Effect of Arsenic Soil Contamination on Stress Response Metabolites, 5-Methylcytosine Level and CDC25 Expression in Spinach
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Analysis of Total Arsenic Content
2.3. Arsenic Speciation Analysis
2.4. 5-Methylcytosine Levels Determination
2.5. Relative Transcript Level Determination
2.6. Phenolic Compounds and Total Antioxidant Capacity
2.7. Microscopy
2.8. Statistical Analyses
3. Results
3.1. Arsenic Contamination Impact on Phenolic Compounds and Total Antioxidant Capacity
3.2. Arsenic Accumulation and Speciation in As(V) Exposed Spinach Plants
3.3. Levels of 5-Methylcytosine under Arsenic Stress
3.4. Anatomic Changes in Tissues of Plants Exposed of Arsenic
3.5. Arsenic-Dependent CDC25 Transcription
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bradl, H.B. Heavy Metals in the Environment: Origin, Interaction and Remediation; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Paul, N.P.; Galván, A.E.; Yoshinaga-Sakurai, K.; Rosen, B.P.; Yoshinaga, M. Arsenic in medicine: Past, present and future. Biometals 2023, 36, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, M.K.; Shukla, A.; Yadav, P.; Srivastava, S. A review of arsenic in crops, vegetables, animals and food products. Food Chem. 2019, 276, 608–618. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; Arcella, D.; Cascio, C.; Gomez Ruiz, J.A. Chronic dietary exposure to inorganic arsenic. EFSA J. 2021, 19, 50. [Google Scholar]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Maciaszczyk-Dziubinska, E.; Wawrzycka, D.; Wysocki, R. Arsenic and Antimony Transporters in Eukaryotes. Int. J. Mol. Sci. 2012, 13, 3527–3548. [Google Scholar] [CrossRef] [PubMed]
- Pandhair, V.; Sekhon, B.S. Reactive oxygen species and antioxidants in plants: An overview. J. Plant Biochem. Biotechnol. 2006, 15, 71–78. [Google Scholar] [CrossRef]
- Zaman, K.P.; Pardini, R.S. An Overview of the Relationship between Oxidative Stress and Mercury and Arsenic. Toxic Subst. Mech. 1996, 15, 151–181. [Google Scholar]
- Sharma, I. Arsenic induced oxidative stress in plants. Biologia 2012, 67, 447–453. [Google Scholar] [CrossRef]
- Souri, Z.; Karimi, N.; Sandalio, L.M. Arsenic Hyperaccumulation Strategies: An Overview. Front. Cell Dev. Biol. 2017, 5, 67. [Google Scholar] [CrossRef]
- Shi, H.; Shi, X.; Liu, K.J. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol. Cell. Biochem. 2004, 255, 67–78. [Google Scholar] [CrossRef]
