Nrf2: A Main Responsive Element of the Toxicity Effect Caused by Trichothecene (T-2) Mycotoxin
Abstract
:1. Introduction
2. Toxicity Mechanism of T-2 Toxin
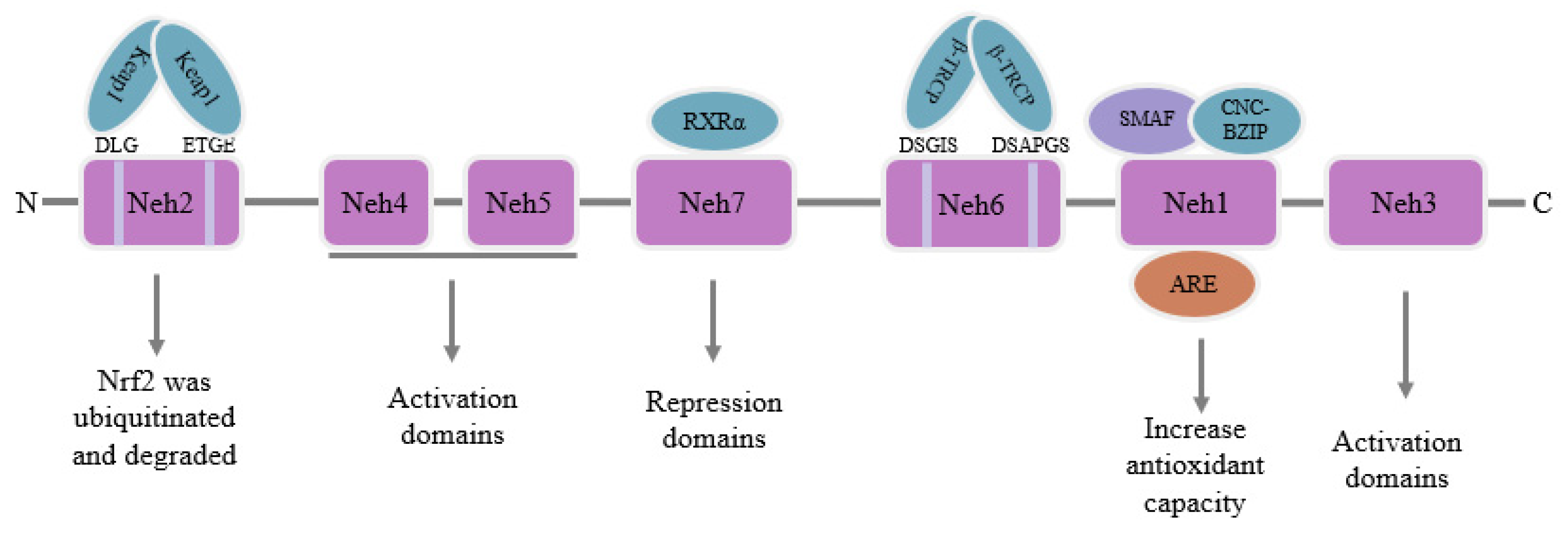
3. Physiological Function of Nrf2
- (1)
- Antioxidant and detoxification abilities: heme oxygenase 1 (HO-1) [40], NADPH-Quinone oxidoreductase 1 (NQO1) [41], superoxide dismutase (SOD), catalase (CAT), glutathione-S-transferase (GST) [42], malic enzyme (ME1) which maintains NADPH stability [43,44,45], glutamate-cysteine ligase catalytic (GCLC) and glutamate cysteine ligase modifier (GCLM) [46].
- (2)
- (3)
- Autophagy: P62/SQSTM1 [51].
| Nrf2 Downstream Target Genes | Nrf2 Non-Downstream Target Genes | |
|---|---|---|
| Antioxidant and detoxification abilities | HO-1 [40], NQO1 [41], SOD, CAT, GST [42], ME1 [43,44,45], GCLC, GCLM [46] | |
| Iron metabolism Homeostasis | FTH [47], FTL [48], SLC7A11 [41], GPX4 [49], Trx [50] | |
| Autophagy | P62/SQSTM1 [51] | PI3K/Akt, mTOR [55,56] |
| Mitochondrial homeostasis | PGC-1α [52], NRF1, TFAM, PINK1 [53] | |
| Endoplasmic reticulum Homeostasis | ATF3 [54] | |
| Apoptosis | caspase molecule, BAX and Bcl-2 [55] | |
| Inflammatory response | NF-κB pathway [57] |
4. The Role of Nrf2 in Toxic Effects Caused by T-2 Toxin
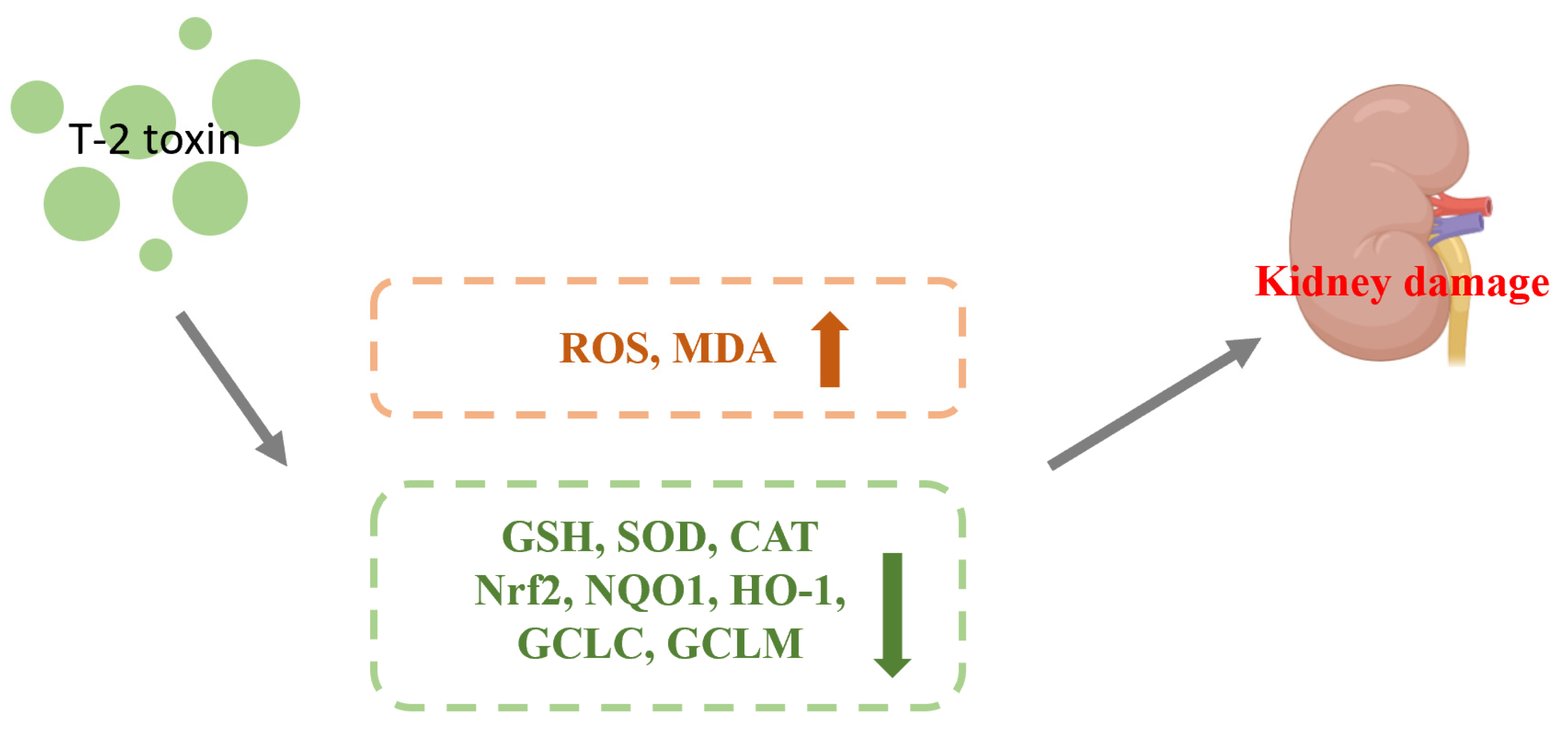
4.1. Effect of Nrf2 on Nephrotoxicity Caused by T-2 Toxin
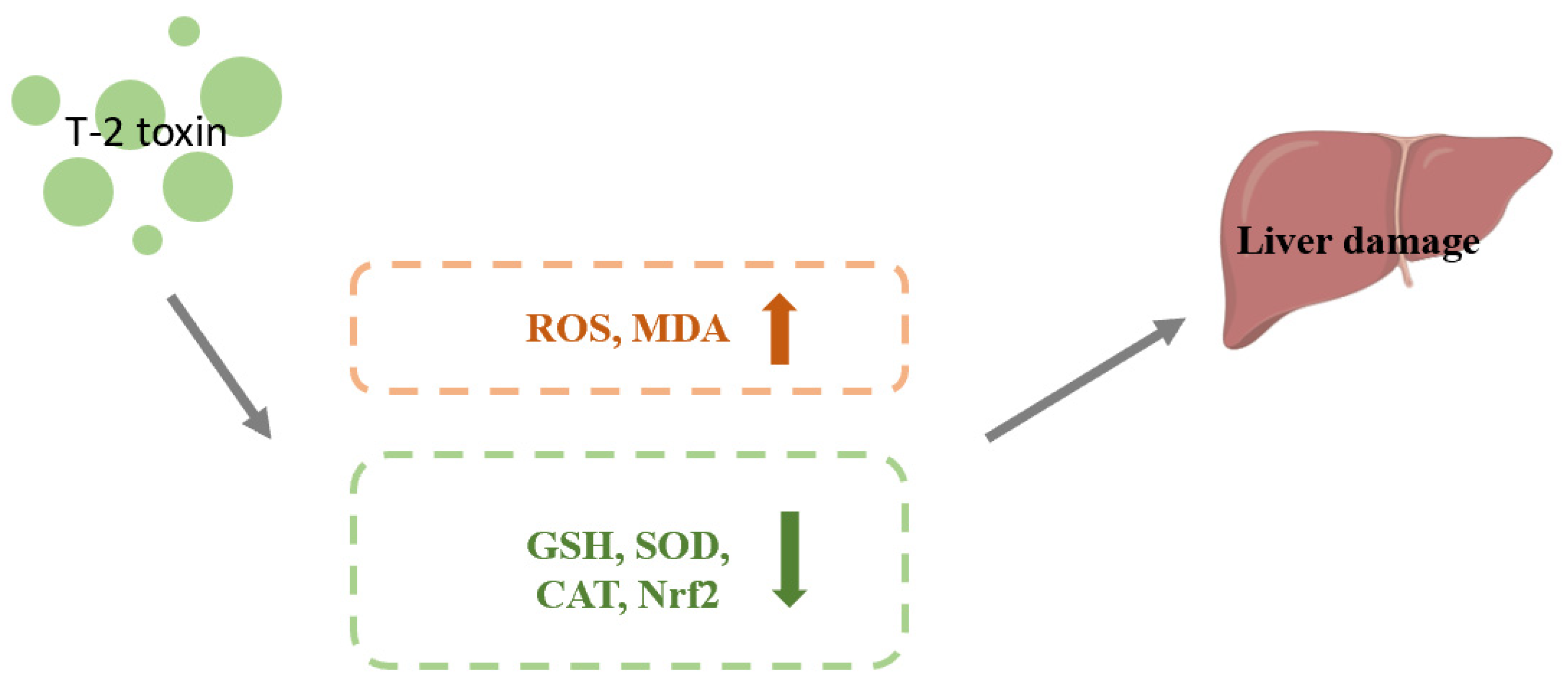
4.2. Effect of Nrf2 on Hepatotoxicity Caused by T-2 Toxin
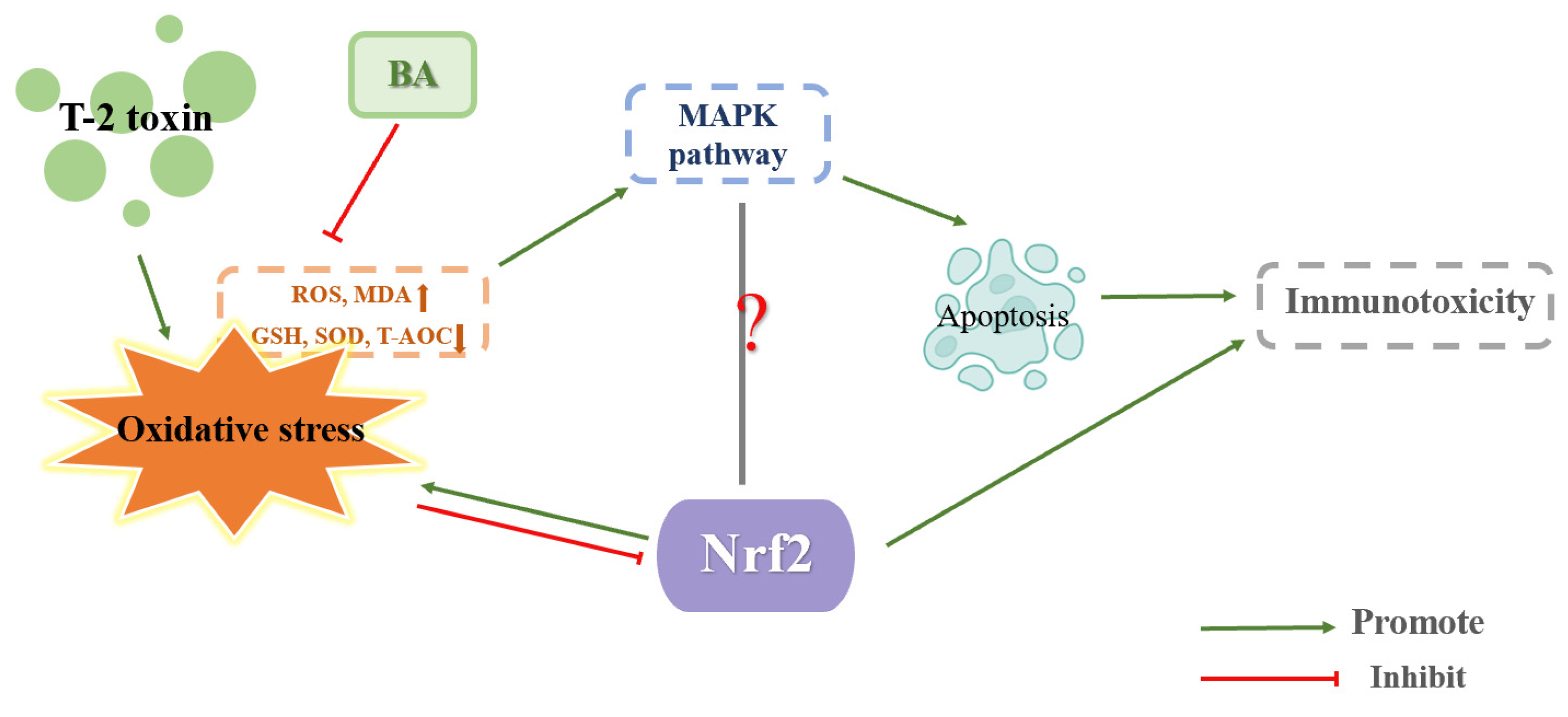
4.3. Effect of Nrf2 on Immunotoxicity Caused by T-2 Toxin
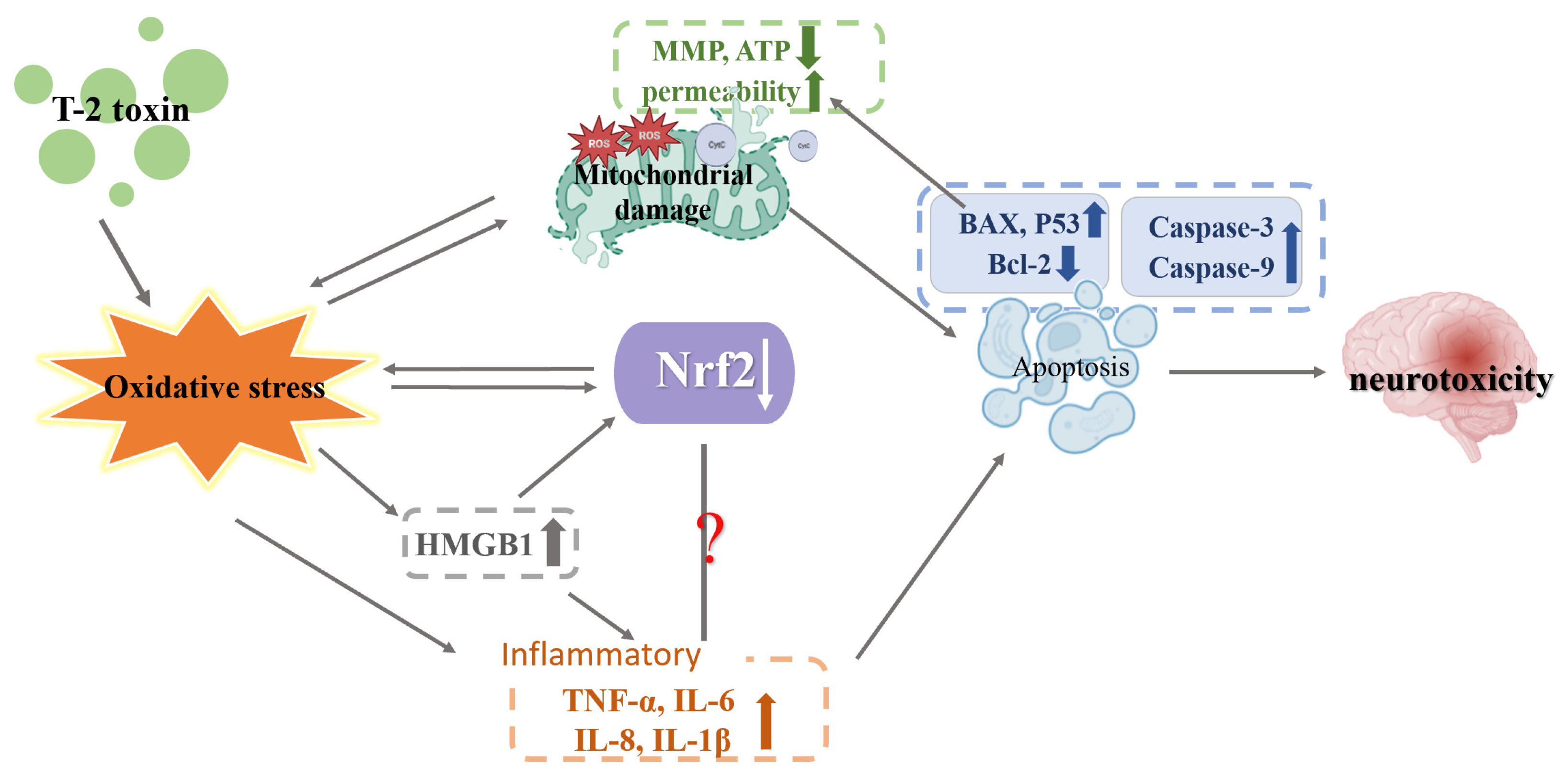
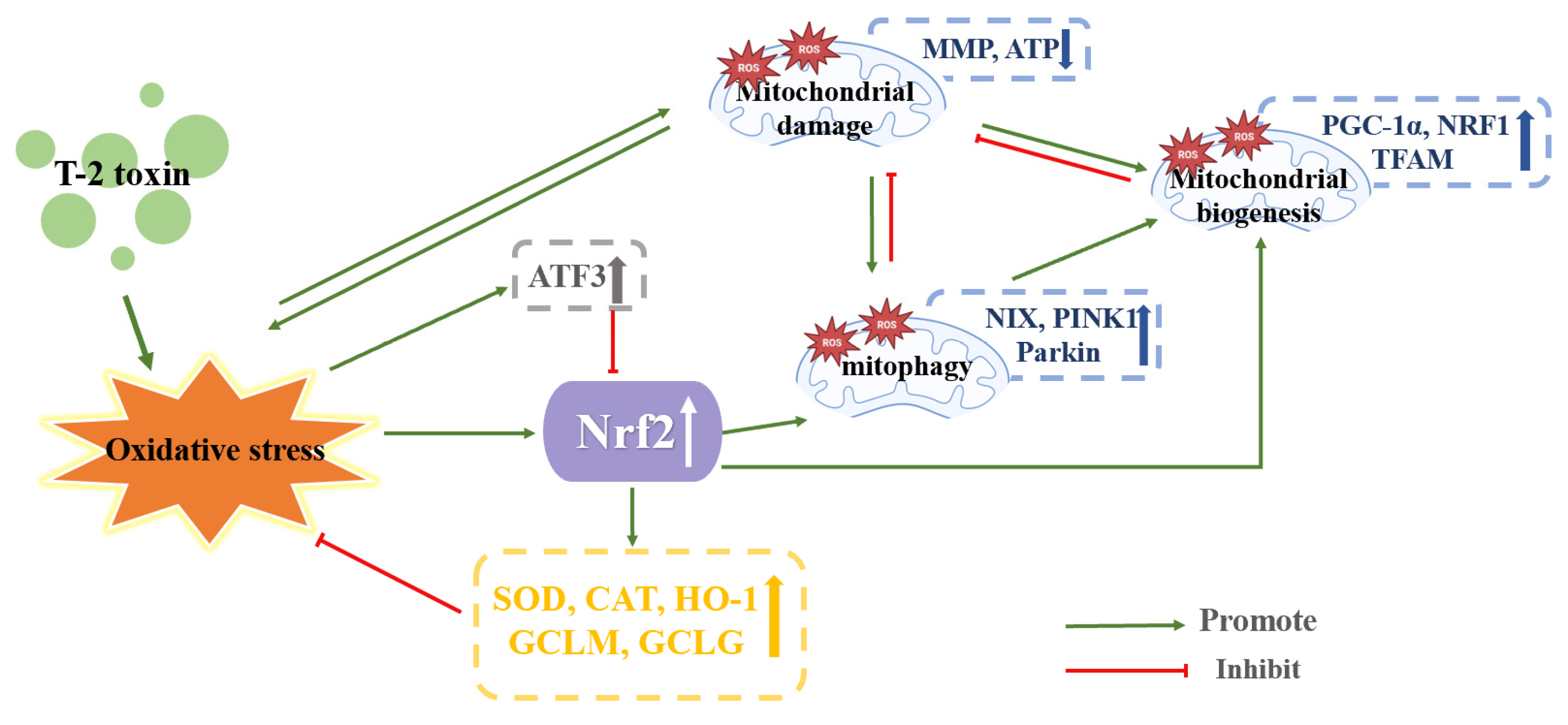
4.4. Effect of Nrf2 on Neurotoxicity Caused by T-2 Toxin
4.5. Effect of Nrf2 on Endocrine Toxicity Caused by T-2 Toxin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pang, Y.; Zhang, L.; Liu, Q.; Peng, H.; He, J.; Jin, H.; Su, X.; Zhao, J.; Guo, J. NRF2/PGC-1α-mediated mitochondrial biogenesis contributes to T-2 toxin-induced toxicity in human neuroblastoma SH-SY5Y cells. Toxicol. Appl. Pharmacol. 2022, 451, 116167. [Google Scholar] [CrossRef]
- Pernica, M.; Kyralová, B.; Svoboda, Z.; Boško, R.; Brožková, I.; Česlová, L.; Benešová, K.; Červenka, L.; Běláková, S. Levels of T-2 toxin and its metabolites, and the occurrence of Fusarium fungi in spring barley in the Czech Republic. Food Microbiol. 2022, 102, 103875. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Q.; Li, M.; Tang, S.; Dai, C. T-2 Toxin Induces Apoptotic Cell Death and Protective Autophagy in Mouse Microglia BV2 Cells. J. Fungi 2022, 8, 761. [Google Scholar] [CrossRef]
- Zhu, L.; Yi, X.; Ma, C.; Luo, C.; Kong, L.; Lin, X.; Gao, X.; Yuan, Z.; Wen, L.; Li, R.; et al. Betulinic Acid Attenuates Oxidative Stress in the Thymus Induced by Acute Exposure to T-2 Toxin via Regulation of the MAPK/Nrf2 Signaling Pathway. Toxins 2020, 12, 540. [Google Scholar] [CrossRef]
- Schmidt, H.S.; Schulz, M.; Focke, C.; Becker, S.; Cramer, B.; Humpf, H.U. Glucosylation of T-2 and HT-2 toxins using biotransformation and chemical synthesis: Preparation, stereochemistry, and stability. Mycotoxin Res. 2018, 34, 159–172. [Google Scholar] [CrossRef]
