Evaluating the Impact of Individual and Combined Toxicity of Imidacloprid, Cycloxaprid, and Tebuconazole on Daphnia magna
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals Used in Testing
2.2. Test Organism Preparation
2.3. Design of Acute and Chronic Exposure Experiments
2.4. Enzyme Activity Assays
2.5. Statistical Analysis
2.6. Concentration Verification
3. Results
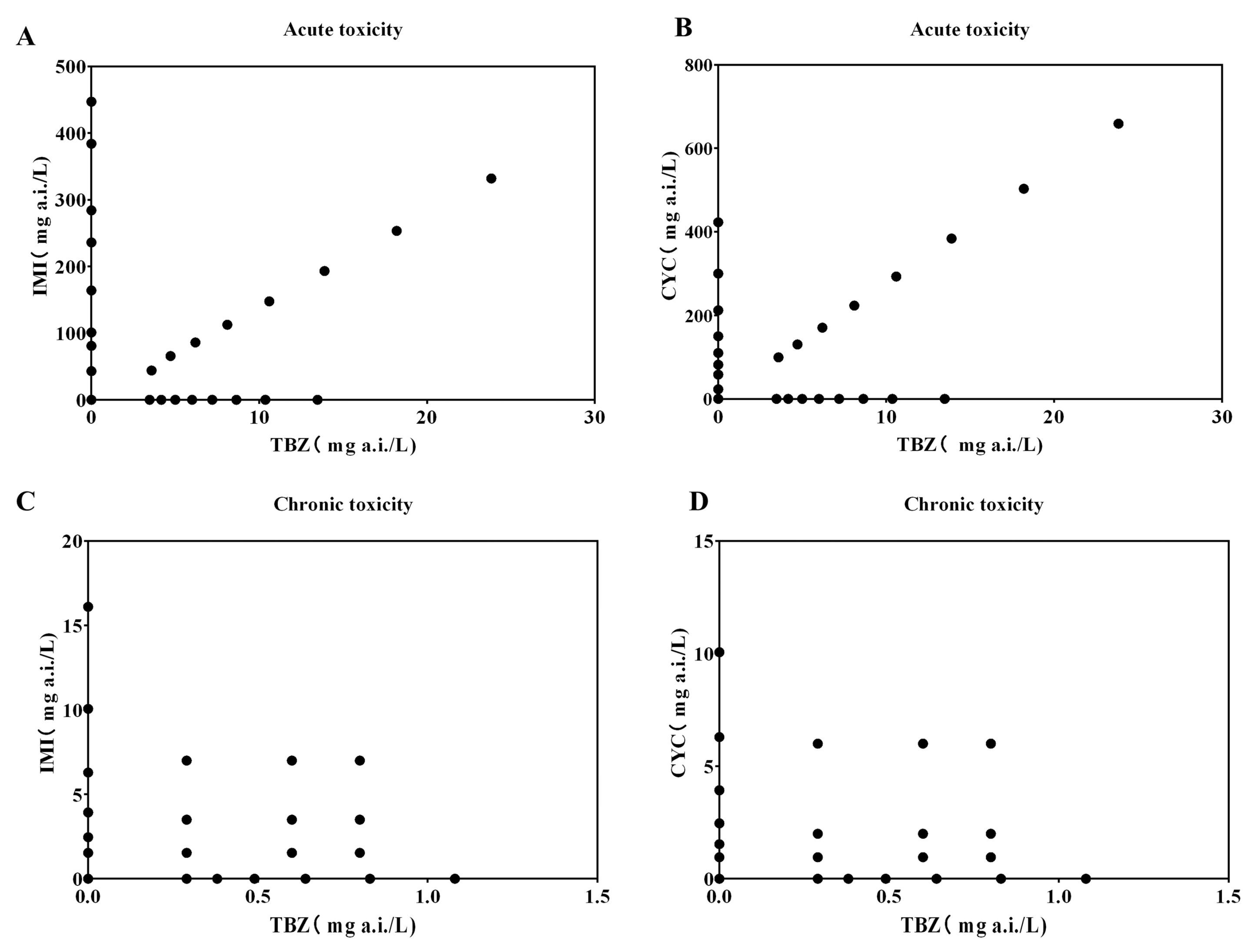
3.1. Concentration in Medium during Exposure
3.2. Interaction in Acute Toxicity
3.2.1. Individual Exposure Toxicity
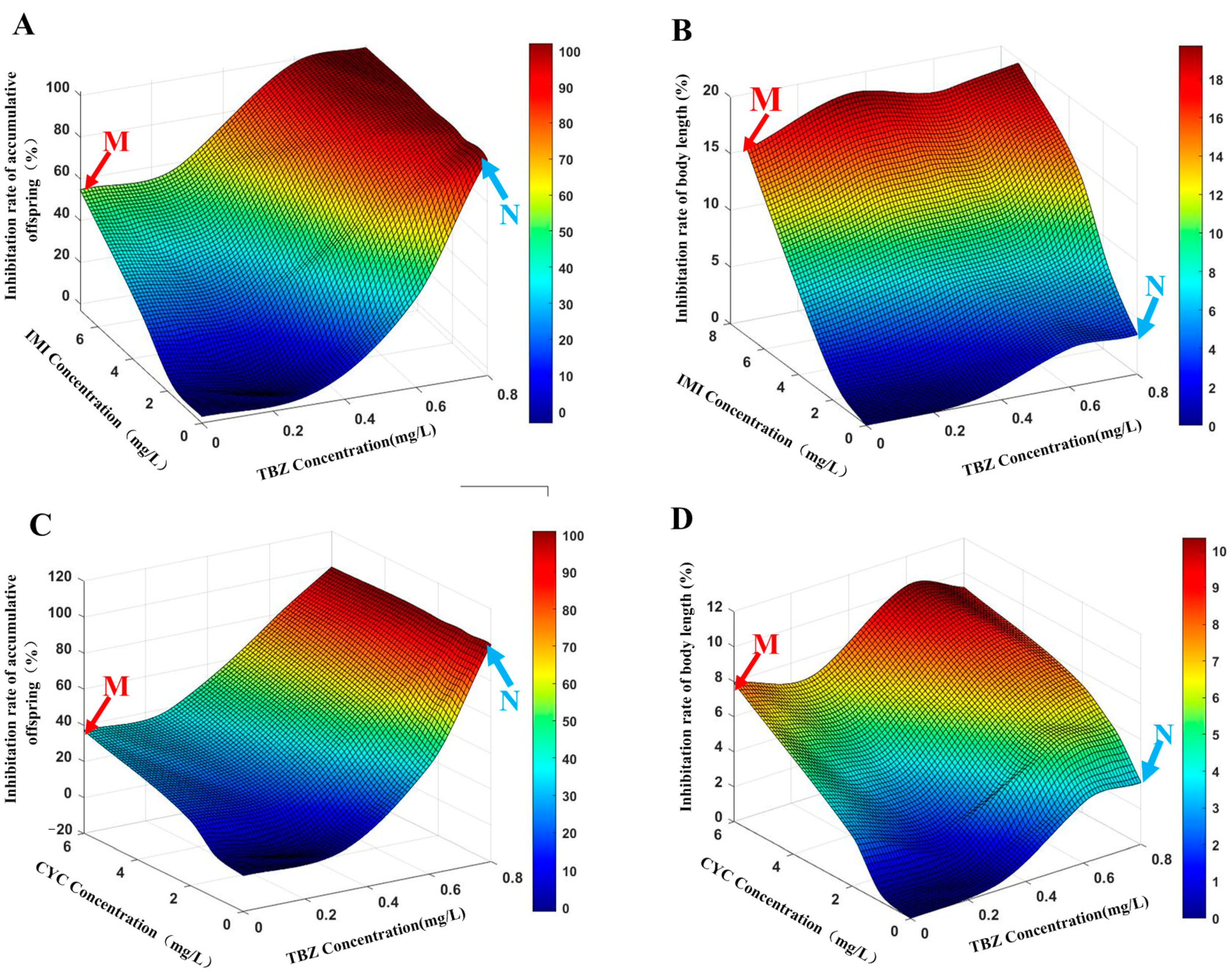
3.2.2. Prediction of Combined Toxicity by MIXTOX
3.3. Chronic Toxic Effect on Reproduction
3.3.1. Individual Chronic Toxicity
3.3.2. Prediction of Combined Toxicity by MIXTOX
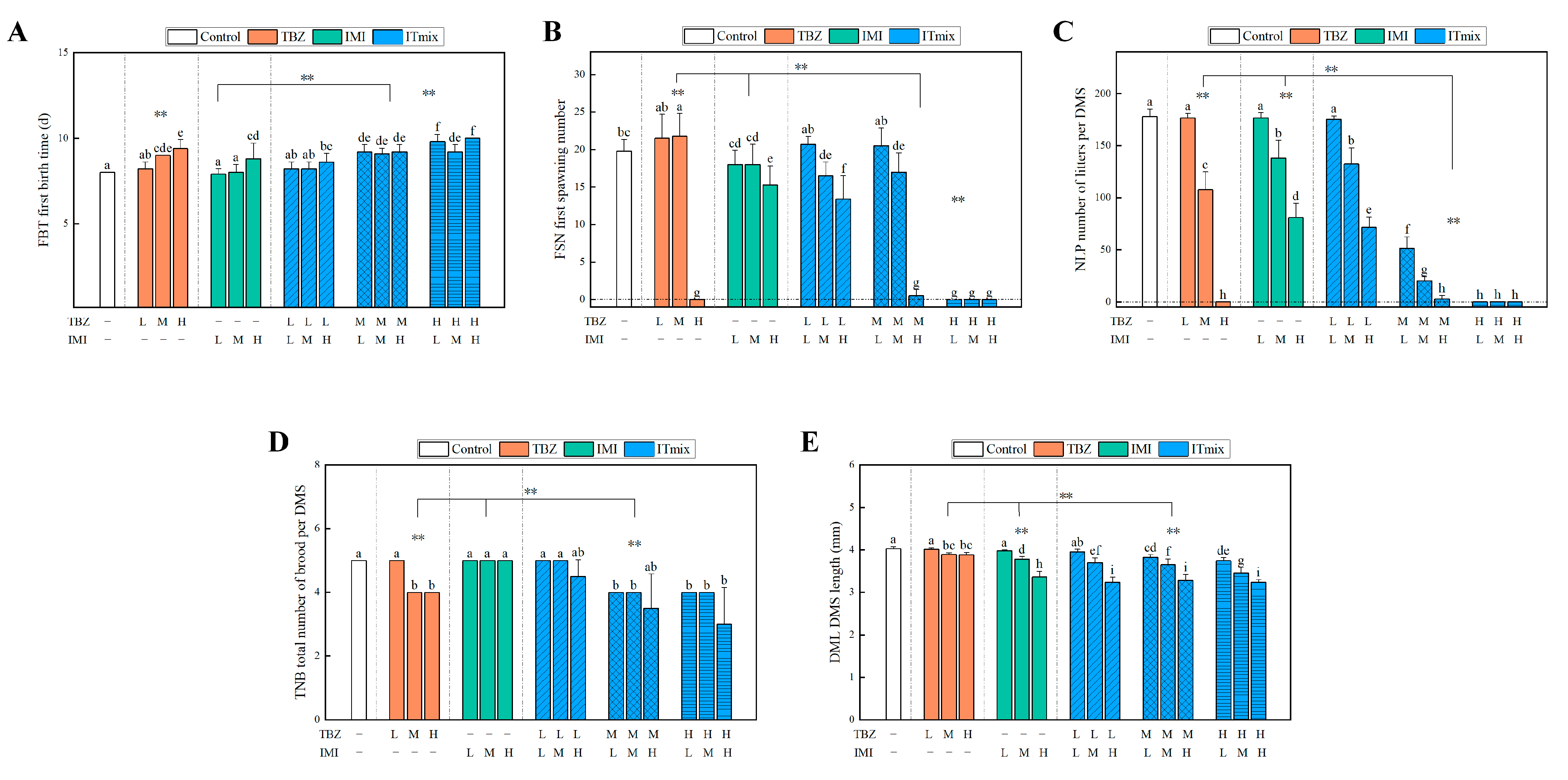
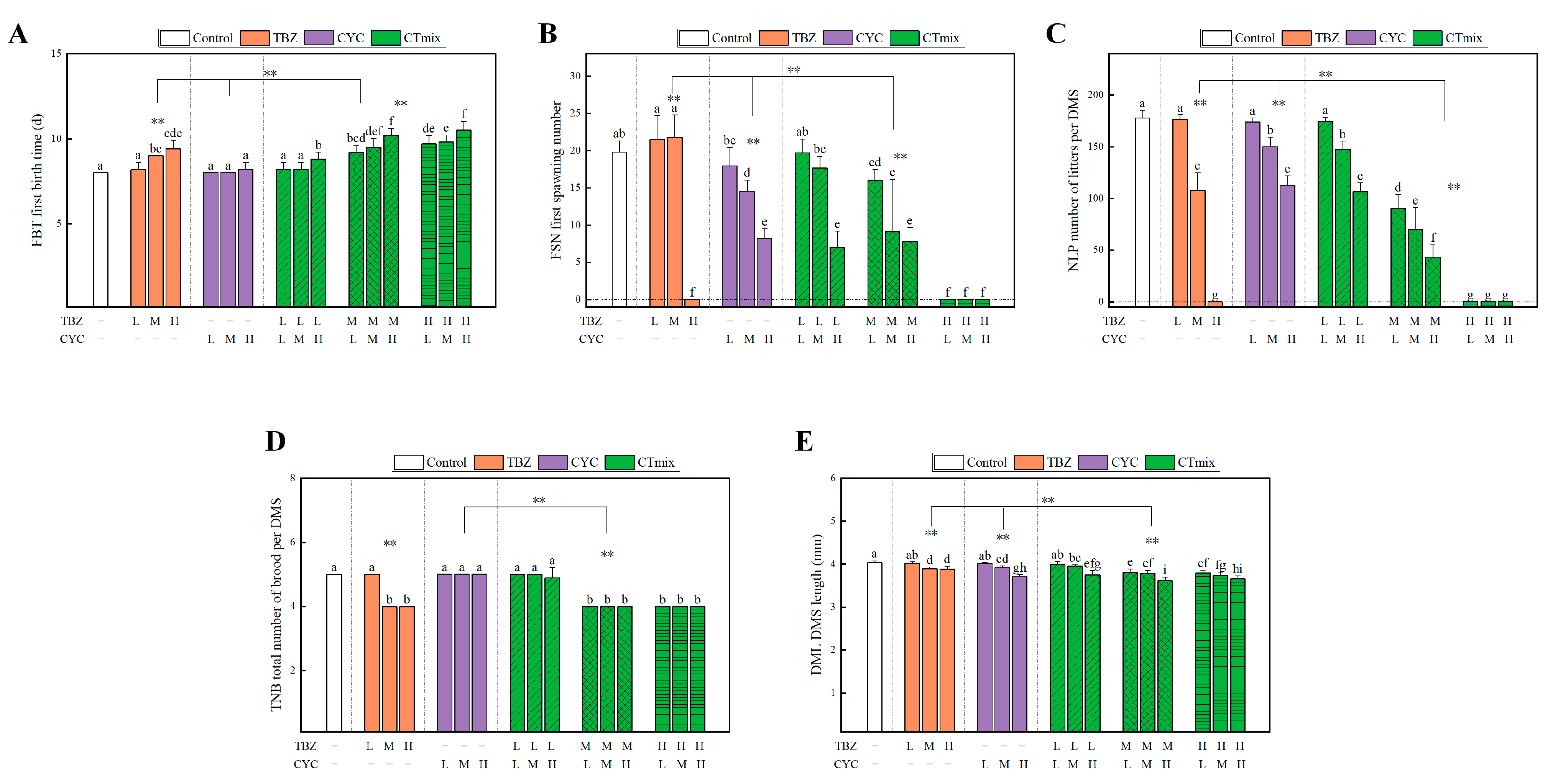
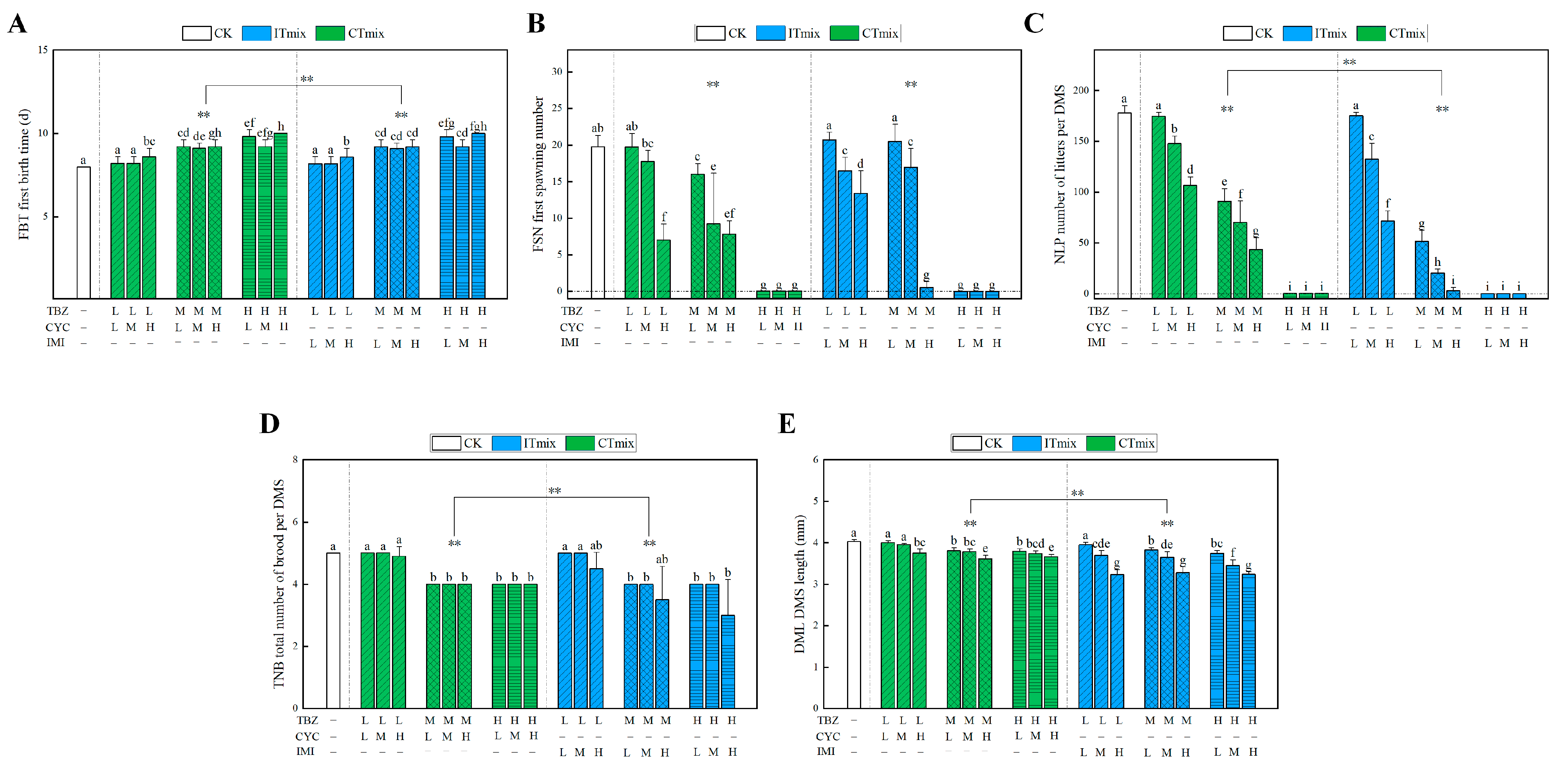
3.3.3. Chronic Toxic Interaction Effect on Growth Parameters
3.3.4. Chronic Toxic Interaction Effect on Enzyme Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borsuah, J.F.; Messer, T.L.; Snow, D.D.; Comfort, S.D.; Mittelstet, A.R. Literature Review: Global Neonicotinoid Insecticide Occurrence in Aquatic Environments. Water 2020, 12, 3388. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the Status and Global Strategy for Neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Shao, X.; Fu, H.; Xu, X.; Xu, X.; Liu, Z.; Li, Z.; Qian, X. Divalent and Oxabridged Neonicotinoids Constructed by Dialdehydes and Nitromethylene Analogues of Imidacloprid: Design, Synthesis, Crystal Structure, and Insecticidal Activities. J. Agric. Food Chem. 2010, 58, 2696–2702. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Yuan, H.; Wang, Q.; Wang, Q.; Rui, C. Sublethal effects of the novel cis-nitromethylene neonicotinoid cycloxaprid on the cotton aphid Aphis gossypii Glover (Hemiptera: Aphididae). Sci. Rep. 2018, 8, 8915. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Bao, H.; Shao, X.; Li, Z.; Liu, Z. The binding properties of cycloxaprid on insect native nAChRs partially explain the low cross-resistance with imidacloprid in Nilaparvata lugens. Pest Manag. Sci. 2019, 75, 246–251. [Google Scholar] [CrossRef]
- Hua, N.Z. Formulation and Application of Fungicide Tebuconazole. Agrochemicals 2013, 52, 781c786–809. [Google Scholar]
