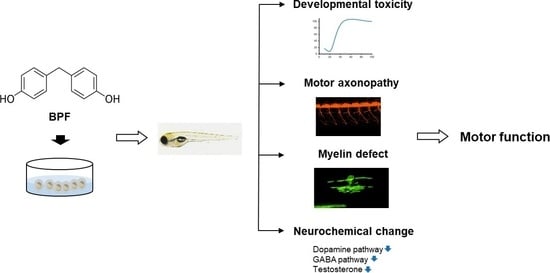
Mechanism of Bisphenol F Affecting Motor System and Motor Activity in Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Lines
2.2. Chemicals
2.3. EC/LC Test
2.4. BPF Uptake Concentration
2.5. Transgenic Zebrafish Live Imaging
2.6. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Staining and Immunohistochemistry
2.7. Behavior Analysis
2.8. Neurochemical Analysis
2.9. Statistical Analysis
3. Results
3.1. Determination of Lethal and Effective Concentration (LC20, EC20) Values of BPF for Zebrafish Larvae
3.2. Accumulation of BPF in the Zebrafish Larvae Body Increased in a Concentration-Dependent Manner
3.3. BPF Impaired the Locomotor Activity in Zebrafish Larvae
3.4. BPF Reduced Startle Response in Zebrafish Larvae
3.5. Effects of BPF Exposure on Motor System
3.6. BPF Affected the Myelination of Zebrafish Larvae
3.7. BPF Exposure Altered Metabolic Neurochemicals in Zebrafish Larvae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Rezg, R.; El-Fazaa, S.; Gharbi, N.; Mornagui, B. Bisphenol A and human chronic diseases: Current evidences, possible mechanisms, and future perspectives. Environ. Int. 2014, 64, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Pirzada, M.; Jahan, S.; Ullah, H.; Khan, M.J. Bisphenol A analogues bisphenol B, bisphenol F, and bisphenol S induce oxidative stress, disrupt daily sperm production, and damage DNA in rat spermatozoa: A comparative in vitro and in vivo study. Toxicol. Ind. Health 2019, 35, 294–303. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Ishibashi, H.; Arizono, K.; Tominaga, N. In vivo and in silico analyses of estrogenic potential of bisphenol analogs in medaka (Oryzias latipes) and common carp (Cyprinus carpio). Ecotoxicol. Environ. Saf. 2015, 120, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Cano-Nicolau, J.; Vaillant, C.; Pellegrini, E.; Charlier, T.D.; Kah, O.; Coumailleau, P. Estrogenic effects of several BPA analogs in the developing zebrafish brain. Front. Neurosci. 2016, 10, 112. [Google Scholar] [CrossRef]
- Martínez, M.Á.; Blanco, J.; Rovira, J.; Kumar, V.; Domingo, J.L.; Schuhmacher, M. Bisphenol A analogues (BPS and BPF) present a greater obesogenic capacity in 3T3-L1 cell line. Food Chem. Toxicol. 2020, 140, 111298. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Pirzada, M.; Afsar, T.; Razak, S.; Almajwal, A.; Jahan, S. Effect of bisphenol F, an analog of bisphenol A, on the reproductive functions of male rats. Environ. Health Prev. Med. 2019, 24, 41. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Oliveri, A.; Levin, E.D. Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Res. C Embryo Today 2013, 99, 14–23. [Google Scholar] [CrossRef]
- Kim, S.S.; Hwang, K.S.; Yang, J.Y.; Chae, J.S.; Kim, G.R.; Kan, H.; Jung, M.H.; Lee, H.Y.; Song, J.S.; Ahn, S.; et al. Neurochemical and behavioral analysis by acute exposure to bisphenol A in zebrafish larvae model. Chemosphere 2020, 239, 124751. [Google Scholar] [CrossRef]
