A Sensitive Response Index Selection for Rapid Assessment of Heavy Metals Toxicity to the Photosynthesis of Chlorella pyrenoidosa Based on Rapid Chlorophyll Fluorescence Induction Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Culture
2.2. Heavy Metals Exposure Experiments
2.3. OJIP Curve Measurement
2.4. Photosynthetic Fluorescence Parameters Acquisition
2.5. Data Processing and Statistical Analysis
3. Results
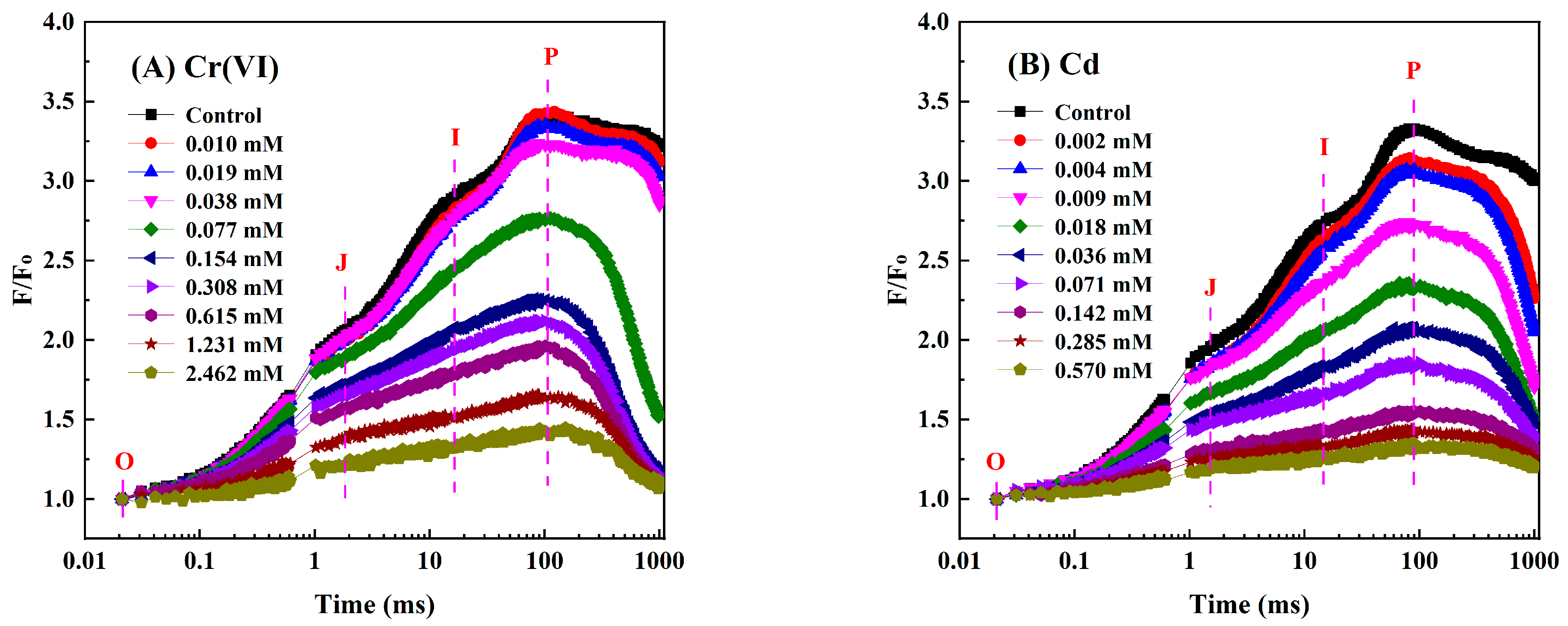
3.1. Influences of Four Heavy Metals on the OJIP Curve
3.2. Changes of Different Parameters with Heavy Metals Concentrations
3.3. Comparison of Response Performances of φPo, FV/FO, PIABS and Sm to Four Heavy Metals Toxicity
3.4. Comparison of the Toxicity of Four Heavy Metals to the Photosynthesis of C. pyrenoidosa during Short-Term Stress within 4 h by PIABS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Appenroth, K.J.; Stöckel, J.; Srivastava, A.; Strasser, R.J. Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probed by OJIP chlorophyll a fluorescence measurements. Environ. Pollut. 2001, 115, 49–64. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Samadani, M.; Perreault, F.; Oukarroum, A.; Dewez, D. Effect of cadmium accumulation on green algae Chlamydomonas reinhardtii and acid-tolerant Chlamydomonas CPCC 121. Chemosphere 2018, 191, 174–182. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Gao, L.; Duan, P.; Wu, H.; Li, M. Evaluating combined toxicity of binary heavy metals to the cyanobacterium Microcystis: A theoretical non-linear combined toxicity assessment method. Ecotoxicol. Environ. Saf. 2020, 187, 109809. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xu, Y.; Hu, N.; Ma, J.; Sun, S.; Cao, W.; Klobučar, G.; Hu, C.; Zhao, Y. To evaluate the toxicity of atrazine on the freshwater microalgae Chlorella sp. using sensitive indices indicated by photosynthetic parameters. Chemosphere 2020, 244, 125514. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.L.G.; Marques, L.G.; Colepicolo, P. Antioxidant enzymes are induced by phenol in the marine microalga Lingulodinium polyedrum. Ecotoxicol. Environ. Saf. 2015, 116, 84–89. [Google Scholar] [CrossRef]
- Dos Reis, L.L.; Alho, L.D.O.G.; de Abreu, C.B.; Melao, M.D.G.G. Using multiple endpoints to assess the toxicity of cadmium and cobalt for chlorophycean Raphidocelis subcapitata. Ecotoxicol. Environ. Saf. 2021, 208, 111628. [Google Scholar] [CrossRef]
