Enhanced Chromium (VI) Adsorption onto Waste Pomegranate-Peel-Derived Biochar for Wastewater Treatment: Performance and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PG and PG-B
2.3. Adsorption Experiments
2.4. Kinetics and Isotherm Analysis
2.5. Characterization of Materials
3. Results and Discussion
3.1. Characterization of PG and PG-B
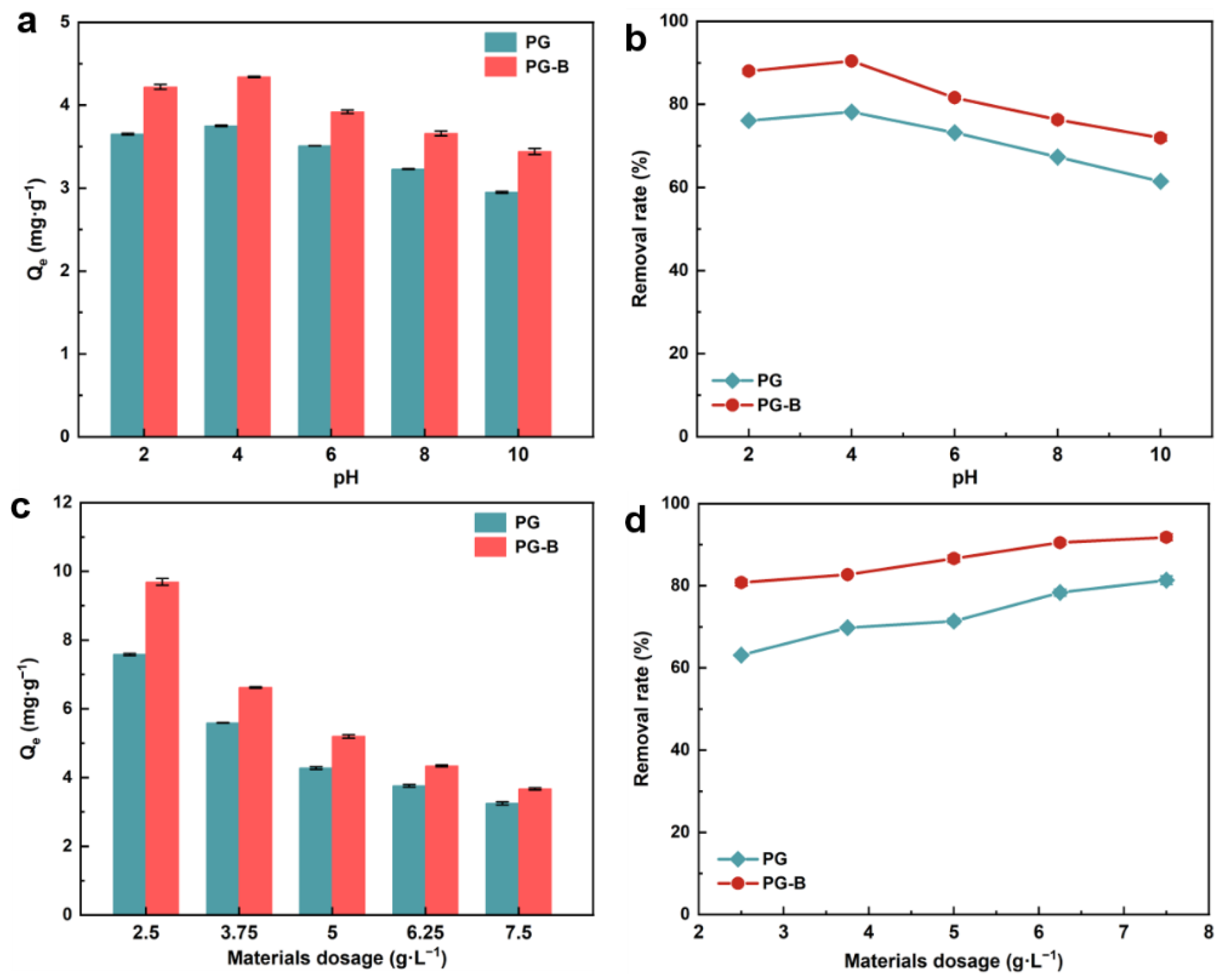
3.2. Effect of pH on the Adsorption Process
3.3. Effect of Material Dosage on the Adsorption Process
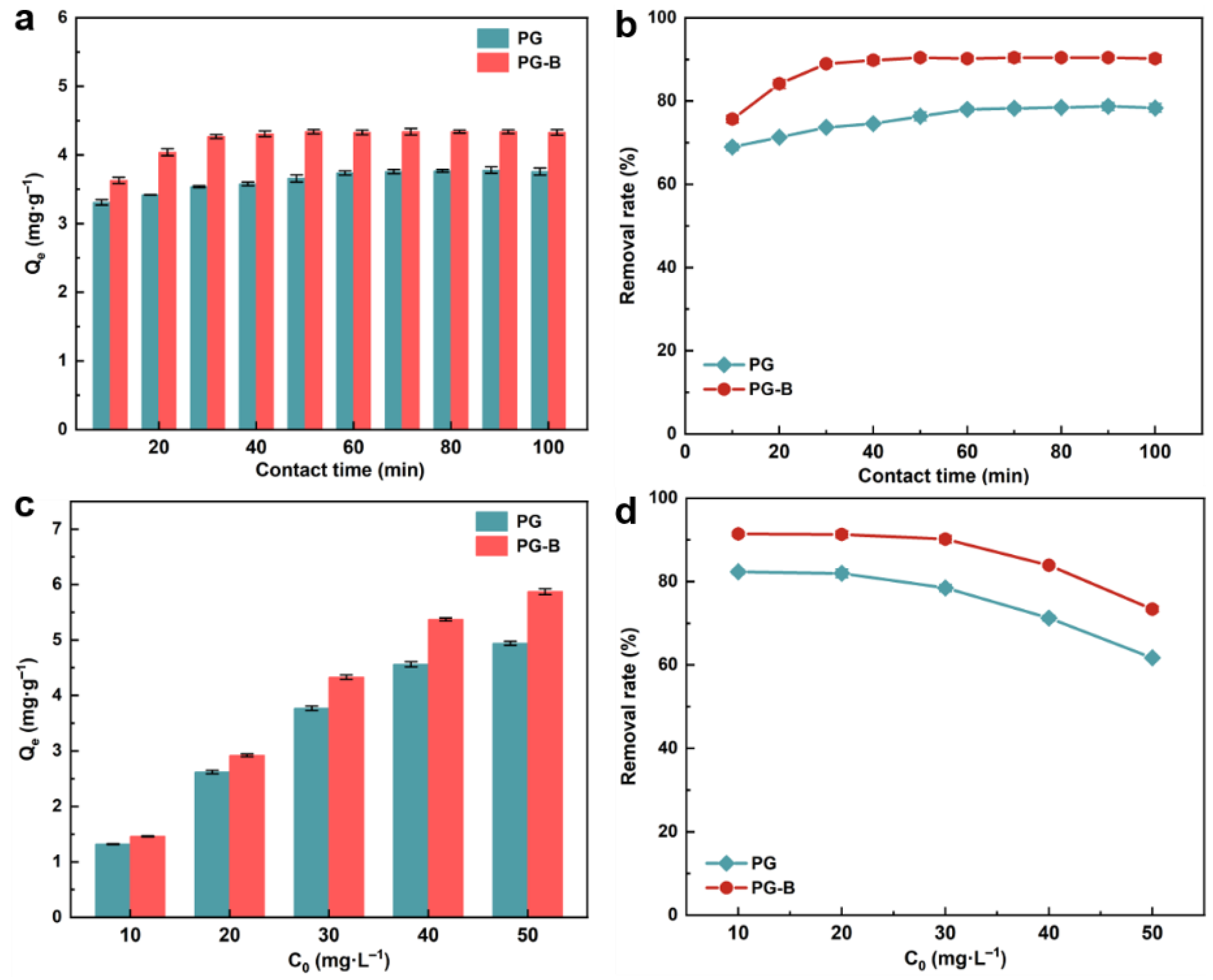
3.4. Effect of Contact Time
3.5. Effect of Initial Concentration
3.6. Mechanism Study
3.6.1. Adsorption Isotherms
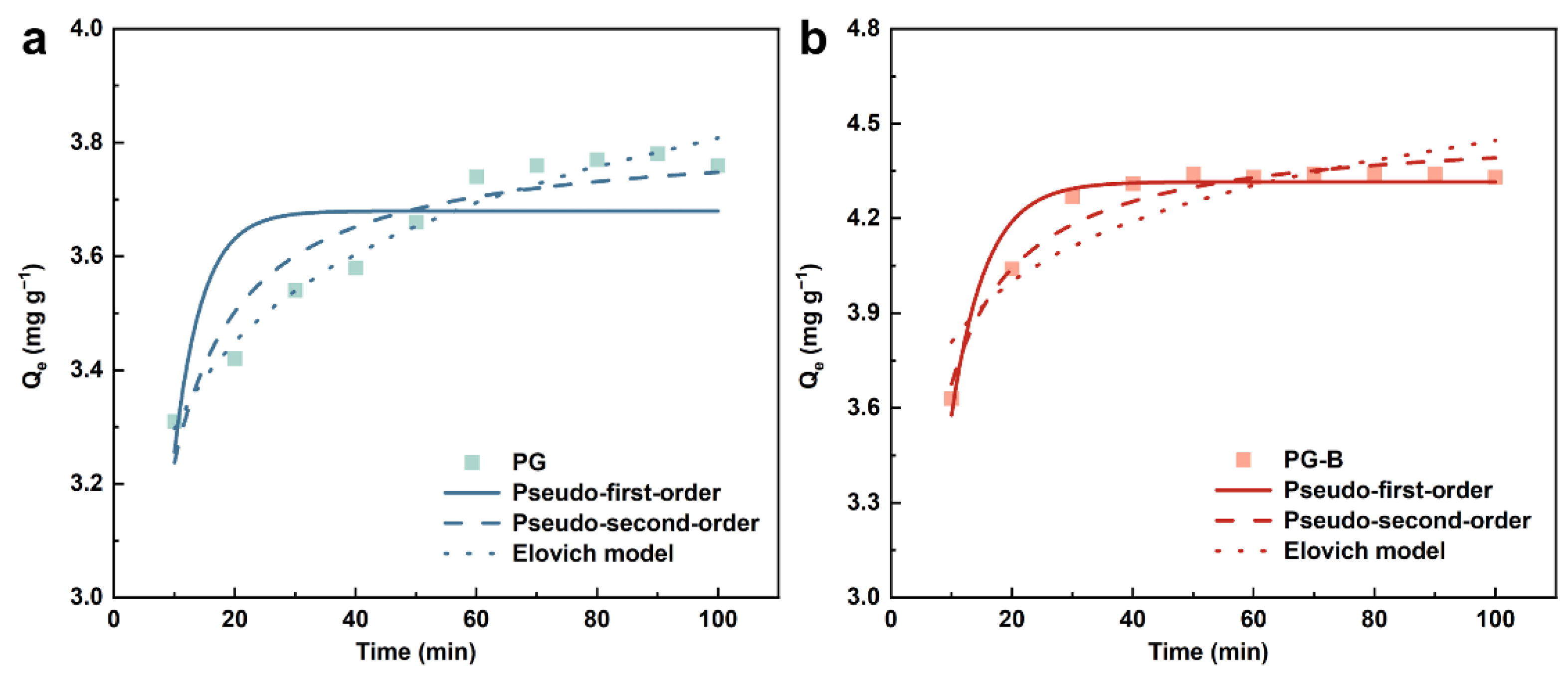
3.6.2. Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, W.; Li, J.; Li, H.; Jin, B.; Li, Z.; Zhang, T.; Zhu, X. Mechanistic insights into ball milling enhanced montmorillonite modification with tetramethylammonium for adsorption of gaseous toluene. Chemosphere 2022, 296, 133962. [Google Scholar] [CrossRef]
- Wang, C.C.; Du, X.D.; Li, J.; Guo, X.X.; Wang, P.; Zhang, J. Photocatalytic Cr(VI) reduction in metal-organic frameworks: A mini-review. Appl. Catal. B-Environ. 2016, 193, 198–216. [Google Scholar] [CrossRef]
- Islam, M.M.; Mohana, A.A.; Rahman, M.A.; Rahman, M.; Naidu, R.; Rahman, M.M. A Comprehensive Review of the Current Progress of Chromium Removal Methods from Aqueous Solution. Toxics 2023, 11, 252. [Google Scholar] [CrossRef]
- Das, S.; Mishra, J.; Das, S.K.; Pandey, S.; Rao, D.S.; Chakraborty, A.; Sudarshan, M.; Das, N.; Thatoi, H. Investigation on mechanism of Cr(VI) reduction and removal by Bacillus amyloliquefaciens, a novel chromate tolerant bacterium isolated from chromite mine soil. Chemosphere 2014, 96, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Xu, J.; Jiang, X.; Liu, C.; McCall, W.; Lu, J. Stabilization of heavy metals in soil using two organo-bentonites. Chemosphere 2017, 184, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, G.; Dai, Z.; Zhou, L.; Bian, P.; Zheng, K.; Wu, Z.; Cai, D. Sandwich-like Nanosystem for Simultaneous Removal of Cr(VI) and Cd(II) from Water and Soil. ACS Appl. Mater. Interfaces 2018, 10, 18316–18326. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lin, H.; Luo, M.; Liu, J.; Dong, Y.; Li, B. Highly efficient remediation of groundwater co-contaminated with Cr(VI) and nitrate by using nano-Fe/Pd bimetal-loaded zeolite: Process product and interaction mechanism. Environ. Pollut. 2020, 263, 114479. [Google Scholar] [CrossRef]
- Fu, L.; Feng, A.; Xiao, J.; Wu, Q.; Ye, Q.; Peng, S. Remediation of soil contaminated with high levels of hexavalent chromium by combined chemical-microbial reduction and stabilization. J. Hazard. Mater. 2021, 403, 123847. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Cao, S.; Zhang, L.; Peng, X.; Wang, X.; Ai, Z.; Zhang, L. Structural dependent Cr(VI) adsorption and reduction of biochar: Hydrochar versus pyrochar. Sci. Total Environ. 2021, 783, 147084. [Google Scholar] [CrossRef]