- Gu, J.; Wu, J.; Xu, S.; Zhang, L.; Fan, D.; Shi, L.; Wang, J.; Ji, G. Bisphenol F exposure impairs neurodevelopment in zebrafish larvae (Danio rerio). Ecotoxicol. Environ. Saf. 2020, 188, 109870. [Google Scholar] [CrossRef]
- Mu, X.; Liu, J.; Yuan, L.; Yang, K.; Huang, Y.; Wang, C.; Yang, W.; Shen, G.; Li, Y. The mechanisms underlying the developmental effects of bisphenol F on zebrafish. Sci. Total Environ. 2019, 687, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Flanagan-Steet, H.; Fox, M.A.; Meyer, D.; Sanes, J.R. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development 2005, 132, 4471–4481. [Google Scholar] [CrossRef] [PubMed]
- Kucenas, S.; Takada, N.; Park, H.C.; Woodruff, E.; Broadie, K.; Appel, B. CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat. Neurosci. 2008, 11, 143–151. [Google Scholar] [CrossRef] [PubMed]
- See, K.; Yadav, P.; Giegerich, M.; Cheong, P.S.; Graf, M.; Vyas, H.; Winkler, C. SMN deficiency alters Nrxn2 expression and splicing in zebrafish and mouse models of spinal muscular atrophy. Human Mol. Genet. 2014, 23, 1754–1770. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Kim, S.; Chung, A.Y.; Kim, H.T.; So, J.H.; Ryu, J.; Park, H.C.; Kim, C.H. Visualization of myelination in GFP-transgenic zebrafish. Dev. Dyn. 2010, 239, 592–597. [Google Scholar] [CrossRef]
- Münzel, E.J.; Schaefer, K.; Obirei, B.; Kremmer, E.; Burton, E.A.; Kuscha, V.; Becker, C.G.; Brösamle, C.; Williams, A.; Becker, T. Claudin k is specifically expressed in cells that form myelin during development of the nervous system and regeneration of the optic nerve in adult zebrafish. Glia 2012, 60, 253–270. [Google Scholar] [CrossRef]
- Kim, S.S.; Im, S.H.; Yang, J.Y.; Lee, Y.R.; Kim, G.R.; Chae, J.S.; Shin, D.S.; Song, J.S.; Ahn, S.; Lee, B.H.; et al. Zebrafish as a screening model for testing the permeability of blood-brain barrier to small molecules. Zebrafish 2017, 14, 322–330. [Google Scholar] [CrossRef]
- Park, C.B.; Kim, G.E.; Kim, Y.J.; On, J.; Park, C.G.; Kwon, Y.S.; Pyo, H.; Yeom, D.H.; Cho, S.H. Reproductive dysfunction linked to alteration of endocrine activities in zebrafish exposed to mono-(2-ethylhexyl) phthalate (MEHP). Environ. Pollut. 2020, 265, 114362. [Google Scholar] [CrossRef]
- Thaler, J.; Harrison, K.; Sharma, K.; Lettieri, K.; Kehrl, J.; Pfaff, S.L. Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron 1999, 23, 675–687. [Google Scholar] [CrossRef]
- Moreno, R.L.; Ribera, A.B. Zebrafish motor neuron subtypes differ electrically prior to axonal outgrowth. J. Neurophysiol. 2009, 102, 2477–2484. [Google Scholar] [CrossRef]
- Liu, B.; Lehmler, H.J.; Sun, Y.; Xu, G.; Sun, Q.; Snetselaar, L.G.; Wallace, R.B.; Bao, W. Association of bisphenol A and its substitutes, bisphenol F and bisphenol S, with obesity in United States children and adolescents. Diabetes Metab. J. 2019, 43, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Lee, M.C.; Yoon, D.S.; Han, J.; Kim, M.; Hwang, U.K.; Jung, J.H.; Lee, J.S. Effects of bisphenol A and its analogs bisphenol F and S on life parameters, antioxidant system, and response of defensome in the marine rotifer Brachionus koreanus. Aquat. Toxicol. 