Dissipation, Bioconcentration and Dietary Risk Assessment of Thiamethoxam and Its Metabolites in Agaricus bisporus and Substrates under Different Application Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Field Trials with TMX Application during A. bisporus Cultivation
2.2.1. A. bisporus Cultivation
2.2.2. TMX Application and Sampling
2.3. Sample Preparation and Purification
2.3.1. Compost and Casing Soil Samples
2.3.2. Fruiting Body Samples
2.4. UPLC-MS/MS Analysis
2.5. Dissipation Dynamics Behavior
2.6. Bioconcentration Factors (BCFs)
2.7. Dietary Risk AssessmentFigur
3. Results and Discussion
3.1. Method Validation
3.1.1. Linearity, Matrix effect (ME), Limit of Detection (LOD), and Limit of Quantification (LOQ)
3.1.2. Accuracy and Precision by Recovery Experiments
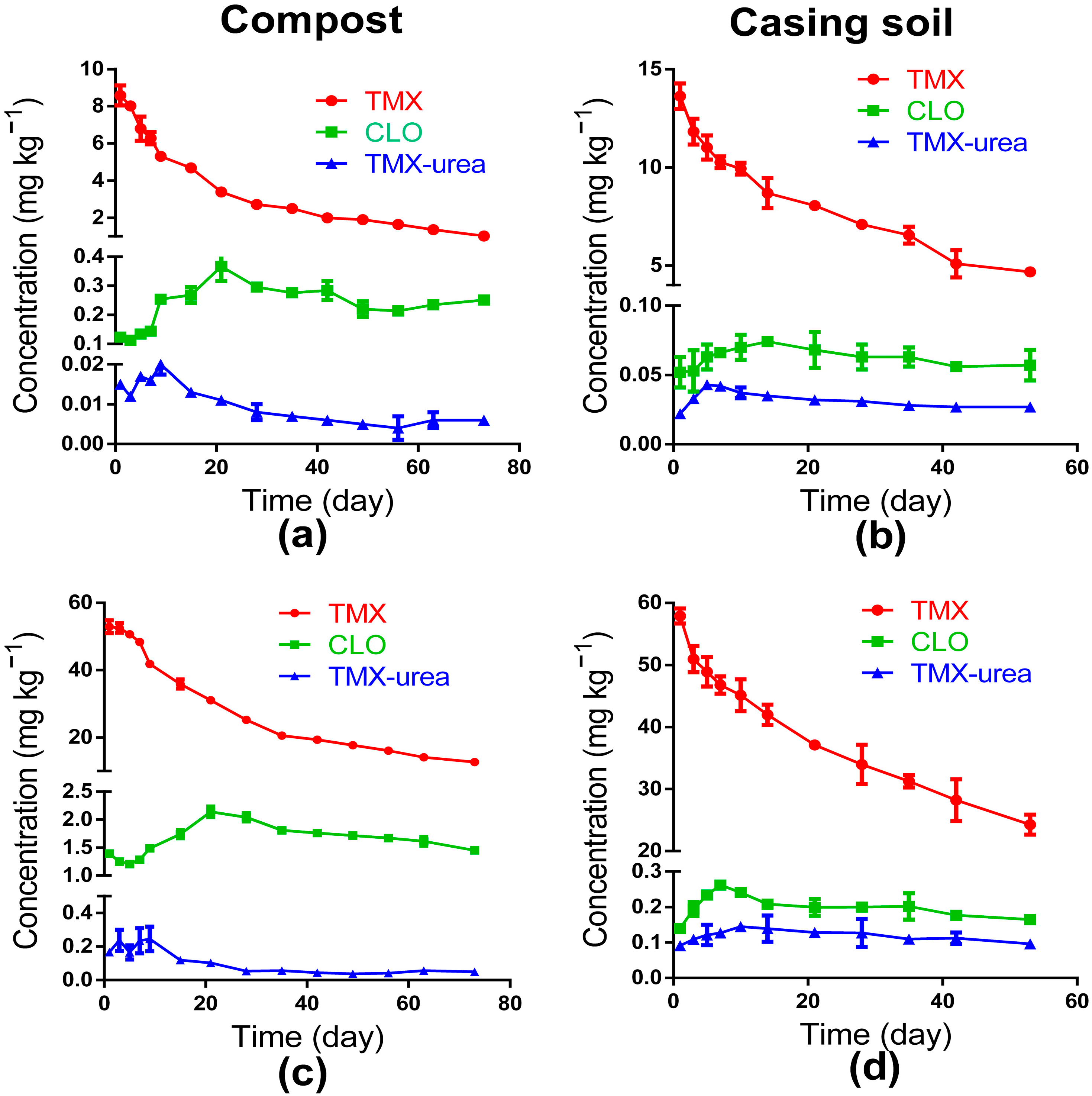
3.2. Dissipation Dynamics of TMX in Compost and Casing Soil
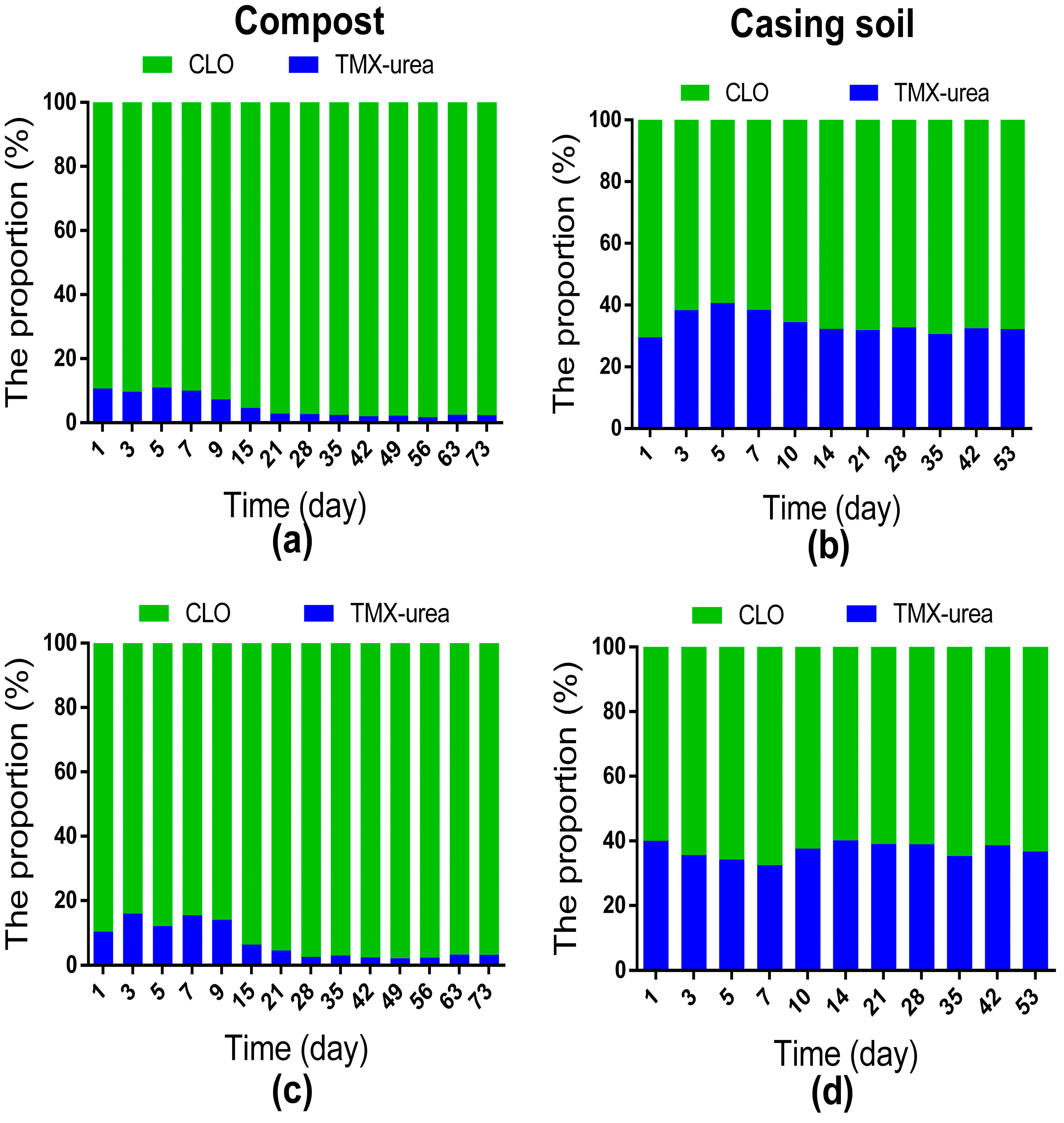
3.3. Residual Fate of CLO and TMX-Urea in Compost and Casing Soil
3.4. Final Residues and Bioconcentration Factors (BCFs) in Fruiting Body
3.5. Dietary Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT, Food and Agriculture Organization of the United Nations Statistical Databases. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 24 March 2023).