- Rocha, G.S.; Parrish, C.C.; Espíndola, E.L.G. Effects of copper on photosynthetic and physiological parameters of a freshwater microalga (Chlorophyceae). Algal Res. 2021, 54, 102223. [Google Scholar] [CrossRef]
- Rocha, G.S.; Lombardi, A.T.; Espíndola, E.L.G. Combination of P-limitation and cadmium in photosynthetic responses of the freshwater microalga Ankistrodesmus densus (Chlorophyceae). Environ. Pollut. 2021, 275, 116673. [Google Scholar] [CrossRef]
- Aksmann, A.; Pokora, W.; Baścik-Remisiewicz, A.; Dettlaff-Pokora, A.; Wielgomas, B.; Dziadziuszko, M.; Tukaj, Z. Time-dependent changes in antioxidative enzyme expression and photosynthetic activity of Chlamydomonas reinhardtii cells under acute exposure to cadmium and anthracene. Ecotoxicol. Environ. Saf. 2014, 110, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Duan, G.; Fang, Y.; Deng, X.; Yin, Y.; Huang, K. Selenium(IV) alleviates chromium(VI)-induced toxicity in the green alga Chlamydomonas reinhardtii. Environ. Pollut. 2021, 272, 116407. [Google Scholar] [CrossRef] [PubMed]
- Didur, O.; Dewez, D.; Popovic, R. Alteration of chromium effect on photosystem II activity in Chlamydomonas reinhardtii cultures under different synchronized state of the cell cycle. Environ. Sci. Pollut. Res. 2013, 20, 1870–1875. [Google Scholar] [CrossRef]
- Da Costa, C.H.; Perreault, F.; Oukarroum, A.; Melegari, S.P.; Popovic, R.; Matias, W.G. Effect of chromium oxide (III) nanoparticles on the production of reactive oxygen species and photosystem II activity in the green alga Chlamydomonas reinhardtii. Sci. Total Environ. 2016, 565, 951–960. [Google Scholar] [CrossRef]
- Wang, S.; Wufuer, R.; Duo, J.; Li, W.; Pan, X. Cadmium caused different toxicity to photosystem I and photosystem II of freshwater unicellular algae Chlorella pyrenoidosa (Chlorophyta). Toxics 2022, 10, 352. [Google Scholar] [CrossRef]
- Oukarroum, A.; Perreault, F.; Popovic, R. Interactive effects of temperature and copper on photosystem II photochemistry in Chlorella vulgaris. J. Photochem. Photobiol. B Biol. 2012, 110, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Dahms, H.-U.; Lee, J.-S.; Kim, H.C.; Lee, W.C.; Shin, K.-H. Algal photosynthetic responses to toxic metals and herbicides assessed by chlorophyll a fluorescence. Ecotoxicol. Environ. Saf. 2014, 104, 51–71. [Google Scholar] [CrossRef]
- Perales-Vela, H.V.; González-Moreno, S.; Montes-Horcasitas, C.; Canizares-Villanueva, R.O. Growth, photosynthetic and respiratory responses to sub-lethal copper concentrations in Scenedesmus incrassatulus (Chlorophyceae). Chemosphere 2007, 67, 2274–2281. [Google Scholar] [CrossRef]