2018, 199, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dong, Q.; Chen, Y.; Jiang, H.; Xiao, Q.; Wang, Y.; Li, W.; Bai, C.; Huang, C.; Yang, D. Bisphenol A affects axonal growth, musculature and motor behavior in developing zebrafish. Aquat. Toxicol. 2013, 142–143, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Liu, S.; Chen, H.; Luo, S.; Xiong, Y.; Wang, X.; Xu, B.; Zheng, C.; Wang, K.J. The comparative toxicities of BPA, BPB, BPS, BPF, and BPAF on the reproductive neuroendocrine system of zebrafish embryos and its mechanisms. J. Hazard. Mater. 2021, 406, 124303. [Google Scholar] [CrossRef]
- Saili, K.S.; Corvi, M.M.; Weber, D.N.; Patel, A.U.; Das, S.R.; Przybyla, J.; Anderson, K.A.; Tanguay, R.L. Neurodevelopmental low-dose bisphenol A exposure leads to early life-stage hyperactivity and learning deficits in adult zebrafish. Toxicology 2012, 291, 83–92. [Google Scholar] [CrossRef]
- Vermeer, L.M.; Gregory, E.; Winter, M.K.; McCarson, K.E.; Berman, N.E. Exposure to bisphenol A exacerbates migraine-like behaviors in a multibehavior model of rat migraine. Toxicol. Sci. 2014, 137, 416–427. [Google Scholar] [CrossRef]
- Ohtani, N.; Iwano, H.; Suda, K.; Tsuji, E.; Tanemura, K.; Inoue, H.; Yokota, H. Adverse effects of maternal exposure to bisphenol F on the anxiety- and depression-like behavior of offspring. J. Vet. Med. Sci. 2017, 79, 432–439. [Google Scholar] [CrossRef]
- Morrice, J.R.; Gregory-Evans, C.Y.; Shaw, C.A. Modeling environmentally-induced motor neuron degeneration in zebrafish. Sci. Rep. 2018, 8, 4890. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Agarwal, S.; Chauhan, L.K.; Mishra, V.N.; Chaturvedi, R.K. Bisphenol-A impairs myelination potential during development in the hippocampus of the rat brain. Mol. Neurobiol. 2015, 51, 1395–1416. [Google Scholar] [CrossRef]
- Xu, X.B.; Fan, S.J.; He, Y.; Ke, X.; Song, C.; Xiao, Y.; Zhang, W.H.; Zhang, J.Y.; Yin, X.P.; Kato, N.; et al. Loss of hippocampal oligodendrocytes contributes to the deficit of contextual fear learning in adult rats experiencing early bisphenol A exposure. Mol. Neurobiol. 2017, 54, 4524–4536. [Google Scholar] [CrossRef]
- Gill, S.; Kumara, V.M.R. Comparative neurodevelopment effects of bisphenol A and bisphenol F on rat fetal neural stem cell models. Cells. 2021, 10, 793. [Google Scholar] [CrossRef]
- Kato, D.; Wake, H.; Lee, P.R.; Tachibana, Y.; Ono, R.; Sugio, S.; Tsuji, Y.; Tanaka, Y.H.; Tanaka, Y.R.; Masamizu, Y.; et al. Motor learning requires myelination to reduce asynchrony and spontaneity in neural activity. Glia 2020, 68, 193–210. [Google Scholar] [CrossRef]
- Kravitz, A.V.; Freeze, B.S.; Parker, P.R.; Kay, K.; Thwin, M.T.; Deisseroth, K.; Kreitzer, A.C. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 2010, 466, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Roseberry, T.K.; Lee, A.M.; Lalive, A.L.; Wilbrecht, L.; Bonci, A.; Kreitzer, A.C. Cell-type-specific control of brainstem locomotor circuits by basal ganglia. Cell. 2016, 164, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Menezes, E.C.; Santos, P.R.; Goes, T.C.; Carvalho, V.C.B.; Teixeira-Silva, F.; Stevens, H.E.; Badauê-Passos, D.J. Effects of a rat model of gestational hypothyroidism on forebrain dopaminergic, GABAergic, and serotonergic systems and related behaviors. Behav. Brain Res. 2019, 366, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Mustard, J.A.; Jones, L.; Wright, G.A. GABA signaling affects motor function in the honey bee. J. Insect Physiol. 2020, 120, 103989. [Google Scholar] [CrossRef]