- Stoyanova, M.; Lacheva, M.; Radoukova, T. Chemical composition and nutritional value of Agaricus bisporus, Bulgaria. Oxid. Commun. 2021, 44, 81–95. [Google Scholar]
- Ramos, M.; Burgos, N.; Barnard, A.; Evans, G.; Jiménez, A. Agaricus bisporus and its by-products as a source of valuable extracts and bioactive compounds. Food Chem. 2019, 292, 176–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, G.; Mrig, K.K.; Singh, R.; Singh, S. Eco-friendly management of phorid fly (Megaselia sandhui) and sciarid fly (Bradysia tritici) on oyster mushroom. Res. on Crops. 2013, 14, 551–554. [Google Scholar]
- Shamshad, A. The development of integrated pest management for the control of mushroom sciarid flies, Lycoriella ingenua (dufour) and Bradysia ocellaris (comstock), in cultivated mushrooms. Pest Manag. Sci. 2010, 66, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Binns, E.S. Field and laboratory observations on the substrates of the mushroom fungus gnat Lycoriella auripila (diptera: Sciaridae). Ann. Appl. Biol. 2010, 96, 143–152. [Google Scholar] [CrossRef]
- Najmeh; Shirvani-Farsani, N.; Zamani, A.A.; Abbasi, S.; Kheradmand, K. Toxicity of three insecticides and tobacco extract against the fungus gnat, Lycoriella auripila and the economic injury level of the gnat on button mushroom. J. Pest. Sci. 2013, 86, 591–597. [Google Scholar]
- Jess, S.; Kirbas, J.M.; Gordon, A.W.; Murchie, A.K. Potential for use of garlic oil to control Lycoriella ingenua (diptera: Sciaridae) and Megaselia halterata (diptera: Phoridae) in commercial mushroom production. Crop Prot. 2017, 102, 1–9. [Google Scholar] [CrossRef]
- Hassani, A.; Dacher, M.; Gary, V.; Lambin, M.; Gauthier, M.; Armengaud, C. Effects of sublethal doses of acetamiprid and thiamethoxam on the behavior of the honeybee (Apis mellifera). Arch. Environ. Contam. Toxicol. 2008, 54, 653–661. [Google Scholar] [CrossRef]
- Thompson, H.; Coulson, M.; Ruddle, N.; Wilkins, S.; Harkin, S. Thiamethoxam: Assessing flight activity of honeybees foraging on treated oilseed rape using radio frequency identification technology. Environ. Toxicol. Chem. 2016, 35, 385–393. [Google Scholar] [CrossRef]
- Basley, K.; Goulson, D. Neonicotinoids thiamethoxam and clothianidin adversely affect the colonisation of invertebrate populations in aquatic microcosms. Environ. Sci. Pollut. Res. 2018, 25, 9593–9599. [Google Scholar] [CrossRef] [Green Version]
- Hallmann, C.A.; Foppen, R.P.B.; Turnhout, C.A.M.V.; Kroon, H.D.; Jongejans, E. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 2014, 511, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.M.D.; Nunes, B.; Barbosa, D.R.; Pallares, A.M.; Faro, L. Effects of the neonicotinoids thiametoxam and clothianidin on in vivo dopamine release in rat striatum. Toxicol. Lett. 2010, 192, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Ford, K.A.; Casida, J.E. Unique and common metabolites of thiamethoxam, clothianidin, and dinotefuran in mice. Chem. Res. Toxicol. 2006, 19, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Michael, C.; Cavallaro; Christy, A.; Morrissey; John, V.H.; Kerry, M.P. Comparative chronic toxicity of imidacloprid, clothianidin, and thiamethoxam to Chironomus dilutus and estimation of toxic equivalency factors. Environ. Toxicol. Chem. 2017, 36, 372–382. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2018/783 of 29 May 2018 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance imidacloprid. Off. J. Eur. Union. 2018, 132, 31–34. [Google Scholar]
- Li, Y.; Su, P.D.; Li, Y.D.; Wen, K.J.; Bi, G.H.; Cox, M. Adsorption-desorption and degradation of insecticides clothianidin and thiamethoxam in agricultural soils. Chemosphere 2018, 207, 708–714. [Google Scholar] [CrossRef]
- Abd-Alrahman, S.H. Residue and dissipation kinetics of thiamethoxam in a vegetable-field ecosystem using QuEChERs methodology combined with HPLC–DAD. Food Chem. 2014, 159, 1–4. [Google Scholar] [CrossRef]
- Fang, Q.; Shi, Y.; Cao, H.; Tong, Z.; Xiao, J.; Liao, M. Degradation dynamics and dietary risk assessments of two neonicotinoid insecticides during Lonicera japonica planting, drying, and tea brewing processes. J. Agric. Food Chem. 2017, 65, 1483–1488. [Google Scholar] [CrossRef]
- Residue and Analytical Aspests. Joint FAO/WHO Meetings on Pesticide Residues (JMPR, 2012). Available online: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Report12/Thiamethoxam_Clothianidin.pdf. (accessed on 21 April 2018).
