Research Progress on Neurodevelopmental Toxicity in Offspring after Indirect Exposure to PFASs in Early Life
Abstract
1. Introduction
2. Characteristics of PFAS Exposure in Early Life
3. PFAS Exposure in Early Life Induced Neurodevelopmental Toxicity in Offspring
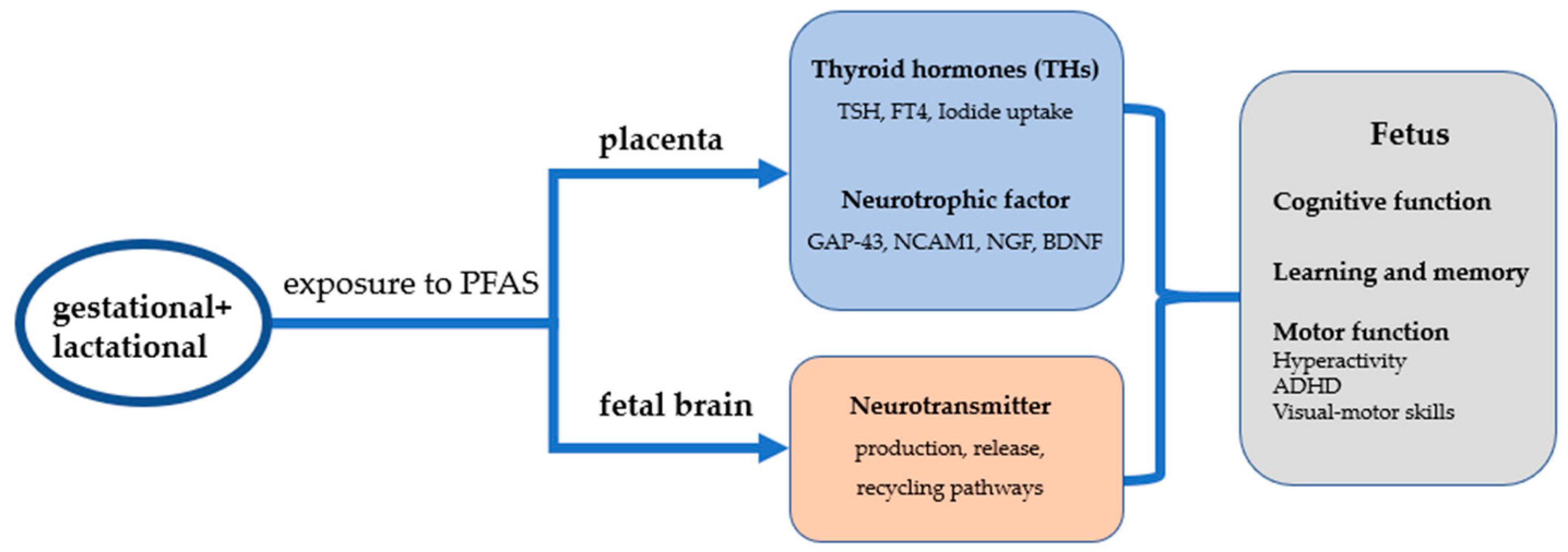
4. Mechanisms of Neurodevelopmental Toxicity after Exposure to PFAS in Early Life
4.1. PFASs Cause Neurodevelopmental Toxicity through Effects on Placental Thyroid Hormones
4.2. PFAS Causes Neurodevelopmental Toxicity by Affecting Placental Neurotrophic Factor Secretion
5. PFAS Causes Neurotoxicity by Altering Nerve Cell Function
6. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADHD | attention deficit hyperactivity disorder |
| BBB | blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| CaMKII | calcium/calmodulin dependent protein kinase II |
| CAT | catalase |
| CREB | cyclic-AMP response binding protein |
| FT4 | free thyroxine |
| GABA | Gamma amino butyric acid |
| GAP-43 | Growth associated protein-43 |
| GenX | 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy) propanoate |
| GGT | γ-Glutamyl Transferase |
| Gln | Glutamine |
| Glu | Glutamic |
| GPx | Glutathione peroxidase |
| HFPO-DA | hexafluoropropylene oxide dimer acid |
| HPT | axis Hypothalamic-pituitary-thyroid axis |
| NCAM1 | Neural Cell Adhesion Molecule 1 |
| NGF | nerve growth factor |
| NMDA | N-methyl-D-aspartic acid |
| PFAAs | perfluoroalkyl acids |
| PFAS | per- and polyfluoroalkyl substances |
| PFBA | perfluorobutanoic acid |
| PFBS | perfluorobutane sulfonic acid |
| PFDA | perfluorodecanoicacid |
| PFHpA | perfluoroheptanoate |
| PFHxA | perfluorohexanoic acid |
| PFHxS | perfluorohexane sulfonic acid |
| PFNA | perfluorononan-1-oic acid |
| PFOA | perfluorooctanoic acid |
| PFOS | perfluorooctane sulfonic acid and its derivatives |
| PFUnA | Perfluoroundecanoic acid |
| PKC | protein kinase C |
| PTE | placental transfer efficiency |
| ROS | Reactive Oxygen Species |
| SOD | Super Oxide Dismutase |
| T3 | 3,3′,5-triiodothyronine |
| T4 | thyroxine |
| TAC | total antioxidant capacity |
| TH | thyroid hormone |
| TSH | thyrotropin |
| TTR | transthyretin |
References
- Panieri, E.; Baralic, K.; Djukic-Cosic, D.; Buha Djordjevic, A.; Saso, L. PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics 2022, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Blake, B.E.; Fenton, S.E. Early Life Exposure to Per- and Polyfluoroalkyl Substances (PFAS) and Latent Health Outcomes: A Review Including the Placenta as a Target Tissue and Possible Driver of Peri- and Postnatal Effects. Toxicology 2020, 443, 152565. [Google Scholar] [CrossRef]