- Sun, C.; Li, W.; Xu, Y.; Hu, N.; Ma, J.; Cao, W.; Sun, S.; Hu, C.; Zhao, Y.; Huang, Q. Effects of carbon nanotubes on the toxicities of copper, cadmium and zinc toward the freshwater microalgae Scenedesmus obliquus. Aquat. Toxicol. 2020, 224, 105504. [Google Scholar] [CrossRef]
- Dao, L.H.T.; Beardall, J. Effects of lead on two green microalgae Chlorella and Scenedesmus: Photosystem II activity and heterogeneity. Algal Res. 2016, 16, 150–159. [Google Scholar] [CrossRef]
- Shivagangaiah, C.P.; Sanyal, D.; Dasgupta, S.; Banik, A. Phycoremediation and photosynthetic toxicity assessment of lead by two freshwater microalgae Scenedesmus acutus and Chlorella pyrenoidosa. Physiol. Plant. 2021, 173, 246–258. [Google Scholar]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborsk, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Durrieu, C.; Tran-Minh, C.; Chovelon, J.M.; Barthet, L.; Chouteau, C.; Védrine, C. Algal biosensors for aquatic ecosystems monitoring. Eur. Phys. J.—Appl. Phys. 2006, 36, 205–209. [Google Scholar] [CrossRef]
- Belaïdi, F.S.; Farouil, L.; Salvagnac, L.; Temple-Boyer, P.; Séguy, I.; Heully, J.L.; Alary, F.; Bedel-Pereira, E.; Launay, J. Towards integrated multi-sensor platform using dual electrochemical and optical detection for on-site pollutant detection in water. Biosens. Bioelectron. 2019, 132, 90–96. [Google Scholar] [CrossRef]
- Connan, S.; Stengel, D.B. Impacts of ambient salinity and copper on brown algae: 1. Interactive effects on photosynthesis, growth, and copper accumulation. Aquat. Toxicol. 2011, 104, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Yang, Y.; Zhao, D.; Qin, L.; Yuan, B.; Liang, N. Time-dependent toxicity and health effects mechanism of cadmium to three green algae. Int. J. Environ. Res. Public Health 2022, 19, 10974. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yin, G.; Zhao, N.; Gan, T.; Feng, C.; Gu, M.; Qi, P.; Ding, Z. Rapid and sensitive detection of water toxicity based on photosynthetic inhibition effect. Toxics 2021, 9, 321. [Google Scholar] [CrossRef]
- OECD. Freshwater Alga and Cyanobacteria, Growth Inhibition Test. OECD Guidelines for the Testing of Chemicals. 2006. Available online: https://www.oecd-ilibrary.org/environment/test-no-201-alga-growth-inhibition-test_9789264069923-en (accessed on 23 March 2006).