- Yalcinkaya, T.; Uzilday, B.; Ozgur, R.; Turkan, I.; Mano, J. Lipid peroxidation-derived reactive carbonyl species (RCS): Their interaction with ROS and cellular redox during environmental stresses. Environ. Exp. Bot. 2019, 165, 139–149. [Google Scholar] [CrossRef]
- Mano, J.; Biswas, M.S.; Sugimoto, K. Reactive Carbonyl Species: A Missing Link in ROS Signaling. Plants 2019, 8, 391. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism, and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolic Acids, flavonoids and Total Antioxidant Capacity of Selected Leafy Vegetables. J. Funct. Foods. 2012, 4, 979–987. [Google Scholar] [CrossRef]
- Lukens, L.N.; Zhan, S. The plant genome’s methylation status and response to stress: Implications for plant improvement. Curr. Opin. Plant Biol. 2007, 10, 317–322. [Google Scholar] [CrossRef]
- Chen, M.; Lv, S.; Meng, Y. Epigenetic performers in plants. Dev. Growth Differ. 2010, 52, 555–566. [Google Scholar] [CrossRef]
- Grativol, C.; Hemerly, A.S.; Ferreira, P.C.G. Genetic and Epigenetic Regulation of Stress Responses in Natural Plant Populations. Biochim. Biophys. Acta-Gene Regul. Mech. 2012, 1819, 176–185. [Google Scholar] [CrossRef]
- Kohli, R.M.; Zhang, Y. TET Enzymes, TDG and the Dynamics of DNA Demethylation. Nature 2013, 502, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wu, H.; Diep, D.; Yamaguchi, S.; D’Alessio, A.C.; Fung, H.L.; Zhang, K.; Zhang, Y. Genome-Wide Analysis Reveals TET- and TDG-Dependent 5-Methylcytosine Oxidation Dynamics. Cell 2013, 153, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Meiqiong, T.; Jiao, Y.; Zhen, H.; Yali, H.; Zengqiang, L.; Dengjie, L.; Shan, C.; Hui, Z.; Jiao, P.; Xia, W.; et al. Physiological and DNA methylation analysis provides epigenetic insights into chromium tolerance in kenaf. J. Exp. Bot. 2022, 194, 104684. [Google Scholar]
- Espinas, N.A.; Saze, H.; Saijo, Y. Epigenetic control of defense signaling and priming in plants. Front. Plant Sci. 2016, 7, 1201. [Google Scholar] [CrossRef] [PubMed]
- Thiebaut, F.; Hemerly, A.S.; Ferreira, P.C.G. A Role for Epigenetic Regulation in the Adaptation and Stress Responses of Non-Model Plants. Front. Plant Sci. 2019, 10, 246. [Google Scholar] [CrossRef]
- Bossdorf, O.; Arcuri, D.; Richards, C.L.; Pigliucci, M. Experimental alteration of DNA methylation affects the phenotypic plasticity of ecologically relevant traits in Arabidopsis thaliana. Evol. Ecol. 2010, 24, 541–553. [Google Scholar] [CrossRef]
- Iwase, Y.; Shiraya, T.; Takeno, K. Flowering and Dwarfism Induced by DNA Demethylation in Pharbitis Nil. Physiol. Plant. 2010, 139, 118–127. [Google Scholar] [CrossRef]