- Fan, K.; Xu, J.; Jiang, K.; Liu, X.; Meng, J.; Di Mavungu, J.D.; Guo, W.; Zhang, Z.; Jing, J.; Li, H.; et al. Determination of multiple mycotoxins in paired plasma and urine samples to assess human exposure in Nanjing, China. Environ. Pollut. 2019, 248, 865–873. [Google Scholar] [CrossRef]
- Dai, C.; Das Gupta, S.; Wang, Z.; Jiang, H.; Velkov, T.; Shen, J. T-2 toxin and its cardiotoxicity: New insights on the molecular mechanisms and therapeutic implications. Food Chem. Toxicol. 2022, 167, 113262. [Google Scholar] [CrossRef]
- Janik, E.; Niemcewicz, M.; Ceremuga, M.; Stela, M.; Saluk-Bijak, J.; Siadkowski, A.; Bijak, M. Molecular Aspects of Mycotoxins-A Serious Problem for Human Health. Int. J. Mol. Sci. 2020, 21, 8187. [Google Scholar] [CrossRef]
- Wu, Q.; Qin, Z.; Kuca, K.; You, L.; Zhao, Y.; Liu, A.; Musilek, K.; Chrienova, Z.; Nepovimova, E.; Oleksak, P.; et al. An update on T-2 toxin and its modified forms: Metabolism, immunotoxicity mechanism, and human exposure assessment. Arch. Toxicol. 2020, 94, 3645–3669. [Google Scholar] [CrossRef]
- Xue, H.L.; Bi, Y.; Tang, Y.M.; Zhao, Y.; Wang, Y. Effect of cultivars, Fusarium strains and storage temperature on trichothecenes production in inoculated potato tubers. Food Chem. 2014, 151, 236–242. [Google Scholar] [CrossRef]
- Arcella, D.; Gergelova, P.; Innocenti, M.L.; Steinkellner, H. Human and animal dietary exposure to T-2 and HT-2 toxin. EFSA J. 2017, 15, e04972. [Google Scholar] [CrossRef]
- Dai, C.; Xiao, X.; Sun, F.; Zhang, Y.; Hoyer, D.; Shen, J.; Tang, S.; Velkov, T. T-2 toxin neurotoxicity: Role of oxidative stress and mitochondrial dysfunction. Arch. Toxicol. 2019, 93, 3041–3056. [Google Scholar] [CrossRef]
- Janik, E.; Ceremuga, M.; Saluk-Bijak, J.; Bijak, M. Biological Toxins as the Potential Tools for Bioterrorism. Int. J. Mol. Sci. 2019, 20, 1181. [Google Scholar] [CrossRef]
- Deyu, H.; Luqing, C.; Xianglian, L.; Pu, G.; Qirong, L.; Xu, W.; Zonghui, Y. Protective mechanisms involving enhanced mitochondrial functions and mitophagy against T-2 toxin-induced toxicities in GH3 cells. Toxicol. Lett. 2018, 295, 41–53. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, G.; Huo, H.; Li, W. Identification of HIF-1α/VEGFA signaling pathway and transcription factors in Kashin-Beck disease by integrated bioinformatics analysis. Exp. Ther. Med. 2021, 22, 1115. [Google Scholar] [CrossRef]
- Shi, Y.; Shao, X.; Sun, M.; Ma, J.; Li, B.; Zou, N.; Li, F. MiR-140 is involved in T-2 toxin-induced matrix degradation of articular cartilage. Toxicon 2023, 222, 106987. [Google Scholar] [CrossRef]
- Lu, Q.; Hu, S.; Guo, P.; Zhu, X.; Ren, Z.; Wu, Q.; Wang, X. PPAR-γ with its anti-fibrotic action could serve as an effective therapeutic target in T-2 toxin-induced cardiac fibrosis of rats. Food Chem. Toxicol. 2021, 152, 112183. [Google Scholar] [CrossRef]
- Pei, X.; Jiang, H.; Liu, X.; Li, L.; Li, C.; Xiao, X.; Li, D.; Tang, S. Targeting HMGB1 inhibits T-2 toxin-induced neurotoxicity via regulation of oxidative stress, neuroinflammation and neuronal apoptosis. Food Chem. Toxicol. 2021, 151, 112134. [Google Scholar] [CrossRef]