- Zhitkovich, A. Chromium in Drinking Water: Sources, Metabolism, and Cancer Risks. Chem. Res. Toxicol. 2011, 24, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Singh, N.; Rai, S.N.; Kumar, A.; Singh, A.K.; Singh, M.P.; Sahoo, A.; Shekhar, S.; Vamanu, E.; Mishra, V. Heavy Metal Contamination in the Aquatic Ecosystem: Toxicity and Its Remediation Using Eco-Friendly Approaches. Toxics 2023, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Shin, J.H.; Oh, W.; Choi, S.J.; Kim, J.; Kim, C.; Jeon, J. Removal of Hexavalent Chromium(VI) from Wastewater Using Chitosan-Coated Iron Oxide Nanocomposite Membranes. Toxics 2022, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhang, H.; Zhao, P.; Zhao, X.; Sun, H.; Geng, Z.; She, D. Synthesis of a novel three-dimensional porous carbon material and its highly selective Cr(VI) removal in wastewater. J. Clean. Prod. 2021, 306, 127204. [Google Scholar] [CrossRef]
- Xia, S.; Song, Z.; Jeyakumar, P.; Bolan, N.; Wang, H. Characteristics and applications of biochar for remediating Cr(VI)-contaminated soils and wastewater. Environ. Geochem. Health 2020, 42, 1543–1567. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cao, G.; Zhu, R. Adsorption of Cr(VI) from aqueous solution by a litchi shell-based adsorbent. Environ. Res. 2021, 196, 110356. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Y.; Liang, H.; Gao, C.; Qin, J.; You, L.; Wang, R.; Li, J.; Yang, S. Adsorption of Cr(VI) from aqueous solutions using novel activated carbon spheres derived from glucose and sodium dodecylbenzene sulfonate. Sci. Total Environ. 2021, 759, 143457. [Google Scholar] [CrossRef]
- Zeng, B.; Xu, W.; Khan, S.B.; Wang, Y.; Zhang, J.; Yang, J.; Su, X.; Lin, Z. Preparation of sludge biochar rich in carboxyl/hydroxyl groups by quenching process and its excellent adsorption performance for Cr(VI). Chemosphere 2021, 285, 131439. [Google Scholar] [CrossRef]
- Basu, M.; Guha, A.K.; Ray, L. Adsorption of Lead on Cucumber Peel. J. Clean. Prod. 2017, 151, 603–615. [Google Scholar] [CrossRef]
- Huang, X.; Wei, D.; Zhang, X.; Fan, D.; Sun, X.; Du, B.; Wei, Q. Synthesis of amino-functionalized magnetic aerobic granular sludge-biochar for Pb(II) removal: Adsorption performance and mechanism studies. Sci. Total Environ. 2019, 685, 681–689. [Google Scholar] [CrossRef]
- Liu, L.; Huang, Y.; Zhang, S.; Gong, Y.; Su, Y.; Cao, J.; Hu, H. Adsorption characteristics and mechanism of Pb(II) by agricultural waste-derived biochars produced from a pilot-scale pyrolysis system. Waste Manag. 2019, 100, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Gaur, R.; Shahabuddin, S.; Tyagi, I. Biochar as Sustainable Alternative and Green Adsorbent for the Remediation of Noxious Pollutants: A Comprehensive Review. Toxics 2023, 11, 117. [Google Scholar] [CrossRef]
