Adverse Effects of Prenatal Exposure to Oxidized Black Carbon Particles on the Reproductive System of Male Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oxidized Black Carbon (OBC) Particles
2.2. Animals
2.3. Particle Administration
2.4. Body and Organ Weights, Testicular Morphology
2.5. Daily Sperm Production
2.6. Serum Testosterone
2.7. Real-Time RT-PCR
2.8. Statistical Analysis
3. Results
3.1. Effects of OBC Administration on Dams and Fetuses
3.2. Effects of Prenatal Exposure to OBC on Body, Testis, and Epididymis Weights in Male Offspring
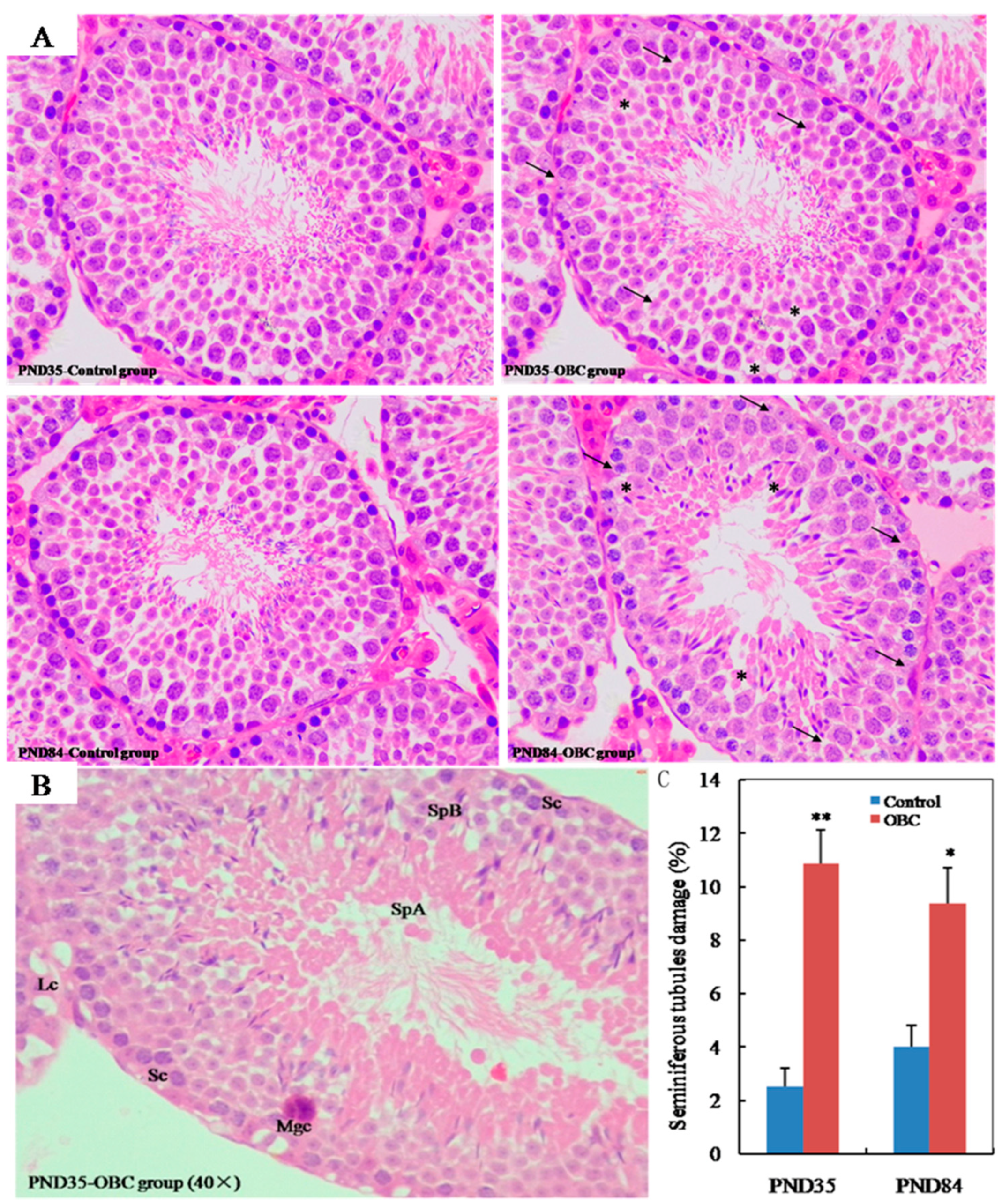
3.3. Effects of Prenatal Exposure OBC on Testicular Histology in Male Offspring
3.4. Effects of Prenatal Exposure to OBC on Daily Sperm Production of Male Offspring
3.5. Effects of Prenatal Exposure to OBC on Serum Testosterone of Male Offspring
3.6. Prenatal Exposure to OBC Disruptstesticular Steroidogenesis in Male Offspring
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virtanen, H.E.; Jorgensen, N.; Toppari, J. Semen quality in the 21(st) Century. Nat. Rev. Urol. 2017, 14, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Jiang, C.; Chen, Q.; Yang, H.; Wang, X.; Zou, P.; Sun, L.; Liu, J.; Li, L.; Li, L.; et al. Exposures to atmospheric PM10 and PM10-2.5 affect male semen quality: Results of MARHCS study. Environ. Sci. Technol. 2018, 52, 1571–1581. [Google Scholar] [CrossRef]
- Radwan, M.; Jurewicz, J.; Polańska, K.; Sobala, W.; Radwan, O.; Bochenek, M.; Hanke, W. Exposure to ambient air pollution–does it affect semen quality and the level of reproductive hormones? Ann. Human. Biol. 2016, 43, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yang, T.; Seyler, B.C.; Wang, X.; Wang, Y.; Jiang, M.; Liu, B.; Li, F. Ambient air pollution and male fecundity: A retrospective analysis of longitudinal data from a Chinese human sperm bank (2013–2018). Environ. Res. 2020, 186, 109528. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Wang, S. Fossil fuel combustion and biomass burning sources of global black carbon from GEOS-Chem simulation and carbon isotope measurements. Atmos. Chem. Phys. 2019, 19, 11545–11557. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.; Guo, S.; Wu, Z.; He, L.; Huang, X.; Hu, M. Impact of aging process on atmospheric black carbon aerosol properties and climate effects. Chin. Sci. Bull. 2020, 65, 4235–4250. [Google Scholar] [CrossRef]
- Antiñolo, M.; Willis, M.D.; Zhou, S.; Abbatt, J.P.D. Connecting the oxidation of soot to its redox cycling abilities. Nat. Commun. 2015, 6, 6812. [Google Scholar] [CrossRef] [Green Version]