- Bertero, A.; Moretti, A.; Spicer, L.J.; Caloni, F. Fusarium Molds and Mycotoxins: Potential Species-Specific Effects. Toxins 2018, 10, 244. [Google Scholar] [CrossRef]
- Yang, L.; Yu, Z.; Hou, J.; Deng, Y.; Zhou, Z.; Zhao, Z.; Cui, J. Toxicity and oxidative stress induced by T-2 toxin and HT-2 toxin in broilers and broiler hepatocytes. Food Chem. Toxicol. 2016, 87, 128–137. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, R.; Yang, Y.; Xie, H.; Huang, Y.; Chen, X.; Wang, D.; Zhang, Z. Protective Effect of Organic Selenium on Oxidative Damage and Inflammatory Reaction of Rabbit Kidney Induced by T-2 Toxin. Biol. Trace Elem. Res. 2021, 199, 1833–1842. [Google Scholar] [CrossRef]
- Yang, J.Y.; Du, J.J.; Zhang, Y.F.; Li, Y.X. Vitamin E and selenium partially prevent cytotoxicity, oxidative stress and DNA damage induced by T-2 toxin in bovine Leydig cells. Theriogenology 2022, 189, 255–261. [Google Scholar] [CrossRef]
- Wu, J.; Tu, D.; Yuan, L.Y.; Yuan, H.; Wen, L.X. T-2 toxin exposure induces apoptosis in rat ovarian granulosa cells through oxidative stress. Environ. Toxicol. Pharmacol. 2013, 36, 493–500. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Yao, Q.; Song, M.; Han, Y.; Shao, B.; Li, Y. T-2 toxin impairs male fertility by disrupting hypothalamic-pituitary-testis axis and declining testicular function in mice. Chemosphere 2019, 234, 909–916. [Google Scholar] [CrossRef]
- Jaćević, V.; Wu, Q.; Nepovimova, E.; Kuča, K. Efficacy of methylprednisolone on T-2 toxin-induced cardiotoxicity in vivo: A pathohistological study. Environ. Toxicol. Pharmacol. 2019, 71, 103221. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Yang, X.; Liu, M.; Huang, W.; Zhang, J.; Song, M.; Shao, B.; Li, Y. The nephrotoxicity of T-2 toxin in mice caused by oxidative stress-mediated apoptosis is related to Nrf2 pathway. Food Chem. Toxicol. 2021, 149, 112027. [Google Scholar] [CrossRef]
- Yin, H.; Han, S.; Chen, Y.; Wang, Y.; Li, D.; Zhu, Q. T-2 Toxin Induces Oxidative Stress, Apoptosis and Cytoprotective Autophagy in Chicken Hepatocytes. Toxins 2020, 12, 90. [Google Scholar] [CrossRef]
- Wang, J.; Yang, C.; Yuan, Z.; Yi, J.; Wu, J. T-2 Toxin Exposure Induces Apoptosis in TM3 Cells by Inhibiting Mammalian Target of Rapamycin/Serine/Threonine Protein Kinase (mTORC2/AKT) to Promote Ca2+ Production. Int. J. Mol. Sci. 2018, 19, 3360. [Google Scholar] [CrossRef]
- Yi, Y.; Zhao, F.; Wang, N.; Liu, H.; Yu, L.; Wang, A.; Jin, Y. Endoplasmic reticulum stress is involved in the T-2 toxin-induced apoptosis in goat endometrium epithelial cells. J. Appl. Toxicol. 2018, 38, 1492–1501. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox. Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Panda, H.; Wen, H.; Suzuki, M.; Yamamoto, M. Multifaceted Roles of the KEAP1-NRF2 System in Cancer and Inflammatory Disease Milieu. Antioxidants 2022, 11, 538. [Google Scholar] [CrossRef]