- Alharbi, H.A.; Alotaibi, K.D.; EL-Saeid, M.H.; Giesy, J.P. Polycyclic Aromatic Hydrocarbons (PAHs) and Metals in Diverse Biochar Products: Effect of Feedstock Type and Pyrolysis Temperature. Toxics 2023, 11, 96. [Google Scholar] [CrossRef]
- Olea-Mejia, O.; Cabral-Prieto, A.; Salcedo-Castillo, U.; Lopez-Tellez, G.; Olea-Cardoso, O.; Lopez-Castanares, R. Orange peel plus nanostructured zero-valent-iron composite for the removal of hexavalent chromium in water. Appl. Surf. Sci. 2017, 423, 170–175. [Google Scholar] [CrossRef]
- Rosales, E.; Meijide, I.; Tavares, T.; Pazos, M.; Sanroman, M.A. Grapefruit peelings as a promising biosorbent for the removal of leather dyes and hexavalent chromium. Process. Saf. Environ. Prot. 2016, 101, 61–71. [Google Scholar] [CrossRef]
- Enniya, I.; Rghioui, L.; Jourani, A. Adsorption of hexavalent chromium in aqueous solution on activated carbon prepared from apple peels. Sustain. Chem. Pharm. 2018, 7, 9–16. [Google Scholar] [CrossRef]
- Shakya, A.; Nunez-Delgado, A.; Agarwal, T. Biochar synthesis from sweet lime peel for hexavalent chromium remediation from aqueous solution. J. Environ. Manag. 2019, 251, 109570. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, A.; Sisnandy, V.O.A.; Trilestari, K.; Sunarso, J.; Indraswati, N.; Ismadji, S. Performance of durian shell waste as high capacity biosorbent for Cr(VI) removal from synthetic wastewater. Ecol. Eng. 2011, 37, 940–947. [Google Scholar] [CrossRef]
- Yin, Z.; Xu, S.; Liu, S.; Xu, S.; Li, J.; Zhang, Y. A novel magnetic biochar prepared by K2FeO4-promoted oxidative pyrolysis of pomelo peel for adsorption of hexavalent chromium. Bioresour. Technol. 2020, 300, 122680. [Google Scholar] [CrossRef]
- Rashtbari, Y.; Hazrati, S.; Azari, A.; Afshin, S.; Fazlzadeh, M.; Vosoughi, M. A novel, eco-friendly and green synthesis of PPAC-ZnO and PPAC-nZVI nanocomposite using pomegranate peel: Cephalexin adsorption experiments, mechanisms, isotherms and kinetics. Adv. Powder Technol. 2020, 31, 1612–1623. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Perez-Alvarez, J.A.; Viuda-Martos, M. Assessment of polyphenolic profile and antibacterial activity of pomegranate peel (Punica granatum) flour obtained from co-product of juice extraction. Food Control. 2016, 59, 94–98. [Google Scholar] [CrossRef]
- Ben-Ali, S.; Jaouali, I.; Souissi-Najar, S.; Ouederni, A. Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J. Clean. Prod. 2017, 142, 3809–3821. [Google Scholar] [CrossRef]
- Seliem, M.K.; Mobarak, M.; Selim, A.Q.; Mohamed, E.A.; Halfaya, R.A.; Gomaa, H.K.; Anastopoulos, I.; Giannakoudakis, D.A.; Lima, E.C.; Bonilla-Petriciolet, A.; et al. A novel multifunctional adsorbent of pomegranate peel extract and activated anthracite for Mn(VII) and Cr(VI) uptake from solutions: Experiments and theoretical treatment. J. Mol. Liq. 2020, 311, 113169. [Google Scholar] [CrossRef]