- Hladik, M.L.; Corsi, S.R.; Kolpin, D.W.; Baldwin, A.K.; Blackwell, B.R.; Cavallin, J.E. Year-round presence of neonicotinoid insecticides in tributaries to the Great Lakes, USA. Env. Pollut 2018, 235, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, J.; Wang, B.; Duan, L.; Zhang, Y.; Zhao, W.; Wang, F.; Sui, Q.; Chen, Z.; Xu, D.; et al. Occurrence, source and ecotoxicological risk assessment of pesticides in surface water of Wujin District (northwest of Taihu Lake), China. Environ. Pollut. 2020, 265, 114953. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zang, L.; Liu, M.; Zhang, C.; Shen, G.; Du, W.; Sun, Z.; Fei, J.; Yang, L.; Wang, Y.; et al. Ecological risk assessment of the increasing use of the neonicotinoid insecticides along the east coast of China. Environ. Int. 2019, 127, 550–557. [Google Scholar] [CrossRef]
- Guarda, P.M.; Gualberto LD, S.; Mendes, D.B.; Guarda, E.A.; da Silva, J.E.C. Analysis of triazines, triazoles, and benzimidazoles used as pesticides in different environmental compartments of the Formoso River and their influence on biodiversity in Tocantins. J. Env. Sci. Health B 2020, 55, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Necibi, M.; Saadaoui, H.; Atayat, A.; Mzoughi, N. Determination of Triazole Pesticides in the Surface Water of the Medjerda River, Tunisia. Anal. Lett. 2020, 54, 742–759. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Conclusion on the peer review of the pesticide risk assessment of the active substance tebuconazole. EFSA Sci. Rep. 2014, 12, 3485. [Google Scholar]
- Pietrzak, D.; Kania, J.; Kmiecik, E.; Malina, G.; Wątor, K. Fate of selected neonicotinoid insecticides in soil–water systems: Current state of the art and knowledge gaps. Chemosphere 2020, 255, 126981. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, E.M.J.; van den Brink, P.J. Review of Recent Literature Concerning Mixture Toxicity of Pesticides to Aquatic Organisms, RIVM Rep. 601400001; RIVM: Bilthoven, The Netherlands, 2010. [Google Scholar]
- Rodney, S.I.; Teed, R.S.; Moore, D.R.J. Estimating the Toxicity of Pesticide Mixtures to Aquatic Organisms: A Review. Hum. Ecol. Risk Assessment Int. J. 2013, 19, 1557–1575. [Google Scholar] [CrossRef]
- Cedergreen, N. Quantifying Synergy: A Systematic Review of Mixture Toxicity Studies within Environmental Toxicology. PLoS ONE 2014, 9, e96580. [Google Scholar] [CrossRef]