- Ba, Q.; Zhang, G.; Wang, J.; Niu, N.; Ma, S.; Wang, J. Gene expression and DNA methylation alterations in chemically induced male sterility anthers in wheat (Triticum aestivum L.). Acta Physiol. Plant. 2014, 36, 503–512. [Google Scholar] [CrossRef]
- Lechat, M.M.; Brun, G.; Montiel, G.; Véronési, C.; Simier, P.; Thoiron, S.; Pouvreau, J.B.; Delavault, P. Seed response to strigolactone is controlled by abscisic acid-independent DNA methylation in the obligate root parasitic plant, Phelipanche ramosa L. Pomel. J. Exp. Bot. 2015, 66, 3129–3140. [Google Scholar] [CrossRef]
- Campos, N.V.; Araújo, T.O.; Arcanjo-Silva, S.; Freitas-Silva, L.; Azevedo, A.A.; Nunes-Nesi, A. Arsenic hyperaccumulation induces metabolic reprogramming in Pityrogramma calomelanos to reduce oxidative stress. Physiol. Plant. 2016, 157, 135–146. [Google Scholar] [CrossRef]
- Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar]
- Fayiga, A.O.; Saha, U.K. Arsenic hyperaccumulating fern: Implications for remediation of arsenic contaminated soils. Geoderma 2016, 284, 132–143. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy Metal Hyperaccumulating Plants: How and Why Do They Do It? And What Makes Them so Interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Zhao, F.J.; McGrath, S.P.; Meharg, A.A. Arsenic as a Food Chain Contaminant: Mechanisms of Plant Uptake and Metabolism and Mitigation Strategies. Annu. Rev. Plant Biol. 2010, 61, 535–559. [Google Scholar] [CrossRef]
- Mendoza-Cózatl, D.G.; Jobe, T.O.; Hauser, F.; Schroeder, J.I. Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Curr. Opin. Plant Biol. 2011, 14, 554–562. [Google Scholar] [CrossRef]
- Duan, G.L.; Zhou, Y.; Tong, Y.P.; Mukhopadhyay, R.; Rosen, B.P.; Zhu, Y.G. A CDC25 homologue from rice functions as an arsenate reductase. New Phytol. 2007, 174, 311–321. [Google Scholar] [CrossRef]
- Bleeker, P.M.; Hakvoort, H.W.; Bliek, M.; Souer, E.; Schat, H. Enhanced arsenate reduction by a CDC25-like tyrosine phosphatase explains increased phytochelatin accumulation in arsenate-tolerant Holcus lanatus. Plant J. 2006, 45, 917–929. [Google Scholar] [CrossRef]
- Ellis, D.R.; Gumaelius, L.; Indriolo, E.; Pickering, I.J.; Banks, J.A.; Salt, D.E. A novel arsenate reductase from the arsenic hyperaccumulating fern Pteris vittata. Plant Physiol. 2006, 141, 1544–1554. [Google Scholar] [CrossRef]
- Dhankher, O.P.; Rosen, B.P.; McKinney, E.C.; Meagher, R.B. Hyperaccumulation of arsenic in the shoots of Arabidopsis silenced for arsenate reductase (ACR2). Proc. Natl. Acad. Sci. USA 2006, 103, 5413–5418. [Google Scholar] [CrossRef]