- Abdelhafez, A.A.; Li, J.H. Removal of Pb(II) from aqueous solution by using biochars derived from sugar cane bagasse and orange peel. J. Taiwan Inst. Chem. E 2016, 61, 367–375. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Preparation, characterization and adsorption studies of the chemically modified Luffa aegyptica peel as a potential adsorbent for the removal of malachite green from aqueous solution. J. Mol. Liq. 2019, 274, 315–327. [Google Scholar] [CrossRef]
- Waqas, M.; Aburiazaiza, A.S.; Miandad, R.; Rehan, M.; Barakat, M.A.; Nizami, A.S. Development of biochar as fuel and catalyst in energy recovery technologies. J. Clean. Prod. 2018, 188, 477–488. [Google Scholar] [CrossRef]
- Allouss, D.; Essamlali, Y.; Chakir, A.; Khadhar, S.; Zahouily, M. Effective removal of Cu(II) from aqueous solution over graphene oxide encapsulated carboxymethylcellulose-alginate hydrogel microspheres: Towards real wastewater treatment plants. Environ. Sci. Pollut. Res. 2020, 27, 7476–7492. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, Z.; Feng, L.; Luo, X.; Wu, P.; Cui, L.; Mao, X. Modified cellulose by polyethyleneimine and ethylenediamine with induced Cu(II) and Pb(II) adsorption potentialities. Carbohydr. Polym. 2018, 202, 470–478. [Google Scholar] [CrossRef]
- Sajjadi, S.-A.; Meknati, A.; Lima, E.C.; Dotto, G.L.; Ileana Mendoza-Castillo, D.; Anastopoulos, I.; Alakhras, F.; Unuabonah, E.I.; Singh, P.; Hosseini-Bandegharaei, A. A novel route for preparation of chemically activated carbon from pistachio wood for highly efficient Pb(II) sorption. J. Environ. Manag. 2019, 236, 34–44. [Google Scholar] [CrossRef]
- Ali, M.E.M.; Abdelsalam, H.; Ammar, N.S.; Ibrahim, H.S. Response surface methodology for optimization of the adsorption capability of ball-milled pomegranate peel for different pollutants. J. Mol. Liq. 2018, 250, 433–445. [Google Scholar] [CrossRef]
- Shang, J.; Guo, Y.; He, D.; Qu, W.; Tang, Y.; Zhou, L.; Zhu, R. A novel graphene oxide-dicationic ionic liquid composite for Cr(VI) adsorption from aqueous solutions. J. Hazard. Mater. 2021, 416, 125706. [Google Scholar] [CrossRef] [PubMed]
- Lan Huong, N.; Huu Tap, V.; Quang Trung, N.; Thu Huong, N.; Thi Bich Lien, N.; Van Quang, N.; Thu Uyen, B.; Hung Le, S. Paper waste sludge derived-hydrochar modified by iron (III) chloride for effective removal of Cr(VI) from aqueous solution: Kinetic and isotherm studies. J. Water Process Eng. 2021, 39, 101877. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y.; Liu, S.; Yin, Y.; Zeng, G.; Tan, X.; Hu, X.; Hu, X.; Jiang, L.; Ding, Y.; et al. Investigation of the adsorption-reduction mechanisms of hexavalent chromium by ramie biochars of different pyrolytic temperatures. Bioresour. Technol. 2016, 218, 351–359. [Google Scholar] [CrossRef]