- Faust, M.; Altenburger, R.; Backhaus, T.; Blanck, H.; Boedeker, W.; Gramatica, P.; Hamer, V.; Scholze, M.; Vighi, M.; Grimme, L.H. Predicting the joint algal toxicity of multi-component s-triazine mixtures at low-effect concentrations of individual toxicants. Aquat. Toxicol. 2001, 56, 13–32. [Google Scholar] [CrossRef]
- Junghans, M.; Backhaus, T.; Faust, M.; Scholze, M.; Grimme, L.H. Application and validation of approaches for the predictive hazard assessment of realistic pesticide mixtures. Aquat. Toxicol. 2006, 76, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.K.; Malik, P.; Tirth, V.; Khan, S.H.; Yadav, K.K.; Islam, S.; Choudhary, N.; Inwati, G.K.; Arabi, A.; Kim, D.-H.; et al. Health and Environmental Risks of Incense Smoke: Mechanistic Insights and Cumulative Evidence. J. Inflamm. Res. 2022, 15, 2665–2693. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, T. Environmental risk assessment of pharmaceutical mixtures: Demands, gaps, and possible bridges. Aaps J. 2016, 18, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, T.; Faust, M. Predictive Environmental Risk Assessment of Chemical Mixtures: A Conceptual Framework. Environ. Sci. Technol. 2012, 46, 2564–2573. [Google Scholar] [CrossRef] [PubMed]
- Loewe, S.; Muischnek, H. Combinated effects I announcement—Implements to the problem. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1926, 114, 313–326. [Google Scholar] [CrossRef]
- Jonker, M.J.; Svendsen, C.; Bedaux, J.J.M.; Bongers, M.; Kammenga, J.E. Significance testing ofsynergistic/antagonistic, dose level-dependent, or dose ratio-dependent effects in mixture dose-response analysis. Env. Toxicol. Chem. 2005, 24, 2701–2713. [Google Scholar] [CrossRef] [PubMed]
- Altshuler, I.; Demiri, B.; Xu, S.; Constantin, A.; Yan, N.D.; Cristescu, M.E. An Integrated Multi-Disciplinary Approach for Studying Multiple Stressors in Freshwater Ecosystems: Daphnia as a Model Organism. Integr. Comp. Biol. 2011, 51, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.R.; Strobel, B.W.; Hama, J.R.; Cedergreen, N. Toxicity and risk of plant-produced alkaloids to Daphnia magna. Environ. Sci. Eur. 2021, 33, 1–12. [Google Scholar] [CrossRef]