- Gui, T.; Jia, G.F.; Xu, J.; Ge, S.J.; Long, X.F.; Zhang, Y.P.; De, Y.H. Determination of the residue dynamics and dietary risk of thiamethoxam and its metabolite clothianidin in citrus and soil by LC-MS/MS. J. Environ. Sci. Health Part B 2019, 54, 326–335. [Google Scholar] [CrossRef]
- Rahman, M.M.; Farha, W.; El-Aty, A.A.; Kabir, M.H.; Im, S.J.; Jung, D.I. Dynamic behaviour and residual pattern of thiamethoxam and its metabolite clothianidin in swiss chard using liquid chromatography-tandem mass spectrometry. Food Chem. 2015, 174, 248–255. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, Z.; Wei, F.; Ren, Y.; Gui, W.; Wu, H. Simultaneous determination of seven neonicotinoid pesticide residues in food by ultraperformance liquid chromatography tandem mass spectrometry. J. Agr. Food Chem. 2010, 58, 3271–3278. [Google Scholar] [CrossRef] [PubMed]
- Teló, G.M.; Senseman, S.A.; Marchesan, E.; Camargo, E.R.; Jones, T.; McCauley, G. Residues of thiamethoxam and chlorantraniliprole in rice grain. J. Agr. Food Chem. 2015, 63, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.C.; Wang, X.L.; Rao, Q.X.; Chen, S.S.; Song, W.G. Imidacloprid dissipation, metabolism and accumulation in Agaricus bisporus fruits, casing soil and compost and dietary risk assessment. Chemosphere 2020, 254, 126837. [Google Scholar] [CrossRef] [PubMed]
- China pesticide information network. Available online: http://www.icama.org.cn/hysj/index.jhtml (accessed on 6 June 2022).
- Li, Y.; Sallach, J.B.; Zhang, W.; Boyd, S.A.; Li, H. Insight into the distribution of pharmaceuticals in soil-water-plant systems. Water Res. 2019, 152, 38–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.Y.; Chen, L.; Bai, B.; Yang, X.L.; Tan, Y.L.; Wang, J.H.; Zhao, X.Y.; Zhou, C.Y. Liquid chromatography-mass spectrometry method for evaluating the dissipation dynamics of cyromazine and its metabolite in Agaricus bisporus and dietary risk assessment. Environ. Sci. Pollut. R. 2018, 25, 2285–2292. [Google Scholar] [CrossRef]
- Li, Y.; Lu, P.; Hu, D.; Bhadury, P.S.; Zhang, Y.; Zhang, K. Determination of dufulin residue in vegetables, rice, and tobacco using liquid chromatography with tandem mass spectrometry. J. AOAC. Int. 2015, 98, 1739–1744. [Google Scholar] [CrossRef]
- He, Z.Y.; Wang, L.; Peng, Y.; Luo, M.; Wang, W.W.; Liu, X.W. Multiresidue analysis of over 200 pesticides in cereals using a QuEChERS and gas chromatography-tandem mass spectrometry-based method. Food Chem. 2015, 169, 372–380. [Google Scholar] [CrossRef]
- China Pesticide Information Network, Announcement No. 2386 of the Ministry of Agriculture, the People’s Republic of China. 2016. Available online: http://www.chinapesticide.org.cn/ssfxpg/9556.jhtml (accessed on 17 November 2022).