- Fang, L.; Ding, L.; Ren, W.; Hu, H.; Huang, Y.; Shao, P.; Yang, L.; Shi, H.; Ren, Z.; Han, K.; et al. High exposure effect of the adsorption site significantly enhanced the adsorption capacity and removal rate: A case of adsorption of hexavalent chromium by quaternary ammonium polymers (QAPs). J. Hazard. Mater. 2021, 416, 125829. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Guan, X.-H.; Xu, X.-H.; Wei, D.-Z. Characteristic and mechanism of Cr(VI) adsorption by ammonium sulfamate-bacterial cellulose in aqueous solutions. Chin. Chem. Lett. 2013, 24, 253–256. [Google Scholar] [CrossRef]
- Sun, Y.; Yue, Q.; Gao, B.; Gao, Y.; Li, Q.; Wang, Y. Adsorption of hexavalent chromium on Arundo donax Linn activated carbon amine-crosslinked copolymer. Chem. Eng. J. 2013, 217, 240–247. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Al-Muhtaseb, A.a.H.; Al-laqtah, N.A.; Walker, G.M.; Allen, S.J.; Ahmad, M.N.M. Biosorption of toxic chromium from aqueous phase by lignin: Mechanism, effect of other metal ions and salts. Chem. Eng. J. 2011, 169, 20–30. [Google Scholar] [CrossRef]
- Pavithra, S.; Thandapani, G.; Sugashini, S.; Sudha, P.N.; Alkhamis, H.H.; Alrefaei, A.F.; Almutairi, M.H. Batch adsorption studies on surface tailored chitosan/orange peel hydrogel composite for the removal of Cr(VI) and Cu(II) ions from synthetic wastewater. Chemosphere 2021, 271, 129415. [Google Scholar] [CrossRef]
- Touihri, M.; Guesmi, F.; Hannachi, C.; Hamrouni, B.; Sellaoui, L.; Badawi, M.; Poch, J.; Fiol, N. Single and simultaneous adsorption of Cr(VI) and Cu (II) on a novel Fe3O4/pine cones gel beads nanocomposite: Experiments, characterization and isotherms modeling. Chem. Eng. J. 2021, 416, 129101. [Google Scholar] [CrossRef]
- Rafiaee, S.; Samani, M.R.; Toghraie, D. Removal of hexavalent chromium from aqueous media using pomegranate peels modified by polymeric coatings: Effects of various composite synthesis parameters. Synth. Met. 2020, 265, 116416. [Google Scholar] [CrossRef]
- Oliveira, M.R.F.; Abreu, K.D.; Romao, A.L.E.; Davi, D.M.B.; Magalhaes, C.E.D.; Carrilho, E.N.V.M.; Alves, C.R. Carnauba (Copernicia prunifera) palm tree biomass as adsorbent for Pb(II) and Cd(II) from water medium. Environ. Sci. Pollut. Res. 2021, 28, 18941–18952. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Application of the biochar derived from orange peel for effective biosorption of copper and cadmium in batch studies: Isotherm models and kinetic studies. Arab. J. Geosci. 2019, 12, 553–566. [Google Scholar] [CrossRef]
- Wang, C.; Gu, L.; Liu, X.; Zhang, X.; Cao, L.; Hu, X. Sorption behavior of Cr(VI) on pineapple-peel-derived biochar and the influence of coexisting pyrene. Int. Biodeterior. Biodegrad. 2016, 111, 78–84. [Google Scholar] [CrossRef]
- Hoang, L.P.; Van, H.T.; Nguyen, L.H.; Mac, D.H.; Vu, T.T.; Ha, L.T.; Nguyen, X.C. Removal of Cr(VI) from aqueous solution using magnetic modified biochar derived from raw corncob. N. J. Chem. 2019, 43, 18663–18672. [Google Scholar] [CrossRef]