- Pereira, A.S.; Cerejeira, M.J.; Daam, M.A. Toxicity of environmentally realistic concentrations of chlorpyrifos and terbuthylazine in indoor microcosms. Chemosphere 2017, 182, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Martins, C.; Pereira, A.S.; Loureiro, S.; Cerejeira, M.J. Toxicity prediction and assessment of an environmentally realistic pesticide mixture to Daphnia magna and Raphidocelis subcapitata. Ecotoxicology 2018, 27, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Borgeraas, J.; Hessen, D.O. Variations of antioxidant enzymes in Daphnia species and populations as related to ambient UV exposure. Hydrobiologia 2002, 477, 15–30. [Google Scholar] [CrossRef]
- Borgeraas, J.; Hessen, D.O. UV-B induced mortality antioxidant enzyme activities in Daphnia magna at different oxygen concentrations temperatures. J. Plankton Res. 2000, 22, 1167–1183. [Google Scholar] [CrossRef]
- Bal, N.; Kumar, A.; Du, J.; Nugegoda, D. Multigenerational effects of two glucocorticoids (prednisolone and dexamethasone) on life-history parameters of crustacean Ceriodaphnia dubia (Cladocera). Environ. Pollut. 2017, 225, 569–578. [Google Scholar] [CrossRef]
- Issa, S.; Simonsen, A.; Jaspers, V.L.B.; Einum, S. Population dynamics and resting egg production in daphnia: Interactive effects of mercury, population density and temperature. Sci. Total Environ. 2020, 755, 143625. [Google Scholar] [CrossRef]
- Barreto, A.; Luis, L.; Paíga, P.; Santos, L.; Delerue-Matos, C.; Soares, A.; Hylland, K.; Loureiro, S.; Oliveira, M. A multibiomarker approach highlights effects induced by the human pharmaceutical gemfibrozil to gilthead seabream Sparus aurata. Aquat. Toxicol. 2018, 200, 266–274. [Google Scholar] [CrossRef]
- OECD. Test No. 202: Daphnia sp. Acute Immobilisation Test, OECD Guidelines for the Testing of Chemicals; Section 2; OECD Publishing: Paris, France, 2004. [Google Scholar] [CrossRef]
- OECD. Test No. 211: Daphnia magna Reproduction Test, OECD Guidelines for the Testing of Chemicals; Section 2; OECD Publishing: Paris, France, 2012. [Google Scholar] [CrossRef]