- Sánchez-Ortega, M.; Carrera, A.C.; Garrido, A. Role of NRF2 in Lung Cancer. Cells 2021, 10, 1879. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, C.Y.; Lin, H.Q.; Hu, J.S.; Ip, T.M.; Chi-Cheong Wan, D. Development of an enzyme-linked immunosorbent assay for Keap1-Nrf2 interaction inhibitors identification. Redox Biol. 2020, 34, 101573. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Chang, M.; Wilson, C.J.; Karunatilleke, N.C.; Moselhy, M.H.; Karttunen, M.; Choy, W.Y. Exploring the Conformational Landscape of the Neh4 and Neh5 Domains of Nrf2 Using Two Different Force Fields and Circular Dichroism. J. Chem. Theory Comput. 2021, 17, 3145–3156. [Google Scholar] [CrossRef]
- Nam, L.B.; Keum, Y.S. Binding partners of NRF2: Functions and regulatory mechanisms. Arch. Biochem. Biophys. 2019, 678, 108184. [Google Scholar] [CrossRef]
- Jiang, H.; Li, R.; Zhang, Z.; Chang, C.; Liu, Y.; Liu, Z.; He, Q.; Wang, Q. Retinoid X receptor α (RXRα)-mediated erythroid-2-related factor-2 (NRF2) inactivation contributes to N,N-dimethylformamide (DMF)-induced oxidative stress in HL-7702 and HuH6 cells. J. Appl. Toxicol. 2020, 40, 470–482. [Google Scholar] [CrossRef]
- Kong, L.; Zhu, L.; Yi, X.; Huang, Y.; Zhao, H.; Chen, Y.; Yuan, Z.; Wen, L.; Wu, J.; Yi, J. Betulinic Acid Alleviates Spleen Oxidative Damage Induced by Acute Intraperitoneal Exposure to T-2 Toxin by Activating Nrf2 and Inhibiting MAPK Signaling Pathways. Antioxidants 2021, 10, 158. [Google Scholar] [CrossRef]
- Wu, X.; Huang, L.; Liu, J. Relationship between oxidative stress and nuclear factor-erythroid-2-related factor 2 signaling in diabetic cardiomyopathy (Review). Exp. Ther. Med. 2021, 22, 678. [Google Scholar] [CrossRef]
- Mundkar, M.; Bijalwan, A.; Soni, D.; Kumar, P. Neuroprotective potential of Moringa oleifera mediated by NF-kB/Nrf2/HO-1 signaling pathway: A review. J. Food Biochem. 2022, 46, e14451. [Google Scholar] [CrossRef]
- Chen, Y.; Bao, M.; Liu, J.T.; Bao, H.; Zhang, S.M.; Lou, Y.; Qi, Y.X. Defective autophagy triggered by arterial cyclic stretch promotes neointimal hyperplasia in vein grafts via the p62/nrf2/slc7a11 signaling pathway. J. Mol. Cell Cardiol. 2022, 173, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cui, Y.; Song, X.; Jin, X.; Sheng, X.; Xu, X.; Li, T.; Chen, H.; Gao, L. Curcumin alleviates ketamine-induced oxidative stress and apoptosis via Nrf2 signaling pathway in rats’ cerebral cortex and hippocampus. Environ. Toxicol. 2023, 38, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Zhao, Y.; Lin, J.; Jiang, S.; Li, W. The Nrf2 antioxidant defense system in intervertebral disc degeneration: Molecular insights. Exp. Mol. Med. 2022, 54, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Cheng, M.Y.; Xun, M.H.; Zhao, Z.W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Cui, J.Y.; Klaassen, C.D. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol. Sci. 2011, 123, 590–600. [Google Scholar] [CrossRef]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Mishra, G.; İlgün, S.; Samarghandian, S. New insights into the role of the Nrf2 signaling pathway in green tea catechin applications. Phytother. Res. 2021, 35, 3078–3112. [Google Scholar] [CrossRef]