- Ao, J.; Yuan, T.; Xia, H.; Ma, Y.; Shen, Z.; Shi, R.; Tian, Y.; Zhang, J.; Ding, W.; Gao, L.; et al. Characteristic and Human Exposure Risk Assessment of Per- and Polyfluoroalkyl Substances: A Study Based on Indoor Dust and Drinking Water in China. Environ. Pollut. 2019, 254, 112873. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim Lazcano, R.; Yousefi, P.; Trim, H.; Lee, L.S. Perfluoroalkyl Acid Characterization in U.S. Municipal Organic Solid Waste Composts. Environ. Sci. Technol. Lett. 2019, 6, 372–377. [Google Scholar] [CrossRef]
- Brown-Leung, J.M.; Cannon, J.R. Neurotransmission Targets of Per- and Polyfluoroalkyl Substance Neurotoxicity: Mechanisms and Potential Implications for Adverse Neurological Outcomes. Chem. Res. Toxicol. 2022, 35, 1312–1333. [Google Scholar] [CrossRef]
- Bonato, M.; Corrà, F.; Bellio, M.; Guidolin, L.; Tallandini, L.; Irato, P.; Santovito, G. PFAS Environmental Pollution and Antioxidant Responses: An Overview of the Impact on Human Field. Int. J. Environ. Res. Public Health 2020, 17, 8020. [Google Scholar] [CrossRef]
- Death, C.; Bell, C.; Champness, D.; Milne, C.; Reichman, S.; Hagen, T. Per- and Polyfluoroalkyl Substances (PFAS) in Livestock and Game Species: A Review. Sci. Total Environ. 2021, 774, 144795. [Google Scholar] [CrossRef]
- Faust, J.A. PFAS on Atmospheric Aerosol Particles: A Review. Environ. Sci. Process. Impacts 2023, 25, 133–150. [Google Scholar] [CrossRef]
- Ding, N.; Harlow, S.D.; Randolph, J.F.; Loch-Caruso, R.; Park, S.K. Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) and Their Effects on the Ovary. Hum. Reprod. Update 2020, 26, 724–752. [Google Scholar] [CrossRef]
- Lange, F.T.; Wenz, M.; Schmidt, C.K.; Brauch, H.-J. Occurrence of Perfluoroalkyl Sulfonates and Carboxylates in German Drinking Water Sources Compared to Other Countries. Water Sci. Technol. 2007, 56, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Gyllenhammar, I.; Berger, U.; Sundström, M.; McCleaf, P.; Eurén, K.; Eriksson, S.; Ahlgren, S.; Lignell, S.; Aune, M.; Kotova, N.; et al. Influence of Contaminated Drinking Water on Perfluoroalkyl Acid Levels in Human Serum—A Case Study from Uppsala, Sweden. Environ. Res. 2015, 140, 673–683. [Google Scholar] [CrossRef]
- Pitter, G.; Da Re, F.; Canova, C.; Barbieri, G.; Zare Jeddi, M.; Daprà, F.; Manea, F.; Zolin, R.; Bettega, A.M.; Stopazzolo, G.; et al. Serum Levels of Perfluoroalkyl Substances (PFAS) in Adolescents and Young Adults Exposed to Contaminated Drinking Water in the Veneto Region, Italy: A Cross-Sectional Study Based on a Health Surveillance Program. Environ. Health Perspect. 2020, 128, 27007. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.-H.; Lee, D.-Y.; Bruce-Vanderpuije, P.; Song, A.-R.; Lee, H.-S.; Park, S.-W.; Lee, J.-H.; Megson, D.; Kim, J.-H. Environmental and Dietary Exposure of Perfluorooctanoic Acid and Perfluorooctanesulfonic Acid in the Nakdong River, Korea. Environ. Geochem. Health 2021, 43, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Sun, Q.; Grandjean, P. PFAS Concentration during Pregnancy in Relation to Cardiometabolic Health and Birth Outcomes. Environ. Res. 2021, 192, 110287. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.; Yang, Z.; Agarwal, M.; Liu, W.; Peng, Z.; Long, Z.; Birbeck, J.; Westrick, J.; Liu, W.; Petriello, M.C. Exposure to a Mixture of Legacy, Alternative, and Replacement per- and Polyfluoroalkyl Substances (PFAS) Results in Sex-Dependent Modulation of Cholesterol Metabolism and Liver Injury. Environ. Int. 2021, 157, 106843. [Google Scholar] [CrossRef]
- Taibl, K.R.; Schantz, S.; Aung, M.T.; Padula, A.; Geiger, S.; Smith, S.; Park, J.-S.; Milne, G.L.; Robinson, J.F.; Woodruff, T.J.; et al. Associations of Per- and Polyfluoroalkyl Substances (PFAS) and Their Mixture with Oxidative Stress Biomarkers during Pregnancy. Environ. Int. 2022, 169, 107541. [Google Scholar] [CrossRef]
- Li, D.; Jiang, L.; Hong, Y.; Cai, Z. Multilayered Glycoproteomic Analysis Reveals the Hepatotoxic Mechanism in Perfluorooctane Sulfonate (PFOS) Exposure Mice. Environ. Pollut. 2021, 268, 115774. [Google Scholar] [CrossRef]
- Rashid, F.; Ramakrishnan, A.; Fields, C.; Irudayaraj, J. Acute PFOA Exposure Promotes Epigenomic Alterations in Mouse Kidney Tissues. Toxicol. Rep. 2020, 7, 125–132. [Google Scholar] [CrossRef]
- Barry, V.; Winquist, A.; Steenland, K. Perfluorooctanoic Acid (PFOA) Exposures and Incident Cancers among Adults Living near a Chemical Plant. Environ. Health Perspect. 2013, 121, 1313–1318. [Google Scholar] [CrossRef]
- Mastrantonio, M.; Bai, E.; Uccelli, R.; Cordiano, V.; Screpanti, A.; Crosignani, P. Drinking Water Contamination from Perfluoroalkyl Substances (PFAS): An Ecological Mortality Study in the Veneto Region, Italy. Eur. J. Public Health 2018, 28, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.M.; Zhang, S.; Hoffman, K.; Miranda, M.L.; Stapleton, H.M. Concentrations of Per- and Polyfluoroalkyl Substances (PFAS) in Human Placental Tissues and Associations with Birth Outcomes. Chemosphere 2022, 295, 133873. [Google Scholar] [CrossRef]
- Bao, J.; Shao, L.-X.; Liu, Y.; Cui, S.-W.; Wang, X.; Lu, G.-L.; Wang, X.; Jin, Y.-H. Target Analysis and Suspect Screening of Per- and Polyfluoroalkyl Substances in Paired Samples of Maternal Serum, Umbilical Cord Serum, and Placenta near Fluorochemical Plants in Fuxin, China. Chemosphere 2022, 307, 135731. [Google Scholar] [CrossRef] [PubMed]
- Mamsen, L.S.; Björvang, R.D.; Mucs, D.; Vinnars, M.-T.; Papadogiannakis, N.; Lindh, C.H.; Andersen, C.Y.; Damdimopoulou, P. Concentrations of Perfluoroalkyl Substances (PFASs) in Human Embryonic and Fetal Organs from First, Second, and Third Trimester Pregnancies. Environ. Int. 2019, 124, 482–492. [Google Scholar] [CrossRef]
- Sagiv, S.K.; Rifas-Shiman, S.L.; Webster, T.F.; Mora, A.M.; Harris, M.H.; Calafat, A.M.; Ye, X.; Gillman, M.W.; Oken, E. Sociodemographic and Perinatal Predictors of Early Pregnancy Per- and Polyfluoroalkyl Substance (PFAS) Concentrations. Environ. Sci. Technol. 2015, 49, 11849–11858. [Google Scholar] [CrossRef] [PubMed]