- Shao, X.; Swenson, T.L.; Casida, J.E. Cycloxaprid Insecticide: Nicotinic Acetylcholine Receptor Binding Site and Metabolism. J. Agric. Food Chem. 2013, 61, 7883–7888. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Xia, S.; Durkin, K.A.; Casida, J.E. Insect nicotinic receptor interactions in vivo with neonicotinoid, organophosphorus, and methylcarbamate insecticides and a synergist. Proc. Natl. Acad. Sci. USA 2013, 110, 17273–17277. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Wang, D.; Zhu, L.; Teng, M.; Wang, C.; Xue, X.; Wu, L. Neonicotinoid insecticides imidacloprid, guadipyr, and cycloxaprid induce acute oxidative stress in Daphnia magna. Ecotoxicol. Environ. Saf. 2018, 148, 352–358. [Google Scholar] [CrossRef]
- Kwok, I.M.Y.; Loeffler, R.T. The biochemical mode of action of some newer azole fungicides. Pestic. Sci. 1993, 39, 1–11. [Google Scholar] [CrossRef]
- De Liguoro, M.; Riga, A.; Fariselli, P. Synergistic toxicity of some sulfonamide mixtures on Daphnia magna. Ecotoxicol. Environ. Saf. 2018, 164, 84–91. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.S.; Liu, H.L. Effect ofionic liquid on the toxicity of pesticide to Vibrioqinghaiensis sp-Q67. J. Hazard. Mater. 2009, 170, 920–927. [Google Scholar] [CrossRef]
- Fu, D.-J.; Li, P.; Song, J.; Zhang, S.-Y.; Xie, H.-Z. Mechanisms of synergistic neurotoxicity induced by two high risk pesticide residues—Chlorpyrifos and Carbofuran via oxidative stress. Toxicol. Vitr. 2019, 54, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Schmuck, R.; Stadler, T.; Schmidt, H.-W. Field relevance of a synergistic effect observed in the laboratory between an EBI fungicide and a chloronicotinyl insecticide in the honeybee (Apis mellifera L., Hymenoptera). Pest Manag. Sci. 2003, 59, 279–286. [Google Scholar] [CrossRef]
- Thompson, H.M.; Fryday, S.L.; Harkin, S.; Milner, S. Potential impacts of synergism in honeybees (Apis mellifera) of exposure to neonicotinoids and sprayed fungicides in crops. Apidologie 2014, 45, 545–553. [Google Scholar] [CrossRef]
- Sgolastra, F.; Medrzycki, P.; Bortolotti, L.; Renzi, M.T.; Tosi, S.; Bogo, G.; Teper, D.; Porrini, C.; Molowny-Horas, R.; Bosch, J. Synergistic mortality between a neonicotinoid insecticide and an ergosterol-biosynthesis-inhibiting fungicide in three bee species. Pest Manag. Sci. 2017, 73, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Eyles, J.L.; Svendsen, C.; Lister, L.; Martin, H.; Hodson, M.E.; Spurgeon, D.J. Measuring and modelling mixture toxicity of imidacloprid and thiacloprid on Caenorhabditis elegans and Eisenia fetida. Ecotoxicol. Environ. Saf. 2009, 72, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, Y.; Yang, L.; Luan, T.; Lin, L. Effects of undissociated SiO2 and TiO2 nano-particles on molting of Daphnia pulex: Comparing with dissociated ZnO nano particles. Ecotoxicol. Environ. Saf. 2021, 222, 112491. [Google Scholar] [CrossRef] [PubMed]
