Inhibition of Histone H3K18 Acetylation-Dependent Antioxidant Pathways Involved in Arsenic-Induced Liver Injury in Rats and the Protective Effect of Rosa roxburghii Tratt Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Arsenic Concentration in Liver
2.3. Analysis of Liver Oxidative Damage
2.4. Analysis of H3K18ac Levels in Liver
2.5. Selection of Representative Heat Shock Proteins
2.6. Quantitative Real-Time PCR
2.7. Chromatin Immunoprecipitation (ChIP) Assay
2.8. Statistical Analysis
3. Results
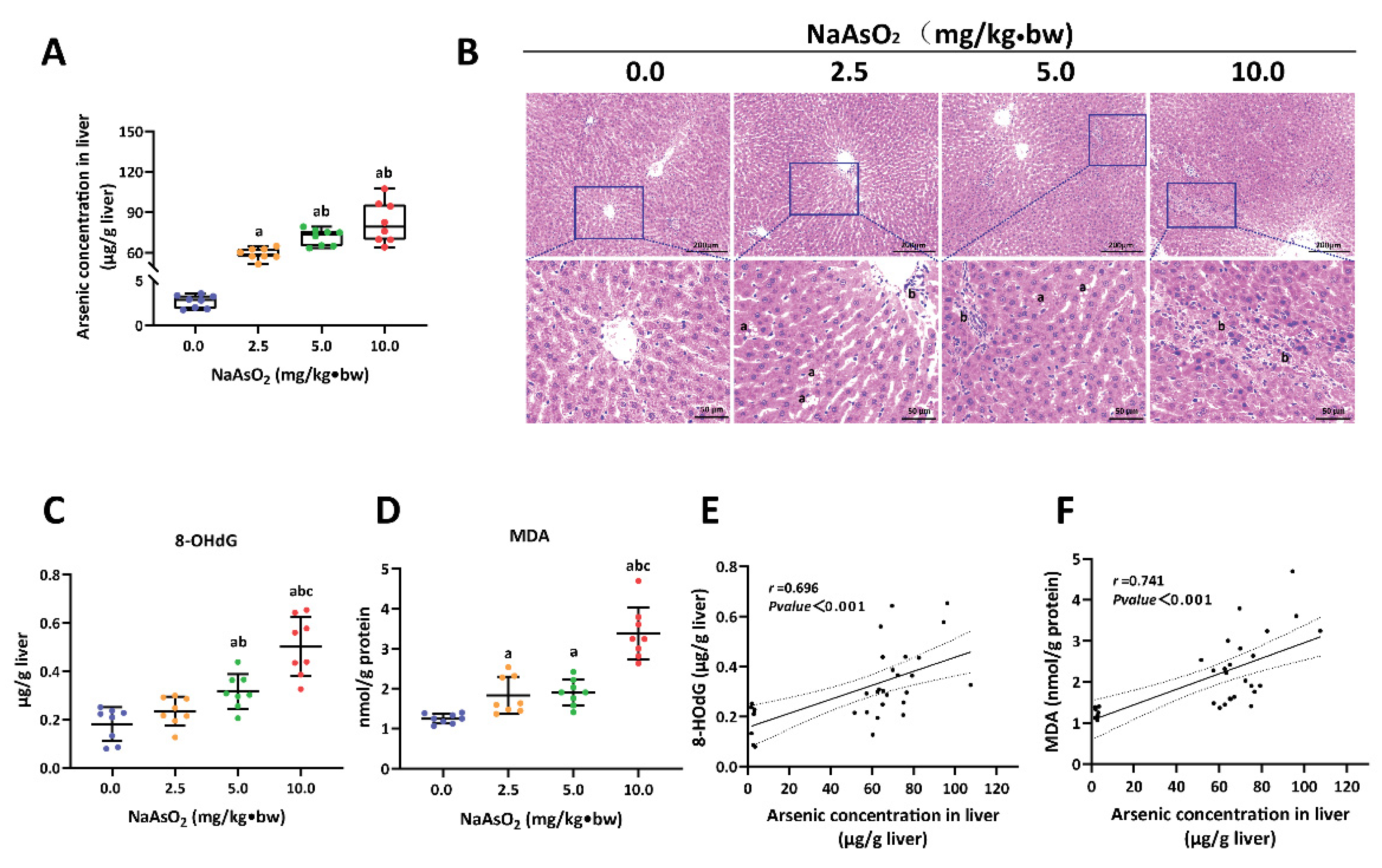
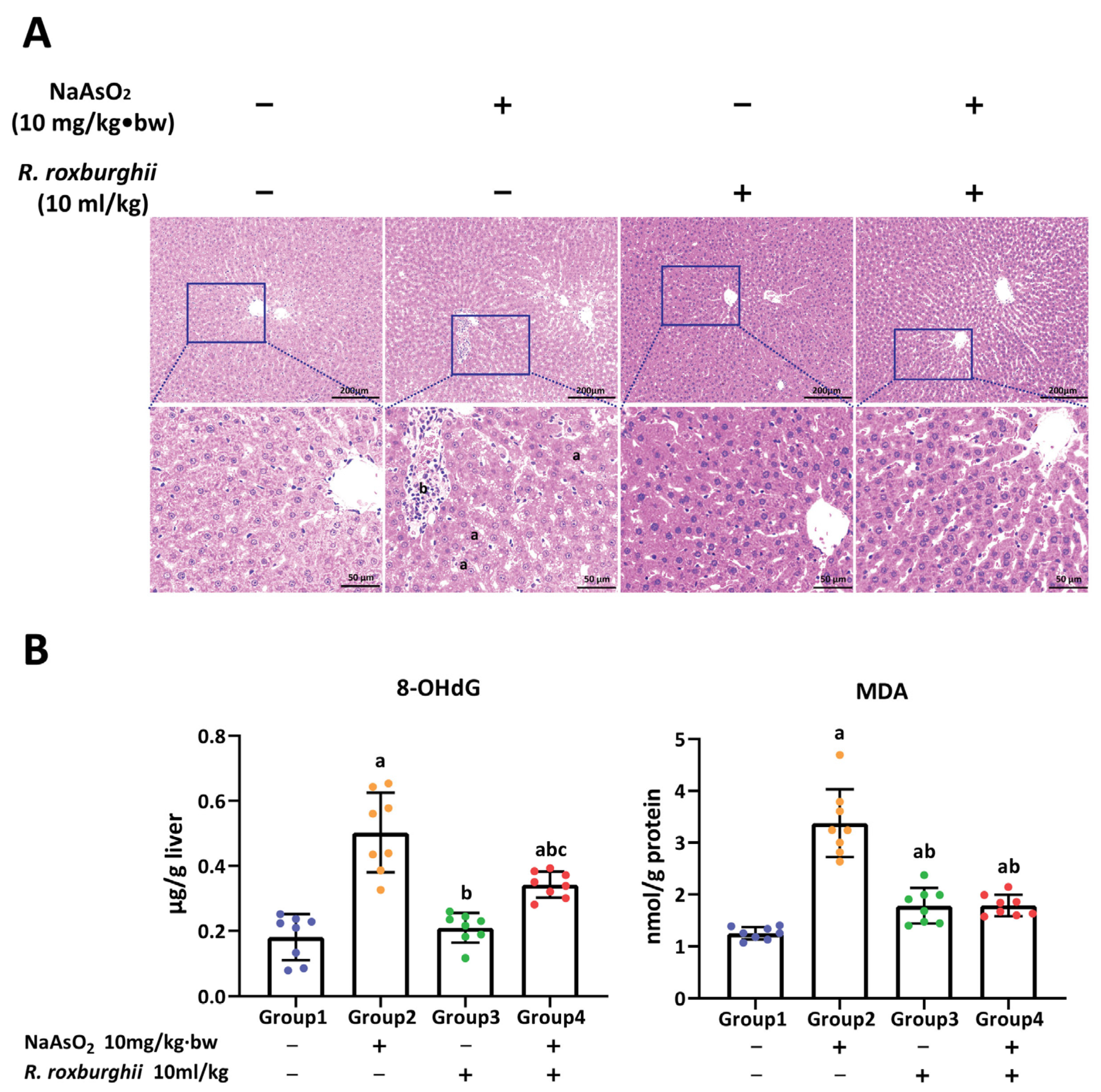
3.1. Arsenic-Exposure-Induced Liver Injury in Rat
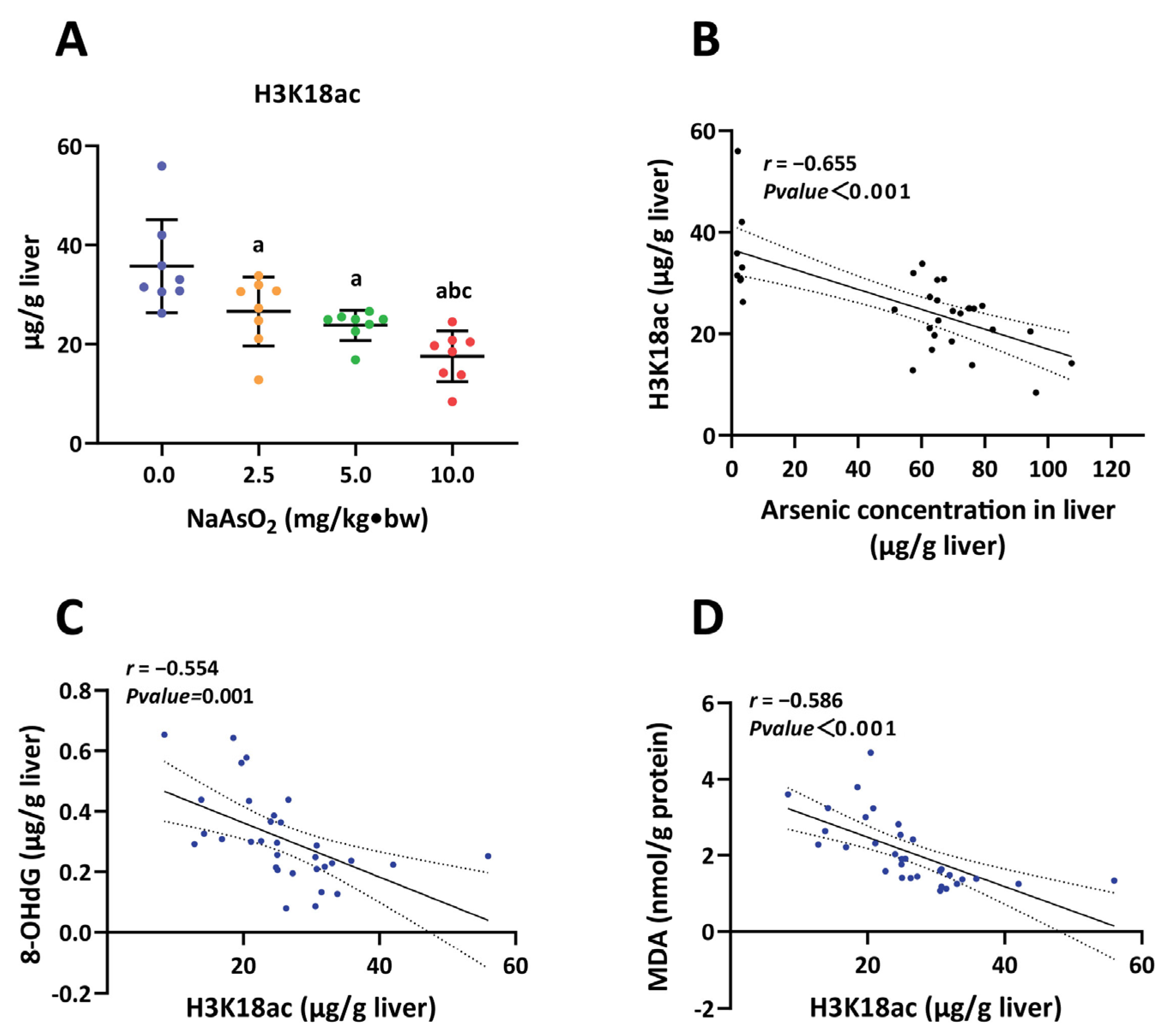
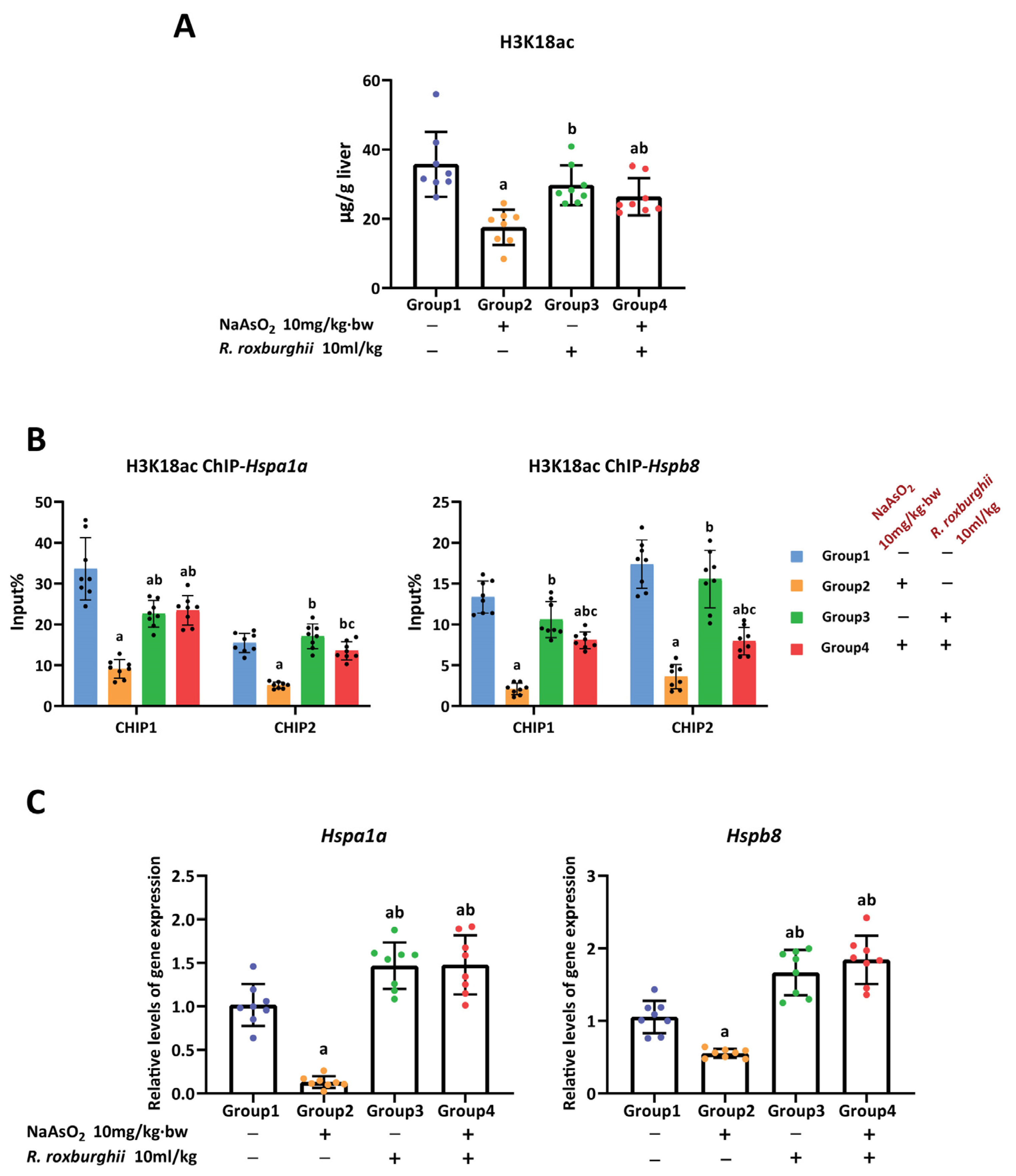
3.2. H3K18ac Was Associated with Hepatic Oxidative Damage Induced by Arsenic
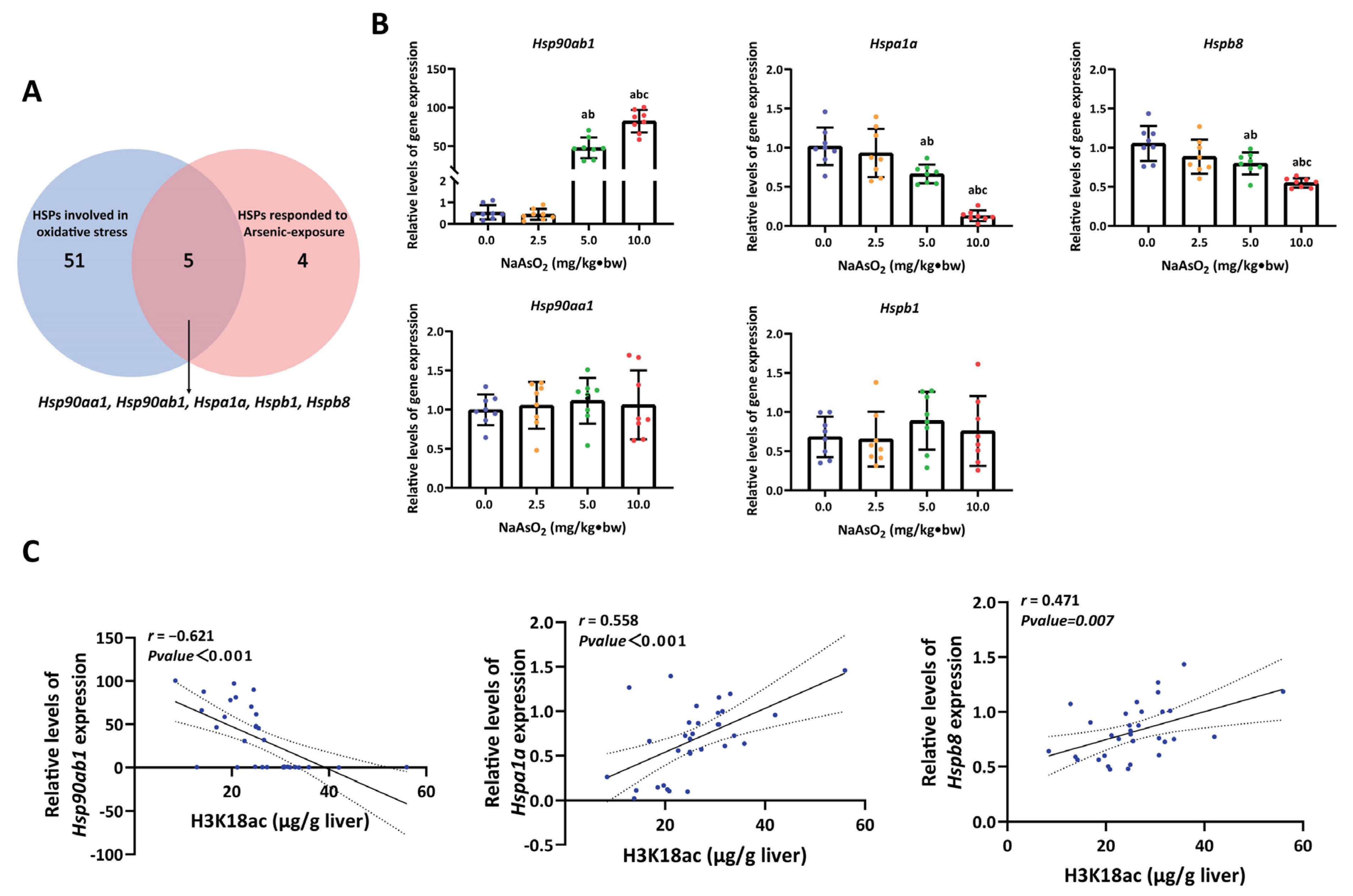
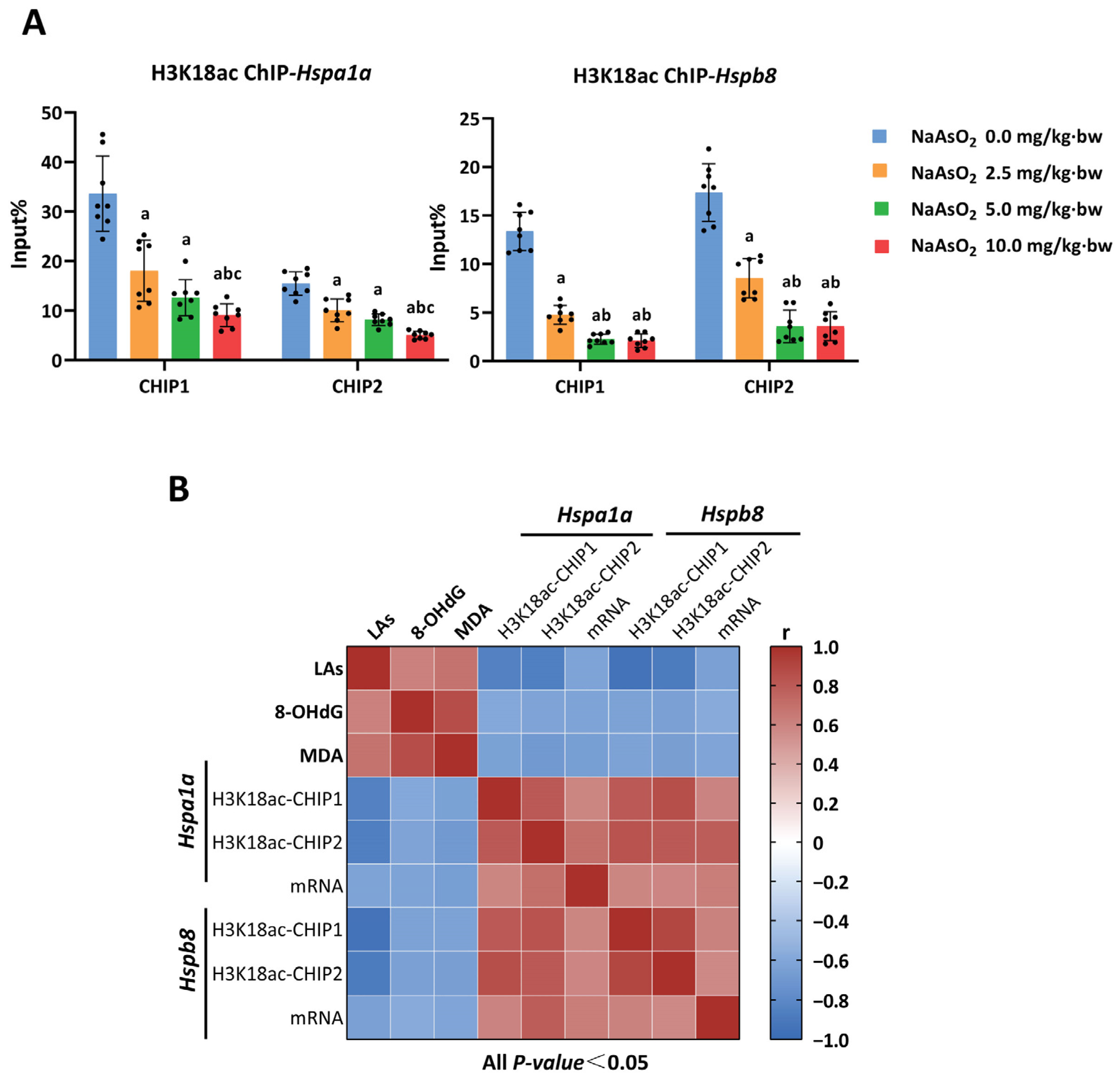
3.3. H3K18ac Might Be Involved in Arsenic-Induced Hepatic Oxidative Damage by Regulating Expression of Heat Shock Proteins
3.4. R. roxburghii Could Alleviate Arsenic-Induced Liver Injury by Declining Oxidative Damage
3.5. The Antioxidative Effect of R. Roxburghii Might Be Involved in Antagonizing Arsenic-induced Inhibition of the H3K18ac–HSPs Axis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Exposure to Arsenic: A Major Public Concern. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/329482/WHO-CED-PHE-EPE-19.4.1-eng.pdf (accessed on 25 January 2022).
- Rahaman, M.S.; Rahman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Uddin, M.K.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, D.N.G. Arsenic and liver disease. J. Indian Med. Assoc. 2001, 99, 314–315, 318–320. [Google Scholar]
- Frediani, J.K.; Naioti, E.A.; Vos, M.B.; Figueroa, J.; Marsit, C.J.; Welsh, J.A. Arsenic exposure and risk of nonalcoholic fatty liver disease (NAFLD) among U.S. adolescents and adults: An association modified by race/ethnicity, NHANES 2005–2014. Environ. Health 2018, 1, 6. [Google Scholar] [CrossRef]