- Sanchez-Bermejo, E.; Castrillo, G.; del Llano, B.; Navarro, C.; Zarco-Fernandez, S.; Martinez-Herrera, D.J.; del Puerto, Y.L.; Munoz, R.; Camara, C.; Paz-Ares, J.; et al. Natural variation in arsenate tolerance identifies an arsenate reductase in Arabidopsis thaliana. Nat. Commun. 2014, 5, 4617. [Google Scholar] [CrossRef]
- Kerk, D.; Templeton, G.; Moorhead, G.B. Evolutionary radiation pattern of novel protein phosphatases revealed by analysis of protein data from the completely sequenced genomes of humans, green algae, and higher plants. Plant Physiol. 2008, 146, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Mfarrej, M.F.B.; Alatawi, A.; Mumtaz, S.; Imran, M.; Ashraf, M.A.; Rizwan, M.; Usman, K.; Ahmad, P.; Ali, S. Silicon enhances morpho-physio-biochemical responses in arsenic stressed spinach (Spinacia oleracea L.) by minimizing its uptake. J. Plant Growth Regul. 2023, 42, 2053–2072. [Google Scholar] [CrossRef]
- Zemanová, V.; Pavlíková, D.; Hnilička, F.; Pavlík, M. Arsenic toxicity-induced physiological and metabolic changes in the shoots of Pteris cretica and Spinacia oleracea. Plants 2021, 10, 2009. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wang, Z.; Li, X.; Wang, H.; Wang, H.; Chen, W. A comprehensive assessment of heavy metal(loid) contamination in leafy vegetables grown in two mining areas in Yunnan, China—A focus on bioaccumulation of cadmium in Malabar spinach. Environ. Sci. Pollut. Res. 2022, 30, 14959–14974. [Google Scholar] [CrossRef]
- Sun, Y.; Mfarrej, B.F.; Song, X.; Ma, J.; Min, B.; Chen, F. New insights in to the ameliorative effects of zinc and iron oxide nanoparticles to arsenic stressed spinach (Spinacia oleracea L.). Plant Physiol. Biochem. 2023, 199, 107715. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.D.; Barman, M.; Mitra, A. Photosynthetic apparatus plays a central role in photosensitive physiological acclimations affecting spinach (Spinacia oleracea L.) growth in response to blue and red photon flux ratios. Environ. Exp. Bot. 2018, 156, 170–182. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Ali, M.; Riaz, M.; Javed, S.; Sehar, A.; Abbas, Z.; Rizwan, M.; El-Sheikh, M.A.; et al. Interactive role of zinc and iron lysine on Spinacia oleracea L. growth, photosynthesis and antioxidant capacity irrigated with tannery wastewater. Physiol. Mol. Biol. Plants 2020, 26, 2435–2452. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Noor, I.; El-Esawi, M.A.; Hayat, K.; Rizwan, M.; Abbas, Z.; El-Sheikh, M.A.; Alyemeni, M.N. Iron–Lysine Mediated Alleviation of Chromium Toxicity in Spinach (Spinacia oleracea L.) Plants in Relation to Morpho-Physiological Traits and Iron Uptake When Irrigated with Tannery Wastewater. Sustainability 2020, 12, 6690. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Favas, P.J.C.; Pratas, J.; Varun, M.; Paul, M.S. Metal(loid) induced toxicity and defense mechanisms in Spinacia oleracea L. Ecological hazard and prospects for phytoremediation Ecotox. Environ. Saf. 2019, 183, 109570. [Google Scholar] [CrossRef]
- Zubair, M.; Khan, Q.U.; Mirza, N.; Sarwar, R.; Khan, A.A.; Baloch, M.S.; Fahad, S.; Shah, A.N. Physiological response of spinach to toxic heavy metal stress. Environ. Sci. Pollut. Res. 2019, 26, 31667–31674. [Google Scholar] [CrossRef]
- Button, M.; Moriarty, M.M.; Watts, M.J.; Zhang, J.; Koch, I.; Reimer, K.J. Arsenic speciation in field-collected and laboratory-exposed earthworms Lumbricus terrestris. Chemosphere 2011, 85, 1277–1283. [Google Scholar] [CrossRef]
- Sácký, J.; Leonhardt, T.; Borovička, J.; Gryndler, M.; Briksí, A.; Kotrba, P. Intracellular Sequestration of Zinc, Cadmium and Silver in Hebeloma mesophaeum and Characterization of Its Metallothionein Genes. Fungal Genet. Biol. 2014, 67, 3–14. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modifified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of Total Flavonoid Content by Aluminum Chloride Assay: A Critical Evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Szaufer-Hajdrych, M. Phenolic acids in leaves of species of the Aquilegia genus. Herba Pol. 2004, 50, 10–14. [Google Scholar]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Smilauer, P.; Leps, J. Multivariate Analysis of Ecological Data Using Canoco 5; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Saha, N.; Zaman, M.R. Evaluation of possible health risks of heavy metals by consumption of foodstuffs available in the central market of Rajshahi City, Bangladesh. Environ. Monit. Assess. 2013, 185, 3867–3878. [Google Scholar] [CrossRef]
- Dahal, B.M.; Fuerhacker, M.; Mentler, A.; Karki, K.B.; Shrestha, R.R.; Blum, W.E.H. Arsenic contamination of soils and agricultural plants through irrigation water in Nepal. Environ. Pollut. 2008, 155, 157–163. [Google Scholar] [CrossRef]
- Hartley, W.; Lepp, N.W. Remediation of arsenic contaminated soils by iron-oxide application, evaluated in terms of plant productivity, arsenic and phytotoxic metal uptake. Sci. Total Environ. 2008, 390, 35–44. [Google Scholar] [CrossRef]
