Mixture of Persistent Organic Pollutants Affects Zebrafish Development, Behavior, and Specifically Eye Formation by Inhibiting the Condensin I Complex. Toxics. 2023; 11(4):357. https://doi.org/10.3390/toxics11040357
Chicago/Turabian StyleGuerrero-Limón, Gustavo, Renaud Nivelle, Nguyen Bich-Ngoc, Dinh Duy-Thanh, and Marc Muller. 2023. "A Realistic Mixture of Persistent Organic Pollutants Affects Zebrafish Development, Behavior, and Specifically Eye Formation by Inhibiting the Condensin I Complex" Toxics 11, no. 4: 357. https://doi.org/10.3390/toxics11040357
APA StyleGuerrero-Limón, G., Nivelle, R., Bich-Ngoc, N., Duy-Thanh, D., & Muller, M. (2023). A Realistic Mixture of Persistent Organic Pollutants Affects Zebrafish Development, Behavior, and Specifically Eye Formation by Inhibiting the Condensin I Complex. Toxics, 11(4), 357. https://doi.org/10.3390/toxics11040357