- Yao, M.; Zeng, Q.; Luo, P.; Sun, B.; Liang, B.; Wei, S.; Xu, Y.; Wang, Q.; Liu, Q.; Zhang, A. Assessing the risk of coal-burning arsenic-induced liver damage: A population-based study on hair arsenic and cumulative arsenic. Environ. Sci. Pollut. Res. Int. 2021, 28, 50489–50499. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Ramakrishna, B.; Zachariah, U.; Sajith, K.; Burad, D.; Kodiatte, T.; Keshava, S.; Balasubramanian, K.; Elias, E.; Eapen, C. What makes non-cirrhotic portal hypertension a common disease in India? Analysis for environmental factors. Indian J. Med. Res. 2019, 149, 468–478. [Google Scholar] [PubMed]
- Hsu, L.; Wang, Y.; Hsieh, F.; Yang, T.; Jeng, R.W.-J.; Liu, C.; Chen, C.; Hsu, K.; Chiou, H.; Wu, M.; et al. Effects of Arsenic in Drinking Water on Risk of Hepatitis or Cirrhosis in Persons With and Without Chronic Viral Hepatitis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2016, 14, 1347–1355.e1344. [Google Scholar]
- Dong, L.; Liu, Y.; Wang, D.; Zhu, K.; Zou, Z.; Zhang, A. Imbalanced inflammatory response in subchronic arsenic-induced liver injury and the protective effects of Ginkgo biloba extract in rats: Potential role of cytokines mediated cell-cell interactions. Environ. Toxicol. 2021, 36, 2073–2092. [Google Scholar] [CrossRef]
- Tao, Y.; Qiu, T.; Yao, X.; Jiang, L.; Wang, N.; Jia, X.; Wei, S.; Wang, Z.; Pei, P.; Zhang, J.; et al. Autophagic-CTSB-inflammasome axis modulates hepatic stellate cells activation in arsenic-induced liver fibrosis. Chemosphere 2020, 242, 124959. [Google Scholar] [CrossRef]
- Martínez-Castillo, M.; García-Montalvo, E.; Arellano-Mendoza, M.; Sánchez-Peña, L.; Jasso, L.S.; Izquierdo-Vega, J.; Valenzuela, O.; Hernández-Zavala, A. Arsenic exposure and non-carcinogenic health effects. Hum. Exp. Toxicol. 2021, 40, S826–S850. [Google Scholar] [CrossRef]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef]