- Pavlík, M.; Pavlíková, D.; Staszková, L.; Neuberg, M.; Kaliszová, R.; Száková, J.; Tlustoš, P. The effect of arsenic contamination on amino acids metabolism in Spinacia oleracea L. Ecotoxicol. Environ. Saf. 2010, 73, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhang, L.; Yao, Z.-L.; Ren, Y.-B.; Wang, L.-Q.; Ou, X.-B. Arsenic accumulation and physiological response of three leafy vegetable varieties to As stress. Int. J. Environ. Res. Public. Health 2022, 19, 2501. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Sharma, Y.K. Altered growth, photosynthetic machinery and induced oxidative stress in spinach in response to arsenic stress. J. Plant. Physiol. Pathol. 2013, 1, 2. [Google Scholar]
- Nihal, A.; Mithun, P.R.; Praveen, N. Effect of heavy metals (Hg, As and La) on biochemical constituents of Spinacia oleracea. J. Pharmacogn. Phytochem. 2019, 8, 669–674. [Google Scholar]
- Finnegan, P.M.; Chen, W. Arsenic toxicity: The effects on plant metabolism. Front. Physiol. 2012, 6, 182. [Google Scholar] [CrossRef]
- González-Moscoso, M.; Juárez-Maldonado, A.; Cadenas-Pliego, G.; Meza-Figueroa, D.; SenGupta, B.; Martínez-Villegas, N. Silicon nanoparticles decrease arsenic translocation and mitigate phytotoxicity in tomato plants. Environ. Sci. Pollut. Res. 2022, 29, 34147–34163. [Google Scholar] [CrossRef]
- Keilig, K.; Ludwig-Mueller, J. Effect of flavonoids on heavy metal tolerance in Arabidopsis thaliana seedlings. Bot. Stud. 2009, 50, 311–318. [Google Scholar]
- Pandey, N.; Patkak, G.C.; Pandey, D.K.; Pandey, R. Heavy metals, Co, Ni, Cu, Zn and Cd, produce oxidative damage and evoke differential antioxidant responses in spinach. Braz. J. Plant Physiol. 2009, 21, 103–111. [Google Scholar]
- Colak, N.; Torun, H.; Gruz, J.; Strnad, M.; Ayaz, F.A. Exogenous N-Acetylcysteine alleviates heavy metal stress by promoting phenolic acids to support antioxidant defence systems in wheat roots. Ecotoxicol. Environ. Saf. 2019, 181, 49–59. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Adeleye, A.S.; Keller, A.A. Metabolomics reveals Cu(OH)2 nanopesticide-activated anti-oxidative pathways and decreased beneficial antioxidants in spinach leaves. Environ. Sci. Technol. 2017, 51, 10184–10194. [Google Scholar] [CrossRef]
- Wu, L.; Yi, H.; Min, Y. Assessment of arsenic toxicity using Allium/Vicia root tip micronucleus assays. J. Hazard. Mater. 2010, 176, 952–956. [Google Scholar] [CrossRef]