- Anderson, J.C.; Dubetz, C.; Palace, V.P. Neonicotinoids in the Canadian aquatic environment: A literature review on current use products with a focus on fate, exposure, and biological effects. Sci. Total Environ. 2015, 505, 409–422. [Google Scholar] [CrossRef]
- Zhang, P.; He, M.; Wei, Y.; Zhao, Y.H.; Mu, W.; Liu, F. Comparative soil distribution and dissipation of phoxim and thiamethoxam and their efficacy in controlling Bradysia odoriphaga Yang and Zhang in Chinese chive ecosystems. Crop Prot. 2016, 90, 1–8. [Google Scholar] [CrossRef]
- Goulson, D. Review: An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Mei, J.; Ge, Q.; Han, L.; Zhang, H.; Long, Z.; Cui, Y.; Hua, R.; Yu, Y.; Hua, F. Deposition, distribution, metabolism, and reduced application dose of thiamethoxam in a pepper-planted ecosystem. J. Agr. Food Chem. 2019, 67, 11848–11859. [Google Scholar] [CrossRef] [PubMed]
- You, X.W.; Jiang, H.T.; Zhao, M.; Suo, F.Y.; Zhang, C.S.; Zheng, H.; Sun, K.; Zhang, G.Y.; Li, F.M.; Li, Y.Q. Biochar reduced Chinese chive (Allium tuberosum) uptake and dissipation of thiamethoxam in an agricultural soil. J. Hazard. Mater. 2020, 390, 121749. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Jindal, V.; Mandal, K.; Kaur, G.; Gupta, V.K. Thiamethoxam degradation by Pseudomonas and Bacillus strains isolated from agricultural soils. Environ. Monit. Assess. 2015, 187, 300. [Google Scholar] [CrossRef] [PubMed]
- Lwaniuk, P.; Kaczyński, P.; Pietkun, M.; Łozowicka, B. Evaluation of titanium and silicon role in mitigation of fungicides toxicity in wheat expressed at the level of biochemical and antioxidant profile. Chemosphere 2022, 308, 136284. [Google Scholar]
- Karmakar, R.; Bhattacharya, R.; Kulshrestha, G. Comparative metabolite profiling of the insecticide thiamethoxam in plant and cell suspension culture of tomato. J. Agr. Food Chem. 2009, 57, 6369–6374. [Google Scholar] [CrossRef]
- Raina-Fulton, R.; Dunn, N.; Xie, Z. Pesticides and their degradation products including metabolites: Chromatography-mass spectrometry methods. Mass Spectrom. 2017, 6, 95–132. [Google Scholar]
- JMPR-Thiamethoxam 2010 Report. Available online: http://www.fao.org/agriculture/crops/core-themes/theme/pests/lpe/en/ (accessed on 24 November 2022).
- GB 2763-2021; National Food Safety Standard Maximum Residue Limits of Pesticides in Food. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2021. Available online: https://agrochemical.chemlinked.com/sites/default/files/preview-doc/preview_sample_en_gb2763-2021.pdf (accessed on 30 November 2022).
- EU-Maximum Residue Limits Database for Pesticides in Food. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/mrls/?event=search.pr (accessed on 10 December 2022).