- Choudhury, S.; Ghosh, S.; Mukherjee, S.; Gupta, P.; Bhattacharya, S.; Adhikary, A.; Chattopadhyay, S. Pomegranate protects against arsenic-induced p53-dependent ROS-mediated inflammation and apoptosis in liver cells. J. Nutr. Biochem. 2016, 38, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Qiu, T.; Yao, X.; Jiang, L.; Wang, N.; Jiang, J.; Jia, X.; Wei, S.; Zhang, J.; Zhu, Y.; et al. IRE1α/NOX4 signaling pathway mediates ROS-dependent activation of hepatic stellate cells in NaAsO-induced liver fibrosis. J. Cell. Physiol. 2021, 236, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xue, Y.; Zheng, B.; Li, L.; Chu, X.; Zhao, Y.; Wu, Y.; Zhang, J.; Han, X.; Wu, Z.; et al. Liquiritigenin protects against arsenic trioxide-induced liver injury by inhibiting oxidative stress and enhancing mTOR-mediated autophagy. Biomed. Pharmacother. 2021, 143, 112167. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Gu, Y.; Yan, N.; Li, Y.; Sun, L.; Li, B. Curcumin functions as an anti-inflammatory and antioxidant agent on arsenic-induced hepatic and kidney injury by inhibiting MAPKs/NF-κB and activating Nrf2 pathways. Environ. Toxicol. 2021, 36, 2161–2173. [Google Scholar] [CrossRef]
- Janasik, B.; Reszka, E.; Stanislawska, M.; Jablonska, E.; Kuras, R.; Wieczorek, E.; Malachowska, B.; Fendler, W.; Wasowicz, W. Effect of Arsenic Exposure on NRF2-KEAP1 Pathway and Epigenetic Modification. Biol. Trace Elem. Res. 2018, 185, 11–19. [Google Scholar] [CrossRef]
- Wang, D.; Ma, Y.; Yang, X.; Xu, X.; Zhao, Y.; Zhu, Z.; Wang, X.; Deng, H.; Li, C.; Gao, F.; et al. Hypermethylation of the Keap1 gene inactivates its function, promotes Nrf2 nuclear accumulation, and is involved in arsenite-induced human keratinocyte transformation. Free Radic. Biol. Med. 2015, 89, 209–219. [Google Scholar] [CrossRef]
- Guo, P.; Chen, S.; Li, D.; Zhang, J.; Luo, J.; Zhang, A.; Yu, D.; Bloom, M.; Chen, L.; Chen, W. SFPQ is involved in regulating arsenic-induced oxidative stress by interacting with the miRNA-induced silencing complexes. Environ. Pollut. Barking Essex 1987 2020, 261, 114160. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, X.; Gu, S.; Zhang, Z. MicroRNA-155 regulates arsenite-induced malignant transformation by targeting Nrf2-mediated oxidative damage in human bronchial epithelial cells. Toxicol. Lett. 2017, 278, 38–47. [Google Scholar] [CrossRef]