- Dong, F.X.; Yan, L.; Zhou, X.H.; Huang, S.T.; Liang, J.Y.; Zhang, W.X.; Guo, Z.W.; Guo, P.R.; Qian, W.; Kong, L.J.; et al. Simultaneous adsorption of Cr(VI) and phenol by biochar-based iron oxide composites in water: Performance, kinetics and mechanism. J. Hazard. Mater. 2021, 416, 125930. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.H.; Abdulhameed, A.S.; Wilson, L.D.; Syed-Hassan SS, A.; Alothman, Z.A.; Khan, M.R. High surface area and mesoporous activated carbon from KOH-activated dragon fruit peels for methylene blue dye adsorption: Optimization and mechanism study. Chin. J. Chem. Eng. 2021, 32, 281–290. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A. Mesoporous activated carbons of low-cost agricultural bio-wastes with high adsorption capacity: Preparation and artificial neural network modeling of dye removal from single and multicomponent (binary and ternary) systems. J. Mol. Liq. 2018, 269, 217–228. [Google Scholar] [CrossRef]
- Amin, N.K. Removal of direct blue-106 dye from aqueous solution using new activated carbons developed from pomegranate peel: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2009, 165, 52–62. [Google Scholar] [CrossRef]
- Dinh, V.-P.; Nguyen, D.-K.; Luu, T.-T.; Nguyen, Q.-H.; Tuyen, L.A.; Phong, D.D.; Kiet HA, T.; Ho, T.-H.; Nguyen TT, P.; Xuan, T.D.; et al. Adsorption of Pb(II) from aqueous solution by pomelo fruit peel-derived biochar. Mater. Chem. Phys. 2022, 285, 126105. [Google Scholar] [CrossRef]
- Romero-Cano, L.A.; Garcia-Rosero, H.; Gonzalez-Gutierrez, L.V.; Baldenegro-Perez, L.A.; Carrasco-Marin, F. Functionalized adsorbents prepared from fruit peels: Equilibrium, kinetic and thermodynamic studies for copper adsorption in aqueous solution. J. Clean. Prod. 2017, 162, 195–204. [Google Scholar] [CrossRef]
- Giri, R.; Kumari, N.; Behera, M.; Sharma, A.; Kumar, S.; Kumar, N.; Singh, R. Adsorption of hexavalent chromium from aqueous solution using pomegranate peel as low-cost biosorbent. Environ. Sustain. 2021, 4, 401–417. [Google Scholar] [CrossRef]
- Ben Khalifa, E.; Rzig, B.; Chakroun, R.; Nouagui, H.; Hamrouni, B. Application of response surface methodology for chromium removal by adsorption on low-cost biosorbent. Chemom. Intell. Lab. Syst. 2019, 189, 18–26. [Google Scholar] [CrossRef]
- Yi, Y.; Lv, J.; Liu, Y.; Wu, G. Synthesis and application of modified Litchi peel for removal of hexavalent chromium from aqueous solutions. J. Mol. Liq. 2017, 225, 28–33. [Google Scholar] [CrossRef]
- Nag, S.; Mondal, A.; Bar, N.; Das, S.K. Biosorption of chromium (VI) from aqueous solutions and ANN modelling. Environ. Sci. Pollut. Res. 2017, 24, 18817–18835. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Chen, L.F.; Li, F.Y.; Chen, K.L.; Wan, W.Y.; Tang, Y.J. Removal of Cr (VI) with wheat-residue derived black carbon: Reaction mechanism and adsorption performance. J. Hazard. Mater. 2010, 175, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Garg, V.K.; Kadirvelu, K. Adsorption of hexavalent chromium from aqueous medium onto carbonaceous adsorbents prepared from waste biomass. J. Environ. Manag. 2010, 91, 949–957. [Google Scholar] [CrossRef]