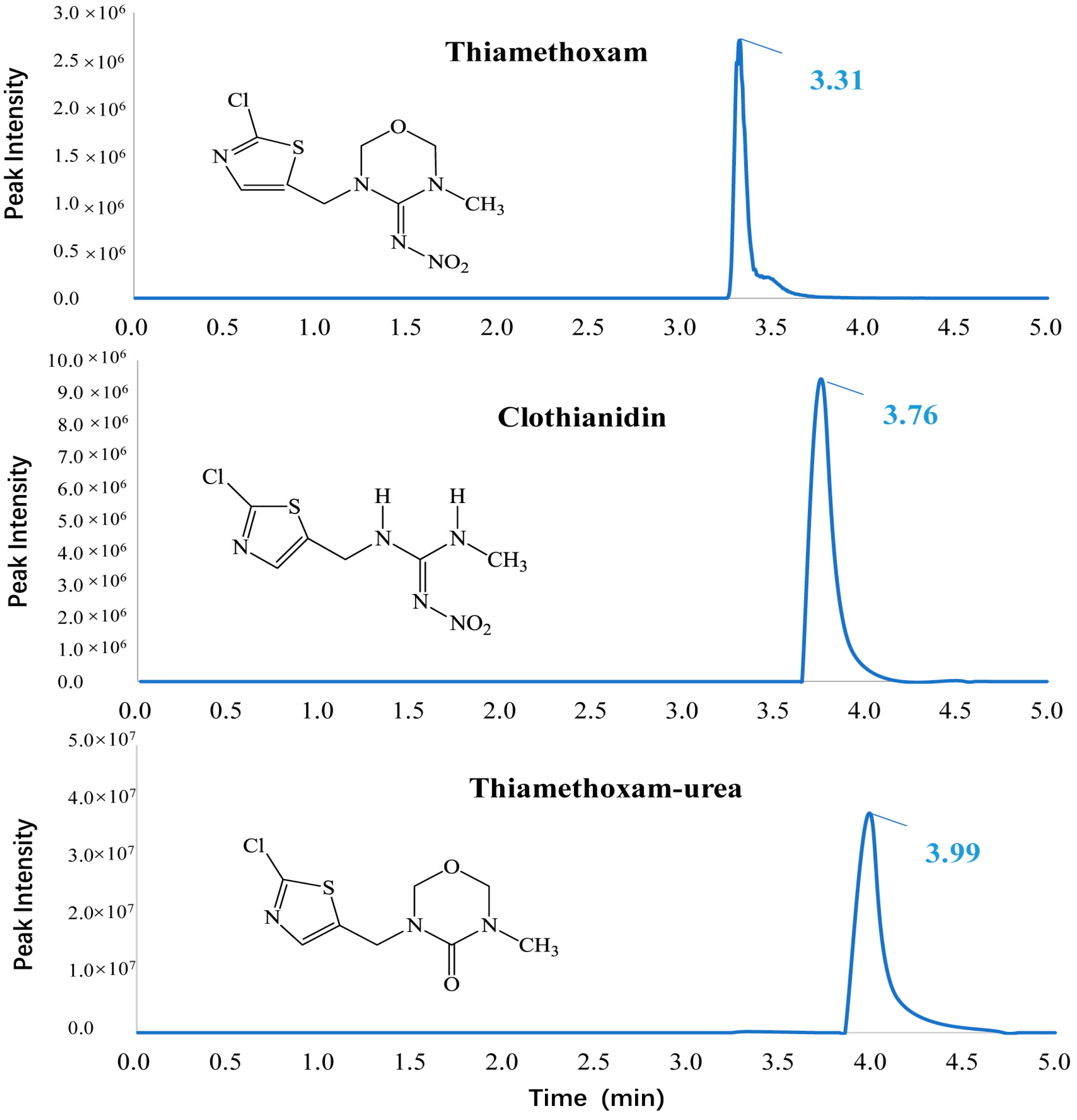


| Compound | Matrix | Calibration Range (mg L−1) | Regression Equation | r | LOD a (mg kg−1) | LOQ b (mg kg−1) | ME c (%) |
|---|---|---|---|---|---|---|---|
| Thiamethoxam | Acetonitrile | 0.0001–1 | y = 16,056.5x + 118,065.0 | 0.9916 | |||
| Fruiting body | 0.001–1 | y = 19,785.9x + 26,133.7 | 0.9990 | 0.001 | 0.002 | 23.2 | |
| Casing soil | 0.005–1 | y = 17,654.9x + 96,874.1 | 0.9915 | 0.005 | 0.01 | 9.9 | |
| Compost | 0.005–1 | y = 18,068.9x + 24,581.9 | 0.9991 | 0.005 | 0.01 | 12.5 | |
| Clothianidin | Acetonitrile | 0.0001–1 | y = 12,036.1x + 50,954.7 | 0.9939 | |||
| Fruiting body | 0.001–1 | y = 13,937.4x + 25,647.3 | 0.9980 | 0.001 | 0.002 | 15.8 | |
| Casing soil | 0.005–1 | y = 13,713.6x + 26,238.5 | 0.9984 | 0.005 | 0.01 | 13.9 | |
| Compost | 0.005–1 | y = 10,246.4x − 2445.0 | 0.9995 | 0.005 | 0.01 | −14.9 | |
| Thiamethoxam-urea | Acetonitrile | 0.0001–0.5 | y = 78,973.9x + 31,132.0 | 0.9958 | |||
| Fruiting body | 0.0005–0.5 | y = 81,351.6x − 4751.4 | 0.9915 | 0.0005 | 0.001 | 3.0 | |
| Casing soil | 0.001–0.5 | y = 89,419.6x + 18,706.2 | 0.9927 | 0.001 | 0.002 | 13.2 | |
| Compost | 0.001–0.5 | y = 61,454.2x + 19,152.3 | 0.9930 | 0.001 | 0.002 | −22.2 |
| Matrix | Dosage (mg kg−1) | Kinetic Equation | R2 | t1/2(d) |
|---|---|---|---|---|
| Compost | 10 | Ct = 8.225e−0.0351t | 0.9629 | 19.74 |
| 50 | Ct = 54.32e−0.024t | 0.9764 | 28.87 | |
| Casing soil | 10 | Ct = 12.53e−0.0207t | 0.9401 | 33.54 |
| 50 | Ct = 53.98e−0.0162t | 0.9438 | 42.89 |
| Matrix | Dosage (mg kg−1) | Compound | Final Residue (μg kg−1) (Days after Thiamethoxam Application) | |||||
|---|---|---|---|---|---|---|---|---|
| First Flush * | BCF | Second Flush * | BCF | Third Flush * | BCF | |||
| Casing soil | 10 | thiamethoxam | 4.3 ± 0.4 | 0.0006 | 2.6 ± 0.3 | 0.0005 | 1.5 ± 0.3 | 0.0003 |