- Hassan, F.; Rehman, M.; Khan, M.; Ali, M.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an Alternative Epigenetic Modulator: Mechanism of Action and Potential Effects. Front. Genet. 2019, 10, 514. [Google Scholar] [CrossRef]
- Grinan-Ferre, C.; Bellver-Sanchis, A.; Izquierdo, V.; Corpas, R.; Roig-Soriano, J.; Chillon, M.; Andres-Lacueva, C.; Somogyvari, M.; Soti, C.; Sanfeliu, C.; et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing Res. Rev. 2021, 67, 101271. [Google Scholar] [CrossRef]
- Dai, X.; Liao, R.; Liu, C.; Liu, S.; Huang, H.; Liu, J.; Jin, T.; Guo, H.; Zheng, Z.; Xia, M.; et al. Epigenetic regulation of TXNIP-mediated oxidative stress and NLRP3 inflammasome activation contributes to SAHH inhibition-aggravated diabetic nephropathy. Redox Biol. 2021, 45, 102033. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Rao, H.; Liu, J.; Li, X.; Feng, W.; Gui, L.; Tang, H.; Xu, J.; Gao, W.Q.; Li, L. The histone methyltransferase SETD2 modulates oxidative stress to attenuate experimental colitis. Redox Biol. 2021, 43, 102004. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y. Cross-talk between the H3K36me3 and H4K16ac histone epigenetic marks in DNA double-strand break repair. J. Biol. Chem. 2017, 292, 11951–11959. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Paul, S.; Bhattacharjee, S.; Giri, A.; Bhattacharjee, P. Association of H3K79 monomethylation (an epigenetic signature) with arsenic-induced skin lesions. Mutat. Res. 2018, 807, 1–9. [Google Scholar] [CrossRef]
- Ma, L.; Li, J.; Zhan, Z.; Chen, L.; Li, D.; Bai, Q.; Gao, C.; Li, J.; Zeng, X.; He, Z.; et al. Specific histone modification responds to arsenic-induced oxidative stress. Toxicol. Appl. Pharmacol. 2016, 302, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Ikwegbue, P.C.; Masamba, P.; Oyinloye, B.E.; Kappo, A.P. Roles of Heat Shock Proteins in Apoptosis, Oxidative Stress, Human Inflammatory Diseases, and Cancer. Pharmaceuticals 2017, 11, 2. [Google Scholar] [CrossRef]