- Ben, B.; Bellouin, N. Black carbon and atmospheric feedbacks. Nature 2015, 519, 167–168. [Google Scholar] [CrossRef]
- Li, Q.; Shang, J.; Zhu, T. Physicochemical characteristics and toxic effects of ozone-oxidized black carbon particles. Atmos. Environ. 2013, 81, 68–75. [Google Scholar] [CrossRef]
- Li, Y.; Henze, D.K.; Jack, D.; Henderson, B.H.; Kinney, P.L. Assessing public health burden associated with exposure to ambient black carbon in the United States. Sci. Total Environ. 2016, 539, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Kirrane, E.F.; Luben, T.J.; Benson, A.; Owens, E.O.; Sacks, J.D.; Dutton, S.J.; Madden, M.; Nichols, J.L. A systematic review of cardiovascular responses associated with ambient black carbon and fine particulate matter. Environ. Int. 2019, 127, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-C.; Cao, J.-J.; Ward, T.-J.; Tian, L.W.; Ning, Z.; Gali, N.K.; Aquilina, N.J.; Yim, S.H.-L.; Qu, L.; Ho, K.-F. Characteristics and toxicological effects of commuter exposure to black carbon and metal components of fine particles (PM2.5) in Hong Kong. Sci. Total Environ. 2020, 742, 140501. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.H.; Hoek, G.; Simic-Lawson, M.; Fischer, P.; van Bree, L.; Brink, H.; Keuken, M.; Atkinson, R.W.; Anderson, H.R.; Brunekreef, B.; et al. Black carbon as an additional indicator of the adverse health effects of airborne particles compared with PM10 and PM2.5. Environ. Health Persp. 2011, 119, 1691–1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garshick, E.; Grady, S.T.; Hart, J.E.; Coull, B.A.; Schwartz, J.D.; Francine Laden, F.; Moy, M.L.; Koutrakis, P. Indoor black carbon and biomarkers of systemic inflammation and endothelial activation in COPD patients. Environ. Res. 2018, 165, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Hao, W.; Cheng, Z.; Huang, Y.; Wang, S.; Shang, J.; Hou, X.; Meng, Q.; Zhang, Q.; Jia, L.; et al. Black carbon particles and ozone-oxidized black carbon particles induced lung damage in mice through an interleukin-33 dependent pathway. Sci. Total Environ. 2018, 644, 217–228. [Google Scholar] [CrossRef]
- Chu, H.; Shang, J.; Jin, M.; Li, Q.; Chen, Y.; Huang, H.; Li, Y.; Pan, Y.; Tao, X.; Cheng, Z.; et al. Comparison of lung damage in mice exposed to black carbon particles and ozone-oxidized black carbon particles. Sci. Total Environ. 2016, 573, 303–312. [Google Scholar] [CrossRef]