- Bansal, M.; Singh, D.; Garg, V.K. A comparative study for the removal of hexavalent chromium from aqueous solution by agriculture wastes’ carbons. J. Hazard. Mater. 2009, 171, 83–92. [Google Scholar] [CrossRef]
- Dobrowolski, R.; Otto, M. Study of chromium(VI) adsorption onto modified activated carbons with respect to analytical application. Adsorption 2010, 16, 279–286. [Google Scholar] [CrossRef]
- Qin, L.; He, L.; Yang, W.; Lin, A. Preparation of a novel iron-based biochar composite for removal of hexavalent chromium in water. Environ. Sci. Pollut. Res. 2020, 27, 9214–9226. [Google Scholar] [CrossRef]
- Shi, J.; Simal-Gandara, J.; Mei, J.; Ma, W.; Peng, Q.; Shi, Y.; Xu, Q.; Lin, Z.; Lv, H. Insight into the pigmented anthocyanins and the major potential co-pigmented flavonoids in purple-coloured leaf teas. Food Chem. 2021, 363, 130278. [Google Scholar] [CrossRef]
- Sun, X.; Li, C.; Ma, J.; Zang, Y.; Huang, J.; Chen, N.; Wang, X.; Zhang, D. New amide alkaloids and carbazole alkaloid from the stems of Clausena lansium. Fitoterapia 2021, 154, 104999. [Google Scholar] [CrossRef]
- Mahindrakar, K.V.; Rathod, V.K. Ultrasonic assisted aqueous extraction of catechin and gallic acid from Syzygium cumini seed kernel and evaluation of total phenolic, flavonoid contents and antioxidant activity. Chem. Eng. Process. 2020, 149, 107841. [Google Scholar] [CrossRef]
- Shahkoomahally, S.; Khadivi, A.; Brecht, J.K.; Sarkhosh, A. Chemical and physical attributes of fruit juice and peel of pomegranate genotypes grown in Florida, USA. Food Chem. 2021, 342, 128302. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhang, Y.; He, Z.; Chang, F.; Zhang, H.; Wagberg, T.; Hu, G. Mesopore-rich badam-shell biochar for efficient adsorption of Cr(VI) from aqueous solution. J. Environ. Chem. Eng. 2021, 9, 105634. [Google Scholar] [CrossRef]
- Ranasinghe, S.H.; Navaratne, A.N.; Priyantha, N. Enhancement of adsorption characteristics of Cr(III) and Ni(II) by surface modification of jackfruit peel biosorbent. J. Environ. Chem. Eng. 2018, 6, 5670–5682. [Google Scholar] [CrossRef]



| Functional Group | Adsorption Peaks of PG (cm−1) | Adsorption Peaks of PG-B (cm−1) | ||
|---|---|---|---|---|
| Before Reaction | After Reaction | Before Reaction | After Reaction | |
| −OH | 3389 | 3387 | 3402 | 3422 |
| C−H | 2931 | 2935 | 2925 | 2929 |
| C=O | 1731 | 1737 | — | — |
| C=C | 1616 | 1640 | 1558 | 1566 |
| C−N | 1353 | 1366 | 1385 | 1395 |
| C−O | 1043 | 1063 | 1112 | 1098 |
| C−H | — | — | 956 | 961 |
| N−H | — | — | 625 | 606 |
| Material | Langmuir Isotherm | Freundlich Isotherm | D-R Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Q0 (mg g−1) | KL (mg L−1) | R2 | KF (mg g−1)(L mg−1)1/n | n | R2 | Q0 (mmol g−1) | E (J mol−1) | R2 | |