- Lin, C.Y.; Hu, C.T.; Cheng, C.C.; Lee, M.C.; Pan, S.M.; Lin, T.Y.; Wu, W.S. Oxidation of heat shock protein 60 and protein disulfide isomerase activates ERK and migration of human hepatocellular carcinoma HepG2. Oncotarget 2016, 7, 11067–11082. [Google Scholar] [CrossRef]
- Mandal, J.; Shiue, C.; Chen, Y.; Lee, M.; Yang, H.; Chang, H.; Hu, C.; Liao, P.; Hui, L.; You, R.; et al. PKCδ mediates mitochondrial ROS generation and oxidation of HSP60 to relieve RKIP inhibition on MAPK pathway for HCC progression. Free. Radic. Biol. Med. 2021, 163, 69–87. [Google Scholar] [CrossRef]
- Moyano, P.; García, J.; Lobo, M.; Anadón, M.; Sola, E.; Pelayo, A.; García, J.; Frejo, M.; Pino, J. Cadmium alters heat shock protein pathways in SN56 cholinergic neurons, leading to Aβ and phosphorylated Tau protein generation and cell death. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 121, 297–308. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Bai, Q.; Ma, L.; Li, D.; Zhang, J.; Gao, C.; Lei, Z.; Zhang, Z.; Xing, X.; et al. PP2A-AMPKα-HSF1 axis regulates the metal-inducible expression of HSPs and ROS clearance. Cell Signal. 2014, 26, 825–832. [Google Scholar] [CrossRef]
- Wang, L.; Lv, M.; An, J.; Fan, X.; Dong, M.; Zhang, S.; Wang, J.; Wang, Y.; Cai, Z.; Fu, Y. Rosa roxburghiiBotanical characteristics, phytochemistry and related biological activities of Tratt fruit, and its potential use in functional foods: A review. Food Funct. 2021, 12, 1432–1451. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yu, C.; Zeng, Q.; Yao, M.; Chen, X.; Zhang, A. Assessing the potential value of Rosa Roxburghii Tratt in arsenic-induced liver damage based on elemental imbalance and oxidative damage. Environ. Geochem. Health 2021, 43, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, J.; Huang, J.; Xu, P.; Huang, H.; Liu, Y.; Yu, L.; Yang, Y.; Zhou, B.; Jiang, H.; et al. p300/CBP inhibitor A-485 alleviates acute liver injury by regulating macrophage activation and polarization. Theranostics 2019, 9, 8344–8361. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, I.; Mondal, P.; Sengupta, A.; Mondal, A.; Singh, V.; Adhikari, S.; Dhang, S.; Roy, S.; Das, C. Epigenetic regulation of Fructose-1,6-bisphosphatase 1 by host transcription factor Speckled 110 kDa during hepatitis B virus infection. FEBS J. 2022, 289, 6694–6713. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Xia, B.; Chen, Z.; Chen, X.; Wu, D.; Lu, W.; Luo, N.; Zhou, L.; Liu, W.; Yang, X.; et al. Short-term and long-term exposure to hexavalent chromium alters 53BP1 via H3K18ac and H3K27ac. Chemosphere 2019, 229, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Han, X.; Nan, B.; Liu, L.; Tian, M.; Shen, H.; Huang, Q. Chronic low-level perfluorooctane sulfonate (PFOS) exposure promotes testicular steroidogenesis through enhanced histone acetylation. Environ. Pollut. Barking Essex 1987 2021, 284, 117518. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Tian, M.; Shliaha, P.; Zhang, J.; Jiang, S.; Nan, B.; Alam, M.; Jensen, O.; Shen, H.; Huang, Q. Real-world particulate matters induce lung toxicity in rats fed with a high-fat diet: Evidence of histone modifications. J. Hazard. Mater. 2021, 416, 126182. [Google Scholar] [CrossRef]
- Dawson, M.; Kouzarides, T. Cancer epigenetics: From mechanism to therapy. Cell 2012, 150, 12–27. [Google Scholar] [CrossRef]
- Hogg, S.J.; Beavis, P.A.; Dawson, D.A.; Johnstone, R.W. Targeting the epigenetic regulation of antitumour immunity. Nat. Rev. Drug Discov. 2020, 19, 776–800. [Google Scholar] [CrossRef]
- Ban, H.; Han, T.; Hur, K.; Cho, H. Epigenetic Alterations of Heat Shock Proteins (HSPs) in Cancer. Int. J. Mol. Sci. 2019, 20, 4758. [Google Scholar] [CrossRef]
- Cui, X.; Wang, N.; Yang, B.; Gao, W.; Lin, Y.; Yao, X.; Ma, X. HSPB8 is methylated in hematopoietic malignancies and overexpression of HSPB8 exhibits antileukemia effect. Exp. Hematol. 2012, 40, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Hayat, B.; Kapuganti, R.; Padhy, B.; Mohanty, P.; Alone, D. Epigenetic silencing of heat shock protein 70 through DNA hypermethylation in pseudoexfoliation syndrome and glaucoma. J. Hum. Genet. 