- Hino, K.; Yanatori, I.; Hara, Y.; Nishina, S. Iron and liver cancer: An inseparable connection. FEBS J. 2022, 289, 7810–7829. [Google Scholar] [CrossRef]
- Noel, S.; Zheng, L.; Navas-Acien, A.; Fuchs, R.J. The effect of ex vivo CDDO-Me activation on nuclear factor erythroid 2-related factor 2 pathway in white blood cells from patients with septic shock. Shock 2014, 42, 392–399. [Google Scholar] [CrossRef]
- Li, J.; Lu, Q.; Peng, M.; Liao, J.; Zhang, B.; Yang, D.; Huang, P.; Yang, Y.; Zhao, Q.; Han, B.; et al. Water extract from Herpetospermum pedunculosum attenuates oxidative stress and ferroptosis induced by acetaminophen via regulating Nrf2 and NF-κB pathways. J. Ethnopharmacol. 2023, 305, 116069. [Google Scholar] [CrossRef]
- Ju, H.; Liu, C.; Zhang, G.; Xu, C.; Wang, H.; Fan, H. Neuroprotective potential of nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element signaling modulator cucurbitacin I upon glucose and oxygen deprivation/reperfusion (OGD/RP). Hum. Exp. Toxicol. 2022, 41, 9603271221104450. [Google Scholar] [CrossRef]
- Jain, A.; Lamark, T.; Sjøttem, E.; Larsen, K.B.; Awuh, J.A.; Øvervatn, A.; McMahon, M.; Hayes, J.D.; Johansen, T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 2010, 285, 22576–22591. [Google Scholar] [CrossRef]
- Astorga, J.; Gasaly, N.; Dubois-Camacho, K.; De la Fuente, M.; Landskron, G.; Faber, K.N.; Urra, F.A.; Hermoso, M.A. The role of cholesterol and mitochondrial bioenergetics in activation of the inflammasome in IBD. Front. Immunol. 2022, 13, 1028953. [Google Scholar] [CrossRef]
- Chen, Q.M. Nrf2 for protection against oxidant generation and mitochondrial damage in cardiac injury. Free Radic. Biol. Med. 2022, 179, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Fu, Y.; Wadgaonkar, P.; Qiu, Y.; Almutairy, B.; Zhang, W.; Seno, A.; Thakur, C.; Chen, F. New Discoveries and Ambiguities of Nrf2 and ATF3 Signaling in Environmental Arsenic-Induced Carcinogenesis. Antioxidants 2021, 11, 77. [Google Scholar] [CrossRef]
- Famurewa, A.C.; Renu, K.; Eladl, M.A.; Chakraborty, R.; Myakala, H.; El-Sherbiny, M.; Elsherbini, D.M.A.; Vellingiri, B.; Madhyastha, H.; Ramesh Wanjari, U.; et al. Hesperidin and hesperetin against heavy metal toxicity: Insight on the molecular mechanism of mitigation. Biomed. Pharmacother. 2022, 149, 112914. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Chen, L.; Ren, B.X.; Tang, F.R. Lycium barbarum Ameliorates Neural Damage Induced by Experimental Ischemic Stroke and Radiation Exposure. Front. Biosci. (Landmark. Ed.) 2023, 28, 38. [Google Scholar] [CrossRef]
- Cao, Z.; Gao, J.; Huang, W.; Yan, J.; Shan, A.; Gao, X. Curcumin mitigates deoxynivalenol-induced intestinal epithelial barrier disruption by regulating Nrf2/p53 and NF-κB/MLCK signaling in mice. Food Chem. Toxicol. 2022, 167, 113281. [Google Scholar] [CrossRef]
- Kövesi, B.; Kulcsár, S.; Zándoki, E.; Szabó-Fodor, J.; Mézes, M.; Balogh, K.; Ancsin, Z.; Pelyhe, C. Short-term effects of deoxynivalenol, T-2 toxin, fumonisin B1 or ochratoxin on lipid peroxidation and glutathione redox system and its regulatory genes in common carp (Cyprinus carpio L.) liver. Fish Physiol. Biochem. 2020, 46, 1921–1932. [Google Scholar] [CrossRef]