| PG | 12.949 | 0.0130 | 0.9859 | 1.226 | 1.533 | 0.9698 | 10.256 | 12.231 | 0.9854 |
| PG-B | 16.229 | 0.0114 | 0.9945 | 1.486 | 0.981 | 0.9750 | 12.564 | 13.875 | 0.9786 |
| Pollutants | Sorbent | Operating Conditions | Adsorption Capacity (mg g−1) | References |
|---|---|---|---|---|
| Methylene blue dye | Dragon fruit peel s activated carbon | pH 10, dose 80 mg, contact time 60 min | 195.2 | [56] |
| Methylene blue dye | Kiwi peel, cucumber peel, and potato peel activated carbon | pH 6.3, dose 25 mg, contact time 180 min | 435, 476, 385 | [57] |
| Blue-106 dye | Pomegranate peel activated carbon | pH 2, dose 250 mg, contact time 120 min | 58.14 | [58] |
| Cu(II) | Raw pomegranate peel | pH 5.8, dose 250 mg, contact time 120 min | 30.12 | [32] |
| Pb(II) | Pomelo fruit peel-derived biochar | pH 5, dose 100 mg, contact time 120 min | 90.3 | [59] |
| Cu(II) | Pineapple peel | pH 5, dose 400 mg, contact time 30 min | 64.33 | [60] |
| Cr(VI) | Pomegranate peel | pH 2, dose 100 mg, contact time 120 min | 38.29 | [61] |
| Cr(VI) | Orange peel | pH 2, dose 112 mg, contact time 300 min | 7.14 | [62] |
| Cr(VI) | Modified Litchi peel | pH 4, dose 80 mg, contact time 100 min | 9.55 | [63] |
| Cr(VI) | Coconut shell | pH 2.3, dose 500 mg, contact time 200 min | 8.73 | [64] |
| Cr(VI) | Pomegranate-Peel-Derived Biochar | pH 4, dose 250 mg, contact time 30 min | 16.23 | This study |
| Material | Pseudo-First-Order Model | Pseudo-Second-Order Model | Elovich | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k1 (min−1) | Qe (mg g−1) | R2 | k2 (g mg−1 min−1) | Qe (mg g−1) | R2 | a (g mg−1 min−1) | b (mg g−1 min0.5) | R2 | |
| PG | 0.017 | 3.679 | 0.5584 | 0.148 | 3.813 | 0.8925 | 6.879 | 3.368 | 0.9668 |
| PG-B | 0.081 | 4.316 | 0.9397 | 0.102 | 4.489 | 0.9547 | 8.265 | 5.283 | 0.7585 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yang, J.; Abbas, A. Enhanced Chromium (VI) Adsorption onto Waste Pomegranate-Peel-Derived Biochar for Wastewater Treatment: Performance and Mechanism. Toxics 2023, 11, 440. https://doi.org/10.3390/toxics11050440
Chen Y, Yang J, Abbas A. Enhanced Chromium (VI) Adsorption onto Waste Pomegranate-Peel-Derived Biochar for Wastewater Treatment: Performance and Mechanism. Toxics. 2023; 11(5):440. https://doi.org/10.3390/toxics11050440
Chicago/Turabian StyleChen, Yingzhou, Jinyan Yang, and Adil Abbas. 2023. "Enhanced Chromium (VI) Adsorption onto Waste Pomegranate-Peel-Derived Biochar for Wastewater Treatment: Performance and Mechanism" Toxics 11, no. 5: 440. https://doi.org/10.3390/toxics11050440
APA StyleChen, Y., Yang, J., & Abbas, A. (2023). Enhanced Chromium (VI) Adsorption onto Waste Pomegranate-Peel-Derived Biochar for Wastewater Treatment: Performance and Mechanism. Toxics, 11(5), 440. https://doi.org/10.3390/toxics11050440