2020, 65, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; White, M.; Choi, W.; Guo, C.; Dinney, C.; McConkey, D.; Siefker-Radtke, A. Inhibition of inducible heat shock protein-70 (hsp72) enhances bortezomib-induced cell death in human bladder cancer cells. PLoS ONE 2013, 8, e69509. [Google Scholar] [CrossRef]
- Smith, C.; Li, B.; Liu, J.; Lee, K.; Aurelian, L. The Levels of H11/HspB8 DNA methylation in human melanoma tissues and xenografts are a critical molecular marker for 5-Aza-2′-deoxycytidine therapy. Cancer Investig. 2011, 29, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Luthold, C.; Varlet, A.; Lambert, H.; Bordeleau, F.; Lavoie, J. Chaperone-Assisted Mitotic Actin Remodeling by BAG3 and HSPB8 Involves the Deacetylase HDAC6 and Its Substrate Cortactin. Int. J. Mol. Sci. 2020, 22, 142. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Q.; Zhang, L.; Feng, L. Inhibition of Histone Deacetylase 3 (HDAC3) Mediates Ischemic Preconditioning and Protects Cortical Neurons against Ischemia in Rats. Front. Mol. Neurosci. 2016, 9, 131. [Google Scholar] [CrossRef]
- Pfister, S.; Ahrabi, S.; Zalmas, L.; Sarkar, S.; Aymard, F.; Bachrati, C.; Helleday, T.; Legube, G.; La Thangue, N.; Porter, A.; et al. SETD2-dependent histone H3K36 trimethylation is required for homologous recombination repair and genome stability. Cell Rep. 2014, 7, 2006–2018. [Google Scholar] [CrossRef]
- Shirane, K.; Miura, F.; Ito, T.; Lorincz, M. NSD1-deposited H3K36me2 directs de novo methylation in the mouse male germline and counteracts Polycomb-associated silencing. Nat. Genet. 2020, 52, 1088–1098. [Google Scholar] [CrossRef]
- Xu, Y.; Zeng, Q.; Sun, B.; Wei, S.; Wang, Q.; Zhang, A. Assessing the Role of Nrf2/GPX4-Mediated Oxidative Stress in Arsenic-Induced Liver Damage and the Potential Application Value of Rosa roxburghii Tratt [Rosaceae]. Oxidative Med. Cell. Longev. 2022, 2022, 9865606. [Google Scholar] [CrossRef]