- Stagg, C.J.; Bachtiar, V.; Johansen-Berg, H. The role of GABA in human motor learning. Curr. Biol. 2011, 21, 480–484. [Google Scholar] [CrossRef]
- Hosie, A.M.; Wilkins, M.E.; Smart, T.G. Neurosteroid binding sites on GABA(A) receptors. Pharmacol. Ther. 2007, 116, 7–19. [Google Scholar] [CrossRef]
- Purves-Tyson, T.D.; Boerrigter, D.; Allen, K.; Zavitsanou, K.; Karl, T.; Djunaidi, V.; Double, K.L.; Desai, R.; Handelsman, D.J.; Weickert, C.S. Testosterone attenuates and the selective estrogen receptor modulator, raloxifene, potentiates amphetamine-induced locomotion in male rats. Horm. Behav. 2015, 70, 73–84. [Google Scholar] [CrossRef]
- Castro, B.; Sánchez, P.; Miranda, M.T.; Torres, J.M.; Ortega, E. Identification of dopamine- and serotonin-related genes modulated by bisphenol A in the prefrontal cortex of male rats. Chemosphere 2015, 139, 235–239. [Google Scholar] [CrossRef]
- Wang, X.H.; Souders, C.L., 2nd; Zhao, Y.H.; Martyniuk, C.J. Paraquat affects mitochondrial bioenergetics, dopamine system expression, and locomotor activity in zebrafish (Danio rerio). Chemosphere 2018, 191, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.; Sánchez, P.; Torres, J.M.; Ortega, E. Bisphenol A, bisphenol F and bisphenol S affect differently 5α-reductase expression and dopamine-serotonin systems in the prefrontal cortex of juvenile female rats. Environ. Res. 2015, 142, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, E.; Yamashita, N.; Taniyasu, S.; Lam, J.; Lam, P.K.; Moon, H.B.; Jeong, Y.; Kannan, P.; Achyuthan, H.; Munuswamy, N.; et al. Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol. Environ. Saf. 2015, 122, 565–572. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kim, S.S.; Park, B.H.; Hwang, K.-S.; Bae, M.A.; Cho, S.-H.; Kim, S.; Park, H.-C. Mechanism of Bisphenol F Affecting Motor System and Motor Activity in Zebrafish. Toxics 2023, 11, 477. https://doi.org/10.3390/toxics11060477
Kim Y, Kim SS, Park BH, Hwang K-S, Bae MA, Cho S-H, Kim S, Park H-C. Mechanism of Bisphenol F Affecting Motor System and Motor Activity in Zebrafish. Toxics. 2023; 11(6):477. https://doi.org/10.3390/toxics11060477
Chicago/Turabian StyleKim, Yeonhwa, Seong Soon Kim, Byeong Heon Park, Kyu-Seok Hwang, Myung Ae Bae, Sung-Hee Cho, Suhyun Kim, and Hae-Chul Park. 2023. "Mechanism of Bisphenol F Affecting Motor System and Motor Activity in Zebrafish" Toxics 11, no. 6: 477. https://doi.org/10.3390/toxics11060477
APA StyleKim, Y., Kim, S. S., Park, B. H., Hwang, K.-S., Bae, M. A., Cho, S.-H., Kim, S., & Park, H.-C. (2023). Mechanism of Bisphenol F Affecting Motor System and Motor Activity in Zebrafish. Toxics, 11(6), 477. https://doi.org/10.3390/toxics11060477