- Zhang, Q.; Meng, X.; Shi, S.; Kan, L.; Chen, R.; Kan, H. Overview of particulate air pollution and human health in China: Evidence, challenges, and opportunities. Innovation 2022, 3, 100312. [Google Scholar] [CrossRef]
- Baumgartner, J.; Zhang, Y.; Schauer, J.J.; Huang, W.; Wang, Y.; Ezzati, M. Highway proximity and black carbon from cookstoves as a risk factor for higher blood pressure in rural China. Proc. Natl. Acad. Sci. USA 2014, 111, 13229–13234. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Song, X.; Han, Y.; Ji, Y.; Gao, S.; Shang, Y.; Lu, S.; Zhu, T.; Huang, W. Size-fractioned ultrafine particles and black carbon associated with autonomic dysfunction in subjects with diabetes or impaired glucose tolerance in Shanghai, China. Part. Fibre Toxicol. 2015, 12, 8. [Google Scholar] [CrossRef] [Green Version]
- Ge, J.; Chu, H.; Xiao, Q.; Hao, W.; Shang, J.; Zhu, T.; Sun, Z.; Wei, X. BC and 1,4NQ-BC up-regulate the cytokines and enhance IL-33 expression in LPS pretreatment of human bronchial epithelial cells. Environ. Pollut. 2021, 273, 116452. [Google Scholar] [CrossRef]
- Bista, S.; Fancello, G.; Chaix, B. Acute ambulatory blood pressure response to short-term black carbon exposure: The MobiliSense sensor-based study. Sci. Total Environ. 2022, 846, 157350. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, W.; Chu, H.; Ge, J.; Wang, X.; Jiang, J.; Xiao, Q.; Meng, Q.; Hao, W.; Wei, X. Exploration of potential mechanism of interleukin-33 up-regulation caused by 1,4-naphthoquinone black carbon in RAW264.7 cell. Sci. Total Environ. 2022, 835, 155357. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Shang, M.; Mu, K.; Jiang, N.; Wen, H.; Wang, R.; Wu, H.; Li, W. In vitro and in vivo toxic effects and inflammatory responses induced by carboxylated black carbon-lead complex exposure. Ecotox. Environ. Safe. 2018, 165, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Wen, H.; Zhou, M.; Lei, T.; Shen, J.; Zhang, D.; Wang, R.; Wu, H.; Jiang, S.; Li, W. Low-dose combined exposure of oxidized black carbon and heavy metal lead induced potentiation of oxidative stress, DNA damage, apoptosis, and inflammation in human bronchial epithelial cells. Ecotox. Environ. Safe 2020, 206, 111388. [Google Scholar] [CrossRef]
- Mäkelä, J.-A.; Koskenniemi, J.J.; Virtanen, H.E.; Toppari, J. Testis Development. Endocr. Rev. 2019, 40, 857–905. [Google Scholar] [CrossRef]
- Johnson, N.M.; Hoffmann, A.R.; Behlen, J.C.; Lau, C.; Pendleton, D.; Harvey, N.; Shore, R.; Li, Y.; Chen, J.; Tian, Y.; et al. Air pollution and children’s health–a review of adverse effects associated with prenatal exposure from fine to ultrafine particulate matter. Environ. Health Prev. 2021, 26, 72. [Google Scholar] [CrossRef]