- Chaudhary, M.; Rao, P.V. Brain oxidative stress after dermal and subcutaneous exposure of T-2 toxin in mice. Food Chem. Toxicol. 2010, 48, 3436–3442. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Velkov, T.; Tang, S.; Dai, C. T-2 toxin-induced toxicity in neuroblastoma-2a cells involves the generation of reactive oxygen, mitochondrial dysfunction and inhibition of Nrf2/HO-1 pathway. Food Chem. Toxicol. 2018, 114, 88–97. [Google Scholar] [CrossRef]
- Chen, X.; Mu, P.; Zhu, L.; Mao, X.; Chen, S.; Zhong, H.; Deng, Y. T-2 Toxin Induces Oxidative Stress at Low Doses via Atf3ΔZip2a/2b-Mediated Ubiquitination and Degradation of Nrf2. Int. J. Mol. Sci. 2021, 22, 7963. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zúñiga-Pflücker, J.C. Thymic Microenvironment: Interactions between Innate Immune Cells and Developing Thymocytes. Front. Immunol. 2022, 13, 885280. [Google Scholar] [CrossRef] [PubMed]
- Kalkal, M.; Das, J. Current understanding of the immune potential of B-cell subsets in malarial pathogenesis. Front. Microbiol. 2023, 14, 1046002. [Google Scholar] [CrossRef]
- Meijles, D.N.; Cull, J.J.; Markou, T.; Cooper, S.T.E.; Haines, Z.H.R.; Fuller, S.J.; O’Gara, P.; Sheppard, M.N.; Harding, S.E.; Sugden, P.H.; et al. Redox Regulation of Cardiac ASK1 (Apoptosis Signal-Regulating Kinase 1) Controls p38-MAPK (Mitogen-Activated Protein Kinase) and Orchestrates Cardiac Remodeling to Hypertension. Hypertension 2020, 76, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Abrishamdar, M.; Farbood, Y.; Sarkaki, A.; Rashno, M.; Badavi, M. Evaluation of betulinic acid effects on pain, memory, anxiety, catalepsy, and oxidative stress in animal model of Parkinson’s disease. Metab. Brain Dis. 2023, 38, 467–482. [Google Scholar] [CrossRef]
- Hayashi, G.; Jasoliya, M.; Sahdeo, S.; Saccà, F.; Pane, C.; Filla, A.; Marsili, A.; Puorro, G.; Lanzillo, R.; Brescia Morra, V.; et al. Dimethyl fumarate mediates Nrf2-dependent mitochondrial biogenesis in mice and humans. Hum. Mol. Genet. 2017, 26, 2864–2873. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Liu, S.; Fan, W.; Ding, C.; Gao, Z.; Tang, Z.; Luo, Y.; Shi, X.; Tan, L.; et al. Bromoacetic acid causes oxidative stress and uric acid metabolism dysfunction via disturbing mitochondrial function and Nrf2 pathway in chicken kidney. Environ. Toxicol. 2022, 37, 2910–2923. [Google Scholar] [CrossRef]
- Wei, X.; Zeng, Y.; Meng, F.; Wang, T.; Wang, H.; Yuan, Y.; Li, D.; Zhao, Y. Calycosin-7-glucoside promotes mitochondria-mediated apoptosis in hepatocellular carcinoma by targeting thioredoxin 1 to regulate oxidative stress. Chem. Biol. Interact. 2023, 374, 110411. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Y.; Yuan, Z.; Yi, J.; Chen, J.; Wang, N.; Tian, Y. Autophagy and Apoptosis Interact to Modulate T-2 Toxin-Induced Toxicity in Liver Cells. Toxins 2019, 11, 45. [Google Scholar] [CrossRef]
- Yfantis, A.; Mylonis, I.; Simos, G. Direct interaction between mortalin and HIF-1α at the mitochondria inhibits apoptosis by blocking recruitment of Bax. FEBS J. 2023. [Google Scholar] [CrossRef]