| 50 | thiamethoxam | 30.4 ± 2.2 | 0.0009 | 26.3 ± 2.4 | 0.0009 | 17.4 ± 1.9 | 0.0007 | |
| Matrix | ADI a (mg kg−1 bw day−1) | First Flush | Second Flush | Third Flush | |||
|---|---|---|---|---|---|---|---|
| Median Residue (mg kg−1) | RQ b | Median Residue (mg kg−1) | RQ | Median Residue (mg kg−1) | RQ | ||
| Casing soil | 0.08 | 0.03 | 0.1064 | 0.026 | 0.1063 | 0.017 | 0.1062 |
| Matrix | ARfD a (mg kg−1 bw day−1) | First Flush | Second Flush | Third Flush | |||
|---|---|---|---|---|---|---|---|
| HR b (mg kg−1) | HQ c | HR (mg kg−1) | HQ | HR (mg kg−1) | HQ | ||
| Casing soil | 1 | 0.033 | 0.000031 | 0.029 | 0.000027 | 0.019 | 0.000018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Zhang, Q.; Rao, Q.; Wang, X.; Du, P.; Song, W. Dissipation, Bioconcentration and Dietary Risk Assessment of Thiamethoxam and Its Metabolites in Agaricus bisporus and Substrates under Different Application Methods. Toxics 2023, 11, 500. https://doi.org/10.3390/toxics11060500
Chen S, Zhang Q, Rao Q, Wang X, Du P, Song W. Dissipation, Bioconcentration and Dietary Risk Assessment of Thiamethoxam and Its Metabolites in Agaricus bisporus and Substrates under Different Application Methods. Toxics. 2023; 11(6):500. https://doi.org/10.3390/toxics11060500
Chicago/Turabian StyleChen, Shanshan, Qicai Zhang, Qinxiong Rao, Xianli Wang, Penghui Du, and Weiguo Song. 2023. "Dissipation, Bioconcentration and Dietary Risk Assessment of Thiamethoxam and Its Metabolites in Agaricus bisporus and Substrates under Different Application Methods" Toxics 11, no. 6: 500. https://doi.org/10.3390/toxics11060500
APA StyleChen, S., Zhang, Q., Rao, Q., Wang, X., Du, P., & Song, W. (2023). Dissipation, Bioconcentration and Dietary Risk Assessment of Thiamethoxam and Its Metabolites in Agaricus bisporus and Substrates under Different Application Methods. Toxics, 11(6), 500. https://doi.org/10.3390/toxics11060500