- Chen, S.; Yang, J.; Zhang, M.; Strasser, R.J.; Qiang, S. Classification and characteristics of heat tolerance in Ageratina adenophora populations using fast chlorophyll a fluorescence rise O-J-I-P. Environ. Exp. Bot. 2016, 122, 126–140. [Google Scholar] [CrossRef]
- Stirbet, A.; Govindjee. On the relation between the Kautsky effect (chlorophyll a fluorescence induction and Photosystem II: Basics and applications of the OJIP fluorescence transient). J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Yang, L.; Li, H.; Zhang, Y.; Jiao, N. Environmental risk assessment of triazine herbicides in the Bohai Sea and the Yellow Sea and their toxicity to phytoplankton at environmental concentrations. Environ. Int. 2019, 133, 105175. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Ru, S.; Wang, J.; Xiong, J.; Yang, L.; Hao, L.; Zhang, J.; Zhang, X. Effects of co-exposure of the triazine herbicides atrazine, prometryn and terbutryn on Phaeodactylum tricornutum photosynthesis and nutritional value. Sci. Total Environ. 2022, 807, 150609. [Google Scholar] [CrossRef]
- Dewez, D.; Geoffroy, L.; Vernet, G.; Popovic, R. Determination of photosynthetic and enzymatic biomarkers sensitivity used to evaluate toxic effects of copper and fludioxonil in alga Scenedesmus obliquus. Aquat. Toxicol. 2005, 74, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Mallick, N.; Mohn, F.H. Use of chlorophyll fluorescence in metal-stress research: A case study with the green microalga Scenedesmus. Ecotoxicol. Environ. Saf. 2003, 55, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Pan, X.; Wang, S.; Zhang, D. Cu2+ inhibits photosystem II activities but enhances photosystem I quantum yield of Microcystis aeruginosa. Biol. Trace Elem. Res. 2014, 160, 268–275. [Google Scholar] [CrossRef]
- Janeczko, A.; Koscielniak, J.; Pilipowicz, M.; Szarek-Lukaszewska, G.; Skoczowski, A. Protection of winter rape photosystem 2 by 24-epibrassinolide under cadmium stress. Photosynthetica 2005, 43, 293–298. [Google Scholar] [CrossRef]
- Xin, J.; Ma, S.; Li, Y.; Zhao, C.; Tian, R. Pontederia cordata, an ornamental aquatic macrophyte with great potential in phytoremediation of heavy-metal-contaminated wetlands. Ecotoxicol. Environ. Saf. 2020, 203, 111024. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Snider, J.L.; Chastain, D.R.; Slaton, W.; Tishchenko, V. Sub-optimal emergence temperature alters thermotolerance of thylakoid component processes in cotton seedlings. Environ. Exp. Bot. 2018, 155, 360–367. [Google Scholar] [CrossRef]
- Rocchetta, I.; Küpper, H. Chromium- and copper-induced inhibition of photosynthesis in Euglena gracilis analysed on the single-cell level by fluorescence kinetic microscopy. New Phytol. 2009, 181, 405–420. [Google Scholar] [CrossRef]
- Eom, H.; Park, M.; Jang, A.; Kim, S.; Oh, S.-E. A simple and rapid algal assay kit to assess toxicity of heavy metal-contaminated water. Environ. Pollut. 2021, 269, 116135. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chai, X.; Shao, Y.; Hu, L.; Xie, Q.; Wu, H. Toxicity of copper, lead, and cadmium on the motility of two marine microalgae Isochrysis galbana and Tetraselmis chui. J. Environ. Sci. 2011, 23, 330–335. [Google Scholar] [CrossRef]
- Tonon, A.P.; Zaini, P.A.; Falcão, V.D.R.; Oliveira, M.C.; Collén, J.; Boyen, C.; Colepicolo, P. Gracilaria tenuistipitata (Rhodophyta) tolerance to cadmium and copper exposure observed through gene expression and photosynthesis analyses. J. Appl. Phycol. 2018, 30, 2129–2141. [Google Scholar] [CrossRef]