- Venereau, E.; De Leo, F.; Mezzapelle, R.; Careccia, G.; Musco, G.; Bianchi, M.E. HMGB1 as biomarker and drug target. Pharmacol. Res. 2016, 111, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Tian, X.; Chen, J.; Wang, Y.; Yao, Y.; Li, X.; Yang, C.; Zhang, S.; Xie, C. α7-nAchR agonist GTS-21 reduces radiation-induced lung injury. Oncol. Rep. 2018, 40, 2287–2297. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, N.; Yuan, Y.; Jin, Y.G.; Guo, H.; Deng, W.; Tang, Q.Z. Activating transcription factor 3 in cardiovascular diseases: A potential therapeutic target. Basic Res. Cardiol. 2018, 113, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Sun, M.; Li, S.; Chen, Z.; Geng, D. Transcription factor ATF3 mediates the radioresistance of breast cancer. J. Cell Mol. Med. 2018, 22, 4664–4675. [Google Scholar] [CrossRef]
- Su, N.; Liu, C.L.; Chen, X.P.; Fan, X.X.; Ma, Y.C. T-2 toxin cytotoxicity mediated by directly perturbing mitochondria in human gastric epithelium GES-1 cells. J. Appl. Toxicol. 2020, 40, 1141–1152. [Google Scholar] [CrossRef]
- Song, C.; Zhang, A.; Zhang, M.; Song, Y.; Huangfu, H.; Jin, S.; Sun, Y.; Zhang, C.; Shi, D.; Wang, J.; et al. Nrf2/PINK1-mediated mitophagy induction alleviates sodium fluoride-induced hepatic injury by improving mitochondrial function, oxidative stress, and inflammation. Ecotoxicol. Environ. Saf. 2023, 252, 114646. [Google Scholar] [CrossRef]


| Experiment Model | Treatment Mode and Time | Results | Intervention Experiment | Reference | |
|---|---|---|---|---|---|
| nephrotoxicity | Mice (kidney) | 0.5, 1, and 2 mg/kg BW of T-2 toxin gavage for 28 d | The levels of ROS, lipid peroxidation end products malondialdehyde (MDA), the Nrf2 protein and its downstream target genes (NQO1, HO-1, SOD, CAT, GCLC, and GCLM) were increased. The levels of glutathione (GSH), and SOD and CAT’s activities were decreased. | [26] | |
| hepatotoxicity | carp juveniles (liver) | 1.82 mg/kg BW T-2 toxin gavage for 8, 16 or 24 h | The amount of ROS and MDA were increased. GSH contents and Nrf2 protein expression were decreased. | [58] | |
| immunotoxicity | Mice (thymus or spleen) | 4 mg/kg BW T-2 toxin intraperitoneal injection for 15 h | ROS level, MDA level, Keap1 protein expression and MAPK pathway were increased. GSH levels, SOD activity, T-AOC activity and Nrf2/HO-1 protein expression were decreased. | BA decreased MDA level, ROS level and MAPK pathway. BA increased SOD activity, CAT activity and Nrf2/HO-1 protein expression. | [4,38] |
| neurotoxicity | Mice (brain) | 1.57 mg/kg BW T-2 toxin Subcutaneous injection for 1, 3 and 7 d 5.74 mg/kg BW T-2 toxin Percutaneous exposure for 1, 3 and 7 d | ROS content, MDA content, protein carbonyl content, SOD activity and CAT activity were increased. GSH content, Nrf2 and its downstream II detoxification genes (NQO1, GCLC, GCLM and HO-1) were decreased. | [59] | |
| SH-SY5Y cells | 5 or 10 ng/mL T-2 toxin for 6 h | The ROS level, LDH level and expression of Nrf2 were increased. GSH/GSSG ratio, PGC-1α, NRF1, TFAM, MMP, ATP and mtDNA copy number were deceased. | After Nrf2 knockdown, cytotoxicity, ROS production, mitochondrial malfunction, and impairment of mitochondrial biogenesis were increased. After Nrf2 knockdown, MMP, ATP, mtDNA copy number, PGC-1, NRF1 and TFAM were decreased. | [1] | |