- Yi, H.; Wu, L.; Jiang, L. Genotoxicity of arsenic evaluated by Allium-root micronucleus assay. Sci. Total Environ. 2007, 383, 232–236. [Google Scholar] [CrossRef]
- Aina, R.; Sgorbati, S.; Santagostino, A.; Labra, M.; Ghiani, A.; Citterio, S. Specific hypomethylation of DNA is induced by heavy metals in white clover and industrial hemp. Physiol. Plant. 2004, 121, 472–480. [Google Scholar] [CrossRef]
- Bolukbasi, E. Methylation Modelling and Epigenetic Analysis of Sunflower (Helianthus annuus L.) Seedlings Exposed to Cadmium Heavy Metal Stress. KSU J. Agric. Nat. 2022, 25, 467–475. [Google Scholar] [CrossRef]


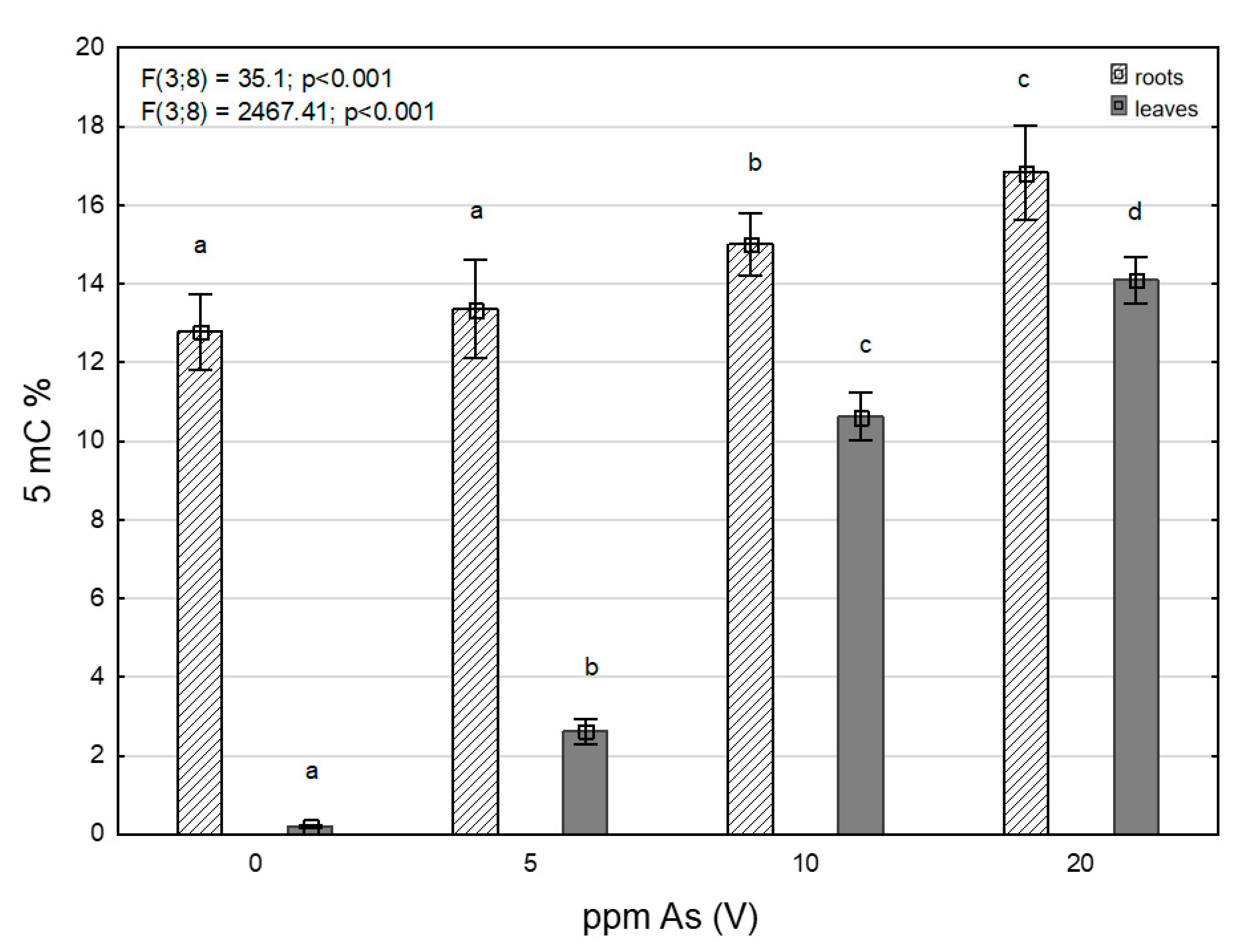

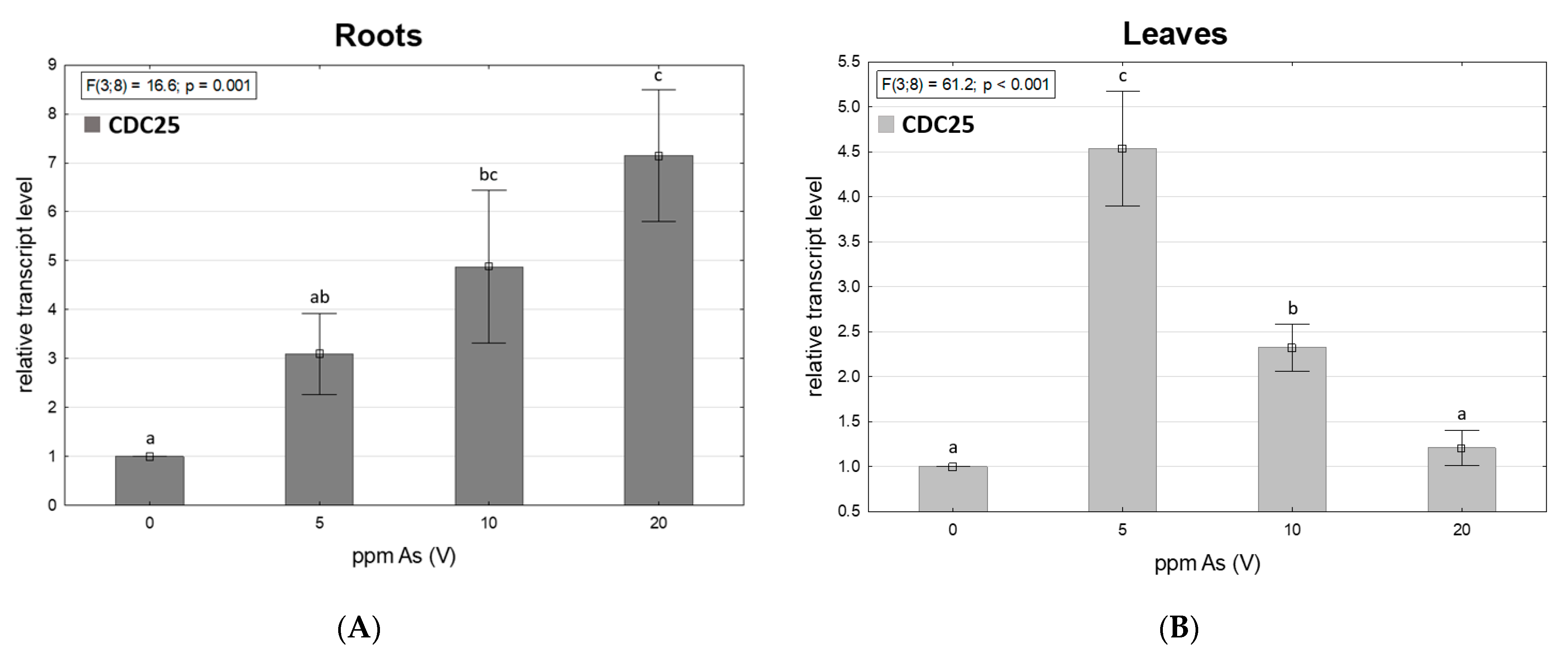

| ppm As(V) | Total Arsenic Content | MDA | ||
|---|---|---|---|---|
| Roots | Leaves | Roots | Leaves | |
| Control | 2.58 ± 0.26 a | 0.31 ± 0.06 b | 11.97 ± 0.54 d | 10.84 ± 0.16 a |
| 5 | 72.99 ± 3.03 b | 5.23 ± 0.30 a | 8.70 ± 0.82 c | 7.87 ± 1.01 b |
| 10 | 180.51 ± 5.06 c | 5.16 ± 0.09 a | 4.72 ± 0.48 b | 10.24 ± 1.04 a |
| 20 | 302.69 ± 11.83 d | 23.39 ± 2.39 c | 2.35 ± 0.43 a | 11.60 ± 0.62 a |
| p | <0.001 | <0.001 | <0.001 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, M.; Kubeš, J.; Vachová, P.; Hnilička, F.; Zemanová, V.; Česká, J.; Praus, L.; Lhotská, M.; Kudrna, J.; Tunklová, B.; et al. Effect of Arsenic Soil Contamination on Stress Response Metabolites, 5-Methylcytosine Level and CDC25 Expression in Spinach. Toxics 2023, 11, 568. https://doi.org/10.3390/toxics11070568
Popov M, Kubeš J, Vachová P, Hnilička F, Zemanová V, Česká J, Praus L, Lhotská M, Kudrna J, Tunklová B, et al. Effect of Arsenic Soil Contamination on Stress Response Metabolites, 5-Methylcytosine Level and CDC25 Expression in Spinach. Toxics. 2023; 11(7):568. https://doi.org/10.3390/toxics11070568
Chicago/Turabian StylePopov, Marek, Jan Kubeš, Pavla Vachová, František Hnilička, Veronika Zemanová, Jana Česká, Lukáš Praus, Marie Lhotská, Jiří Kudrna, Barbora Tunklová, and et al. 2023. "Effect of Arsenic Soil Contamination on Stress Response Metabolites, 5-Methylcytosine Level and CDC25 Expression in Spinach" Toxics 11, no. 7: 568. https://doi.org/10.3390/toxics11070568
APA StylePopov, M., Kubeš, J., Vachová, P., Hnilička, F., Zemanová, V., Česká, J., Praus, L., Lhotská, M., Kudrna, J., Tunklová, B., Štengl, K., Krucký, J., & Turnovec, T. (2023). Effect of Arsenic Soil Contamination on Stress Response Metabolites, 5-Methylcytosine Level and CDC25 Expression in Spinach. Toxics, 11(7), 568. https://doi.org/10.3390/toxics11070568