- Ni, H.; Yu, L.; Zhao, X.; Wang, L.; Zhao, C.; Huang, H.; Zhu, H.; Efferth, T.; Gu, C.; Fu, Y. Seed oil of Rosa roxburghii Tratt against non-alcoholic fatty liver disease in vivo and in vitro through PPARα/PGC-1α-mediated mitochondrial oxidative metabolism. Phytomed. Int. J. Phytother. Phytopharm. 2022, 98, 153919. [Google Scholar] [CrossRef]
- Yang, S.; Huang, X.; Zhou, N.; Wu, Q.; Liu, J.; Shi, J. Rosa roxburghiiRNA-Seq Analysis of Protection against Chronic Alcohol Liver Injury by Fruit Juice (Cili) in Mice. Nutrients 2022, 14, 1974. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Akan, O.; Luo, F.; Lin, Q. Dietary polysaccharides exert biological functions via epigenetic regulations: Advance and prospectives. Crit. Rev. Food Sci. Nutr. 2021, 63, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Izzo, S.; Naponelli, V.; Bettuzzi, S. Flavonoids as Epigenetic Modulators for Prostate Cancer Prevention. Nutrients 2020, 12, 1010. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Hou, T.; Zhu, K.; Zhang, A. Inhibition of Histone H3K18 Acetylation-Dependent Antioxidant Pathways Involved in Arsenic-Induced Liver Injury in Rats and the Protective Effect of Rosa roxburghii Tratt Juice. Toxics 2023, 11, 503. https://doi.org/10.3390/toxics11060503
Ma L, Hou T, Zhu K, Zhang A. Inhibition of Histone H3K18 Acetylation-Dependent Antioxidant Pathways Involved in Arsenic-Induced Liver Injury in Rats and the Protective Effect of Rosa roxburghii Tratt Juice. Toxics. 2023; 11(6):503. https://doi.org/10.3390/toxics11060503
Chicago/Turabian StyleMa, Lu, Teng Hou, Kai Zhu, and Aihua Zhang. 2023. "Inhibition of Histone H3K18 Acetylation-Dependent Antioxidant Pathways Involved in Arsenic-Induced Liver Injury in Rats and the Protective Effect of Rosa roxburghii Tratt Juice" Toxics 11, no. 6: 503. https://doi.org/10.3390/toxics11060503
APA StyleMa, L., Hou, T., Zhu, K., & Zhang, A. (2023). Inhibition of Histone H3K18 Acetylation-Dependent Antioxidant Pathways Involved in Arsenic-Induced Liver Injury in Rats and the Protective Effect of Rosa roxburghii Tratt Juice. Toxics, 11(6), 503. https://doi.org/10.3390/toxics11060503