| BV2 cells | 1, 2, and 5 ng/mL T-2 toxin for 24 h | ROS level, MDA level, BAX, cleaved-caspase-3 and cleaved-RARP-1 were increased. CAT activity, SOD activity, Nrf2/ HO-1 protein, MMP and Bcl-2 were decreased. | [3] | ||
| N2a cells | 10, 20, 40 or 80 ng/mL T-2 toxin for 24 h | ROS level, MDA level caspase-3, caspase-8, caspase-9, cleaved-PARP-1, pro-apoptotic protein BAX and p53 were increased. GSH content, CAT activity, SOD activity, MMP, Bcl-XL and Nrf2/HO-1 were decreased. | After inhibiting the expression of Nrf2, the cell activity was decreased. After inhibiting the expression of Nrf2, caspase-3 and caspase-9 were increased. | [60] | |
| PC12 cells | 0.75, 1, 3, 6 or 12 ng/mL T-2 toxin for 24 h | ROS level, MDA level, CytC, cleaved caspase-3, caspase-9, BAX and HMGB1 were increased. SOD activity, CAT activity and GSH level were decreased. Nrf2/HO-1 was increased at T-2 toxin < 6 ng/mL, was decreased at T-2 toxin > 6 ng/mL. | After inhibiting HMGB1, apoptosis, oxidative stress and inflammation were decreased. After inhibiting HMGB1, Nrf2 was increased. | [18] | |
| Endocrine toxicity | MCF-7 cells | 5 ng/mL T-2 toxin for 24 h | ROS synthesis, ATF3 were increased. Nitric oxide synthase, CAT, GST, SOD, Nrf2 were decreased | After overexpressing Nrf2, ROS synthesis was decreased. After inhibiting ATF3, ROS level was decreased. After inhibiting ATF3, Nrf2 was increased. After overexpressing ATF3, Nrf2 was decreased. | [61] |
| GH3 cells | 10 and 40 nM T-2 toxin for 24 h | ROS level, GSH-Px, SOD, CAT, UCP, Nrf2, mitochondrial complex I, ATP, 8-OHDG level, PGC-1α, NRF1, TFAM, LC II/LC I, autophagy-related 3, NIX, PINK1 and Parkin were increased. GSH level, MMP were decreased. | After inhibiting Nrf2, PINK1 expression was decreased. After inhibiting PKA, Nrf2 and PINK1 were decreased. | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, Y.; Huang, T.; Chen, Y.; Song, W.; Chen, F.; Jiang, Y.; Zhang, C.; Yang, X. Nrf2: A Main Responsive Element of the Toxicity Effect Caused by Trichothecene (T-2) Mycotoxin. Toxics 2023, 11, 393. https://doi.org/10.3390/toxics11040393
Wang Y, Liu Y, Huang T, Chen Y, Song W, Chen F, Jiang Y, Zhang C, Yang X. Nrf2: A Main Responsive Element of the Toxicity Effect Caused by Trichothecene (T-2) Mycotoxin. Toxics. 2023; 11(4):393. https://doi.org/10.3390/toxics11040393
Chicago/Turabian StyleWang, Youshuang, Yu Liu, Tingyu Huang, Yunhe Chen, Wenxi Song, Fengjuan Chen, Yibao Jiang, Cong Zhang, and Xu Yang. 2023. "Nrf2: A Main Responsive Element of the Toxicity Effect Caused by Trichothecene (T-2) Mycotoxin" Toxics 11, no. 4: 393. https://doi.org/10.3390/toxics11040393
APA StyleWang, Y., Liu, Y., Huang, T., Chen, Y., Song, W., Chen, F., Jiang, Y., Zhang, C., & Yang, X. (2023). Nrf2: A Main Responsive Element of the Toxicity Effect Caused by Trichothecene (T-2) Mycotoxin. Toxics, 11(4), 393. https://doi.org/10.3390/toxics11040393