- Chaudhuri, I.; Fruijtier-Pölloth, C.; Ngiewih, Y.; Levy, L. Evaluating the evidence on genotoxicity and reproductive toxicity of carbon black: A critical review. Crit. Rev. Toxicol. 2018, 48, 143–169. [Google Scholar] [CrossRef] [Green Version]
- Pires, A.; de Melo, E.N.; Mauad, T.; Saldiva, P.H.N.; de Siqueira Bueno, H.M. Pre- and postnatal exposure to ambient levels of urban particulate matter (PM2.5) affects mice spermatogenesis. Inhal. Toxicol. 2011, 23, 237–245. [Google Scholar] [CrossRef]
- Umezawa, M.; Bondarenko, O.; Mortimer, M.; Kahru, A.; Feliu, N.; Javedg, I.; Kakinen, A.; Lin, S.; Xia, T.; Song, Y.; et al. Nanotoxicology and nanomedicine: The Yin and Yang of nano-bio interactions for the new decade. Nano Today 2021, 39, 101184. [Google Scholar] [CrossRef]
- Skovmand, A.; Jensen, A.C.Ø.; Maurice, C.; Marchetti, C.F.; Lauvås, A.J.; Koponen, I.K.; Jensen, K.A.; Goericke-Pesch, S.; Vogel, U.; Hougaard, K.S. Effects of maternal inhalation of carbon black nanoparticles on reproductive and fertility parameters in a four-generation study of male mice. Part. Fibre Toxicol. 2019, 16, 13. [Google Scholar] [CrossRef] [Green Version]
- Bové, H.; Bongaerts, E.; Slenders, E.; Bijnens, E.M.; Saenen, N.D.; Gyselaers, W.; Eyken, P.V.; Plusquin, M.; Roeffaers, M.B.J.; Ameloot, M.; et al. Ambient black carbon particles reach the fetal side of human placenta. Nat. Commun. 2019, 10, 3866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongaerts, E.; Lecante, L.L.; Bové, H.; Roeffaers, M.B.J.; Ameloot, M.; Fowler, P.A.; Nawrot, T.S. Maternal exposure to ambient black carbon particles and their presence in maternal and fetal circulation and organs: An analysis of two independent population-based observational studies. Lancet. Planet. Health 2022, 6, e804–e811. [Google Scholar] [CrossRef]
- Skovmand, A.; Lauvås, A.J.; Christensen, P.; Vogel, U.; Hougaard, K.S.; GoerickePesch, S. Pulmonary exposure to carbonaceous nanomaterials and sperm quality. Part. Fibre Toxicol. 2018, 15, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, S.; Yamada, H.; Sugawara, I.; Takeda, K. Effect of dibromochloropropane (DBCP) on the hormone receptors of the male rat reproductive system. Biosci. Biotechnol. Biochem. 1998, 62, 479–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, N.; Oshio, S.; Niwata, Y.; Yoshida, S.; Tsukue, N.; Sugawara, I.; Takano, H.; Takeda, K. Prenatal exposure to diesel exhaust impairs mouse spermatogenesis. Inhal. Toxicol. 