- Cedergreen, N.; Streibig, J. Can the choice of endpoint lead to contradictory results of mixture-toxicity experiments? Environ. Toxicol. Chem. 2005, 24, 1676–1683. [Google Scholar] [CrossRef]
- Livingstone, D. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar. Pollut. Bull 2001, 42, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Gorbi, G.; Moroni, F.; Sei, S.; Rossi, V. Anticipatory maternal effects in two different clones of Daphnia magna in response to food shortage. J. Limnol. 2011, 70, 222–230. [Google Scholar] [CrossRef]
- Parolini, M.; DE Felice, B.; Ferrario, C.; Salgueiro-González, N.; Castiglioni, S.; Finizio, A.; Tremolada, P. Benzoylecgonine exposure induced oxidative stress and altered swimming behavior and reproduction in Daphnia magna. Environ. Pollut. 2018, 232, 236–244. [Google Scholar] [CrossRef]
- Arzate-Cárdenas, M.A.; Martínez-Jerónimo, F. Energy reserve modification in different age groups of Daphnia schoedleri (Anomopoda: Daphniidae) exposed to hexavalent chromium. Environ. Toxicol. Pharmacol. 2012, 34, 106–116. [Google Scholar] [CrossRef]
- Arzate-Cárdenas, M.A.; Martínez-Jerónimo, F. Energy resource reallocation in Daphnia schodleri (Anomopoda: Daphniidae) reproduction induced by exposure to hexavalent chromium. Chemosphere 2012, 87, 326–332. [Google Scholar] [CrossRef]

| Insecticide Type | Exposure Time | Toxic Regression Equation | EC50/(mg/L) | 95% Confidence Interval | R2 | NOEC |
|---|---|---|---|---|---|---|
| TBZ | 48 h | y = −10.077 + 12.752x | 5.74 | 5.355~6.027 | 0.9861 | |
| 21 d | y = 1.605 + 10.398x | 0.70 | 0.558~0.935 | 0.957 | 0.294 | |
| IMI | 48 h | y = −26.192 + 11.448x | 194.0 | 183.976~204.008 | 0.9953 | |
| 21 d | y = −3.628 + 4.406x | 6.66 | 5.999~7.438 | 0.9843 | 1.54 | |
| CYC | 48 h | y = −24.858 + 11.029x | 190.0 | 185.1~195.2 | 0.9973 | |
| 21 d | y = −2.711 + 4.113x | 4.56 | 3.127~7.588 | 0.9942 | 0.96 |
| Acute | CA | IA | ||||||
|---|---|---|---|---|---|---|---|---|