| Parameter and Equation | Definition |
|---|---|
| VJ = (FJ − FO)/(FM − FO) | Relative variable fluorescence at J-step |
| VI = (FI − FO)/(FM − FO) | Relative variable fluorescence at I-step |
| MO = 4·(F300μs − FO)/(FM − FO) | Approximate value of the initial slope of the relative variable fluorescence curve |
| Area | Area between the OJIP curve and the line F = FM |
| FV/FO | PSII photochemical parameter |
| Sm = Area/FV | Normalized area of the OJIP curve |
| φPo = 1 − FO/FM = FV/FM | Maximum quantum yield of primary PSII photochemistry |
| φEo = φPo·(1-VJ) = 1 − FJ/FM | Quantum yield of the electron transport flux from QA to QB |
| φRo = φPo·(1-VI) = 1 − FI/FM | Quantum yield of the electron transport flux until the PSI electron acceptors |
| ΨEo = 1 − VJ | Efficiency with which a PSII trapped electron is transferred from QA to QB |
| ΨRo = 1 − VI | Efficiency with which a PSII trapped electron is transferred until PSI acceptors |
| δRo = (1 − VI)/(1-VJ) | Efficiency with which an electron from QB is transferred until PSI acceptors |
| PIABS = [γRC/(1 − γRC)]·[φPo/(1 − φPo)]·[ΨEo/(1 − ΨEo)] | Performance index for energy conservation from photons absorbed by PSII antenna to the reduction of QB |
| Heavy Metal | 3 h EC10 (mM) | 3 h EC50 (mM) | ||||||
|---|---|---|---|---|---|---|---|---|
| φPo | FV/FO | PIABS | Sm | φPo | FV/FO | PIABS | Sm | |
| Cr(VI) | 0.086 (0.064–0.116) | 0.024 (0.019–0.030) | 0.014 (0.013–0.015) | 0.043 (0.042–0.044) | 1.835 (1.548–2.309) | 0.272 (0.223–0.336) | 0.054 (0.049–0.059) | 0.403 (0.322–0.491) |
| Cd | 0.010 (0.008–0.015) | 0.003 (0.002–0.004) | 0.002 (0.002–0.003) | 0.004 (0.003–0.005) | 0.164 (0.127–0.212) | 0.032 (0.025–0.041) | 0.023 (0.020–0.027) | 0.090 (0.089–0.091) |
| Hg | 0.013 (0.011–0.014) | 0.007 (0.006–0.008) | 0.005 (0.004–0.006) | 0.010 (0.009–0.011) | 0.016 (0.016–0.017) | 0.013 (0.013–0.014) | 0.013 (0.012–0.014) | 0.014 (0.011–0.017) |
| Cu | 0.019 (0.015–0.023) | 0.015 (0.011–0.017) | 0.013 (0.010–0.014) | 0.019 (0.017–0.022) | 0.039 (0.034–0.046) | 0.025 (0.024–0.027) | 0.022 (0.021–0.023) | 0.029 (0.027–0.031) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, T.; Yin, G.; Zhao, N.; Tan, X.; Wang, Y. A Sensitive Response Index Selection for Rapid Assessment of Heavy Metals Toxicity to the Photosynthesis of Chlorella pyrenoidosa Based on Rapid Chlorophyll Fluorescence Induction Kinetics. Toxics 2023, 11, 468. https://doi.org/10.3390/toxics11050468
Gan T, Yin G, Zhao N, Tan X, Wang Y. A Sensitive Response Index Selection for Rapid Assessment of Heavy Metals Toxicity to the Photosynthesis of Chlorella pyrenoidosa Based on Rapid Chlorophyll Fluorescence Induction Kinetics. Toxics. 2023; 11(5):468. https://doi.org/10.3390/toxics11050468
Chicago/Turabian StyleGan, Tingting, Gaofang Yin, Nanjing Zhao, Xiaoxuan Tan, and Ying Wang. 2023. "A Sensitive Response Index Selection for Rapid Assessment of Heavy Metals Toxicity to the Photosynthesis of Chlorella pyrenoidosa Based on Rapid Chlorophyll Fluorescence Induction Kinetics" Toxics 11, no. 5: 468. https://doi.org/10.3390/toxics11050468
APA StyleGan, T., Yin, G., Zhao, N., Tan, X., & Wang, Y. (2023). A Sensitive Response Index Selection for Rapid Assessment of Heavy Metals Toxicity to the Photosynthesis of Chlorella pyrenoidosa Based on Rapid Chlorophyll Fluorescence Induction Kinetics. Toxics, 11(5), 468. https://doi.org/10.3390/toxics11050468