2007, 19, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Luyten, L.J.; Saenen, N.D.; Janssen, B.G.; Vrijens, K.; Plusquin, M.; Roles, H.A.; Debacq-Chainiaux, F.; Nawrot, T.S. Air pollution and the fetal origin of disease: A systematic review of the molecular signatures of air pollution exposure in human placenta. Environ. Res. 2018, 166, 310–323. [Google Scholar] [CrossRef]
- Morales-Rubio, R.A.; Alvarado-Cruz, I.; Manzano-León, N.; Andrade-Oliva, M.A.; Uribe-Ramirez, M.; Quintanilla-Vega, B.; Osornio-Vargas, Á.; Vizcaya-Ruiz, A.D. In utero exposure to ultrafine particles promotes placental stress-induced programming of renin-angiotensin systemrelated elements in the offspring results in altered blood pressure in adult mice. Part. FibreToxicol. 2019, 16, 7. [Google Scholar] [CrossRef]
- Kyjovska, Z.O.; Boisen, A.M.Z.; Jackson, P.; Wallin, H.; Vogel, U.; Hougaard, K.S. Daily sperm production: Application in studies of prenatal exposure to nanoparticles in mice. Reprod. Toxicol. 2013, 36, 88–97. [Google Scholar] [CrossRef]
- Martenies, S.E.; Keller, J.P.; WeMott, S.; Kuiper, G.; Ross, Z.; Allshouse, W.B.; Adgate, J.L.; Starling, A.P.; Dabelea, D.; Magzamen, S. A spatiotemporal prediction model for black carbon in the Denver Metropolitan Area, 2009–2020. Environ. Sci. Technol. 2021, 55, 3112–3123. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiyoshi, K.; Oshio, S.; Takano, H.; Takeda, K.; Ichinose, T. Effects of fetal exposure to carbon nanoparticles on reproductive function in male offspring. FertilSteril. 2010, 93, 1695–1699. [Google Scholar] [CrossRef]
- Lui, W.Y.; Mruk, D.; Lee, W.M.; Cheng, C.Y. Sertoli cell tight junction dynamics: Their regulation during spermatogenesis. Biol. Reprod. 2003, 68, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef] [Green Version]
- Stocco, D.M.; Barbara, J.; Clark, B.J. Role of the steroidogenic acute regulatory protein (StAR) in steroidogenesis. Biochem. Pharmacol. 1996, 51, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, X.; Jigami, J.; Hasegawa, C.; Akira, K.; Suzuki, A.K.; Zhang, Y.; Fujitani, Y.; Nagaoka, K.; Watanabe, G.; et al. Effect of nanoparticle-rich diesel exhaust on testosterone biosynthesis in adult male mice. Inhal. Toxicol. 2012, 24, 599–608. [Google Scholar] [CrossRef]



| Gene | Forward Primer (5′–3′) | Reverse Primer (3′–5′) | Probe |
|---|---|---|---|
| P450scc | ACTAGCAGTCCTAGGTCCTTCAATGA | TGGATTTTCTGTGTGCCACTCC | CTGGCGACAATGGTTGGCTAAACCTGT |