| Refer | S/A | DR | DL | Refer | S/A | DR | DL | |
| Vmax | 96.18 | 94.86 | 102.29 | 101.71 | 94.87 | 95.02 | 95.10 | 94.98 |
| β (TBZ) | 3.85 | 5.50 | 5.05 | 2.59 | 5.33 | 5.27 | 5.27 | 5.24 |
| β (IMI) | 4.28 | 5.50 | 3.22 | 2.55 | 5.31 | 5.14 | 5.13 | 5.12 |
| EC50 (TBZ) | 6.96 | 6.07 | 14.98 | 79.18 | 6.10 | 6.07 | 6.07 | 6.07 |
| EC50 (IMI) | 226.85 | 196.32 | 124.92 | 118.76 | 196.90 | 195.43 | 195.32 | 195.49 |
| a | / | 2.10 | −0.42 | −5.15 | / | 0.29 | 22.63 | 0.47 |
| b | / | / | −1.85 | 0.25 | / | −40.81 | 0.75 | |
| SS | 5451.51 | 20,585.91 | 12,141.54 | 159,675.57 | 237.02 | 230.24 | 228.90 | 229.49 |
| χ2 | / | 80.71 | 81.01 | 83.50 | / | 0.75 | 0.91 | 0.84 |
| df | / | 1.00 | 2.00 | 2.00 | / | 1.00 | 2.00 | 2.00 |
| p (χ2) | / | 2.612 × 10−19 | 2.56 × 10−18 | 7.391 × 10−19 | / | 0.39 | 0.64 | 0.66 |
| r2 | 0.8434 | 0.9948 | 0.9950 | 0.9965 | 0.9951 | 0.9953 | 0.9953 | 0.9955 |
| Vmax | 104.04 | 96.07 | 96.07 | 95.85 | 90.59 | 94.83 | 94.83 | 96.93 |
| β (TBZ) | 2.35 | 4.78 | 4.78 | 5.14 | 4.70 | 4.92 | 4.92 | 4.86 |
| β (CYC) | 0.89 | 4.41 | 4.41 | 4.97 | 6.95 | 4.60 | 4.60 | 4.52 |
| EC50 (TBZ) | 6.97 | 6.05 | 6.05 | 6.05 | 7.41 | 6.07 | 6.07 | 6.02 |
| EC50 (CYC) | 314.78 | 183.43 | 183.43 | 184.14 | 233.72 | 184.49 | 184.49 | 182.59 |
| a | / | 4.74 | 4.74 | 3.81 | / | 9.70 | 9.70 | 1.12 |
| b | / | / | 0.00 | −0.07 | / | / | −1980.93 | −7.68 |
| SS | 15,806.37 | 616.74 | 616.74 | 518.89 | 8776.48 | 693.34 | 32,033.94 | 547.01 |
| χ2 | / | 84.59 | 84.75 | 89.08 | / | 65.88 | 67.19 | 72.17 |
| df | / | 1.00 | 2.00 | 2.00 | / | 1.00 | 2.00 | 2.00 |
| p (χ2) | / | 3.67 × 10−20 | 3.95 × 10−19 | 4.53 × 10−20 | / | 4.79 × 10−16 | 2.57 × 10−15 | 2.13 × 10−16 |
| r2 | 0.9791 | 0.9716 | 0.9702 | 0.9724 | 0.7734 | 0.9705 | 0.9656 | 0.9727 |
| Chronic | CA | IA | ||||||
|---|---|---|---|---|---|---|---|---|
| Refer | S/A | DR | DL | Refer | S/A | DR | DL | |
| Vmax | 2196.17 | 2196.45 | 2198.02 | 2198.81 | 206.50 | 179.97 | 178.08 | 186.42 |
| β (TBZ) | 1.68 | 1.68 | 1.89 | 1.61 | 4.44 | 5.73 | 6.79 | 4.76 |
| β (IMI) | 4.65 | 4.62 | 4.72 | 4.54 | 1.48 | 3.16 | 3.40 | 2.00 |
| EC50(TBZ) | 54.35 | 54.56 | 50.91 | 54.17 | 0.57 | 0.69 | 0.74 | 0.68 |
| EC50(IMI) | 669.43 | 671.31 | 680.18 | 667.96 | 5.16 | 8.00 | 7.55 | 7.73 |
| a | / | −0.02 | −2.47 | 0.22 | / | −4.57 | 2.18 | −0.03 |