| P450c17 | CTCATCCCACACAAGGCTAACA | TTATCGTGATGCAGTGCCCA | TTGCCATCCCGAAGGACACACATGT |
| 17β-HSD | AGCCTATTCATTTGAGTTGGCC | TGGTCCTCTCAATCTCTTCTGCA | AGCCGGACACTGGAAAAGCTACAGACCA |
| 3β-HSD | ATTCCCAGGCAGACCATCCTA | TGAGCTGCAGAAGATGAAGGC | TCTGAAAGGTACCCAGAACCTATTGGAGGC |
| StAR | TCACTTGGCTGCTCAGTATTGAC | TGGTTGGCGAACTCTATCTGG | TGGCTGCCGAAGACAATCATCAACC |
| LHR | GGTGCTGGCAATGCTGG | CGCAGTCGCAGGGCTC | TCTCCAGAGTTGTCAGGGTCGCGC |
| GAPDH | TGCACCACCAACTGCTTAG | GGATGCAGGGATGATGTTC | CAGAAGACTGTGGATGGCCCCTC |
| Number of Dam | Number of Offspring per Dam | Total Offspring | Sex Ratio (%) | |||
|---|---|---|---|---|---|---|
| Male | Female | Litter Size | ||||
| Control | 15 | 7.26 ± 0.55 | 5.49 ± 0.62 | 12.83 ± 0.65 | 183 | 56.59 |
| OBC | 12 | 5.64 ± 0.61 | 6.38 ± 0.51 | 11.59 ± 0.68 | 154 | 48.66 |
| PND 8 | PND 16 | PND 21 | PND 35 | PND 84 | ||
|---|---|---|---|---|---|---|
| No. animal examined | Control | 12 | 12 | 10 | 10 | 10 |
| OBC | 11 | 10 | 9 | 9 | 9 | |
| BW(g) | Control | 5.26 ± 0.15 | 7.23 ± 0.51 | 11.12 ± 0.78 | 29.87 ± 0.85 | 41.75 ± 1.02 |
| OBC | 4.31 ± 0.22 ** | 6.08 ± 0.46 | 9.01 ± 0.83 | 26.45 ± 1.03 * | 39.11 ± 1.38 | |
| Testis/BW (mg/g) | Control | 1.62 ± 0.05 | 2.81 ± 0.12 | 3.56 ± 0.13 | 4.92 ± 0.16 | 6.12 ± 0.16 |
| OBC | 1.55 ± 0.08 | 2.75 ± 0.09 | 3.41 ± 0.18 | 4.51 ± 0.19 | 5.83 ± 0.19 | |
| Epididymis/BW (mg/g) | Control | 0.63 ± 0.05 | 0.73 ± 0.04 | 0.89 ± 0.05 | 1.32 ± 0.05 | 2.41 ± 0.06 |
| OBC | 0.70 ± 0.03 | 0.76 ± 0.03 | 0.92 ± 0.06 | 1.30 ± 0.03 | 2.52 ± 0.09 | |
| Access glands/BW (mg/g) | Control | 0.18 ± 0.02 | 0.29 ± 0.02 | 0.33 ± 0.04 | 2.87 ± 0.19 | 7.06 ± 0.42 |
| OBC | 0.12 ± 0.04 * | 0.23 ± 0.07 * | 0.28 ± 0.06 | 2.59 ± 0.27 | 6.83 ± 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Chen, L.; Shen, J.; Zhang, D.; Wu, H.; Wang, R.; Zhang, S.; Jiang, N.; Li, W. Adverse Effects of Prenatal Exposure to Oxidized Black Carbon Particles on the Reproductive System of Male Mice. Toxics 2023, 11, 556. https://doi.org/10.3390/toxics11070556
Jiang S, Chen L, Shen J, Zhang D, Wu H, Wang R, Zhang S, Jiang N, Li W. Adverse Effects of Prenatal Exposure to Oxidized Black Carbon Particles on the Reproductive System of Male Mice. Toxics. 2023; 11(7):556. https://doi.org/10.3390/toxics11070556
Chicago/Turabian StyleJiang, Shuanglin, Li Chen, Jianyun Shen, Di Zhang, Hai Wu, Rong Wang, Shangrong Zhang, Nan Jiang, and Wenyong Li. 2023. "Adverse Effects of Prenatal Exposure to Oxidized Black Carbon Particles on the Reproductive System of Male Mice" Toxics 11, no. 7: 556. https://doi.org/10.3390/toxics11070556