| bcd | / | / | 4.96 | 0.89 | / | / | −13.62 | −339.9469 |
| SS | 383,154.75 | 383,059.67 | 258,571.93 | 381,424.58 | 12,815.76 | 7097.33 | 3804.39 | 4857.12 |
| χ2 | / | 2.27 | 17.87 | 3.77 | / | 13.00 | 26.72 | 21.35 |
| df | / | 2.00 | 1.00 | 1.00 | / | 2.00 | 1.00 | 1.00 |
| p (χ2) | / | 0.13 | 1.32 × 10−4 | 0.15 | / | 3.11 × 10−4 | 1.58 × 10−6 | 2.32 × 10−5 |
| r2 | 0.936 | 0.937 | 0.996 | 0.938 | 0.967 | 0.912 | 0.986 | 0.931 |
| Vmax | 171.43 | 172.17 | 173.77 | 172.61 | 189.03 | 185.03 | 173.04 | 186.63 |
| β (TBZ) | 142.00 | 2227.12 | 2197.37 | 2704.00 | 6.34 | 6.57 | 8.73 | 6.11 |
| β (CYC) | 1.78 | 1.58 | 1.60 | 1.56 | 1.45 | 1.68 | 3.08 | 1.46 |
| EC50 (TBZ) | 0.83 | 0.83 | 0.83 | 0.83 | 0.64 | 0.67 | 0.77 | 0.67 |
| EC50 (CYC) | 6.40 | 6.54 | 5.51 | 6.57 | 4.94 | 5.50 | 5.72 | 5.53 |
| a | / | −0.08 | 2.35 | −0.19 | / | −1.27 | 6.42 | −4.11 × 10−3 |
| bcd | / | / | −3.14 | 0.37 | / | / | −18.21 | −651.76 |
| SS | 8436.32 | 8194.40 | 5370.80 | 8138.52 | 9255.67 | 8468.10 | 3130.21 | 8025.17 |
| χ2 | / | 0.16 | 23.32 | 0.18 | / | 1.96 | 23.85 | 3.14 |
| df | / | 1.00 | 2.00 | 2.00 | / | 1.00 | 2.00 | 2.00 |
| p (χ2) | / | 0.69 | 8.62 × 10−6 | 0.91 | / | 0.16 | 6.62 × 10−6 | 0.21 |
| r2 | 0.863 | 0.863 | 0.991 | 0.865 | 0.944 | 0.902 | 0.995 | 0.900 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, Y.; Sun, T.; Wu, C.; Liu, X.; He, M. Evaluating the Impact of Individual and Combined Toxicity of Imidacloprid, Cycloxaprid, and Tebuconazole on Daphnia magna. Toxics 2023, 11, 428. https://doi.org/10.3390/toxics11050428
Man Y, Sun T, Wu C, Liu X, He M. Evaluating the Impact of Individual and Combined Toxicity of Imidacloprid, Cycloxaprid, and Tebuconazole on Daphnia magna. Toxics. 2023; 11(5):428. https://doi.org/10.3390/toxics11050428
Chicago/Turabian StyleMan, Yanli, Tian Sun, Chi Wu, Xingang Liu, and Mingyuan He. 2023. "Evaluating the Impact of Individual and Combined Toxicity of Imidacloprid, Cycloxaprid, and Tebuconazole on Daphnia magna" Toxics 11, no. 5: 428. https://doi.org/10.3390/toxics11050428
APA StyleMan, Y., Sun, T., Wu, C., Liu, X., & He, M. (2023). Evaluating the Impact of Individual and Combined Toxicity of Imidacloprid, Cycloxaprid, and Tebuconazole on Daphnia magna. Toxics, 11(5), 428. https://doi.org/10.3390/toxics11050428