- Aylward, L.L.; Hays, S.M.; Kirman, C.R.; Marchitti, S.A.; Kenneke, J.F.; English, C.; Mattison, D.R.; Becker, R.A. Relationships of Chemical Concentrations in Maternal and Cord Blood: A Review of Available Data. J. Toxicol. Environ. Health B Crit. Rev. 2014, 17, 175–203. [Google Scholar] [CrossRef]
- Verner, M.-A.; Ngueta, G.; Jensen, E.T.; Fromme, H.; Völkel, W.; Nygaard, U.C.; Granum, B.; Longnecker, M.P. A Simple Pharmacokinetic Model of Prenatal and Postnatal Exposure to Perfluoroalkyl Substances (PFASs). Environ. Sci. Technol. 2016, 50, 978–986. [Google Scholar] [CrossRef]
- Wang, Z.; Cousins, I.T.; Berger, U.; Hungerbühler, K.; Scheringer, M. Comparative Assessment of the Environmental Hazards of and Exposure to Perfluoroalkyl Phosphonic and Phosphinic Acids (PFPAs and PFPiAs): Current Knowledge, Gaps, Challenges and Research Needs. Environ. Int. 2016, 89–90, 235–247. [Google Scholar] [CrossRef]
- Varsi, K.; Huber, S.; Averina, M.; Brox, J.; Bjørke-Monsen, A.-L. Quantitation of Linear and Branched Perfluoroalkane Sulfonic Acids (PFSAs) in Women and Infants during Pregnancy and Lactation. Environ. Int. 2022, 160, 107065. [Google Scholar] [CrossRef]
- Eryasa, B.; Grandjean, P.; Nielsen, F.; Valvi, D.; Zmirou-Navier, D.; Sunderland, E.; Weihe, P.; Oulhote, Y. Physico-Chemical Properties and Gestational Diabetes Predict Transplacental Transfer and Partitioning of Perfluoroalkyl Substances. Environ. Int. 2019, 130, 104874. [Google Scholar] [CrossRef]
- Ma, D.; Lu, Y.; Liang, Y.; Ruan, T.; Li, J.; Zhao, C.; Wang, Y.; Jiang, G. A Critical Review on Transplacental Transfer of Per- and Polyfluoroalkyl Substances: Prenatal Exposure Levels, Characteristics, and Mechanisms. Environ. Sci. Technol. 2022, 56, 6014–6026. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Zhuang, T.; Liu, X.; Fu, J.; Zhang, J.; Fu, J.; Wang, L.; Zhang, A.; Liang, Y.; Song, M.; et al. Prenatal Exposure to Per- and Polyfluoroalkyl Substances (PFASs) and Association between the Placental Transfer Efficiencies and Dissociation Constant of Serum Proteins-PFAS Complexes. Environ. Sci. Technol. 2019, 53, 6529–6538. [Google Scholar] [CrossRef]
- Cai, D.; Li, Q.-Q.; Chu, C.; Wang, S.-Z.; Tang, Y.-T.; Appleton, A.A.; Qiu, R.-L.; Yang, B.-Y.; Hu, L.-W.; Dong, G.-H.; et al. High Trans-Placental Transfer of Perfluoroalkyl Substances Alternatives in the Matched Maternal-Cord Blood Serum: Evidence from a Birth Cohort Study. Sci. Total Environ. 2020, 705, 135885. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Schreder, E.; Dempsey, J.C.; Uding, N.; Chu, V.; Andres, G.; Sathyanarayana, S.; Salamova, A. Per- and Polyfluoroalkyl Substances (PFAS) in Breast Milk: Concerning Trends for Current-Use PFAS. Environ. Sci. Technol. 2021, 55, 7510–7520. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Choi, K.; Lee, H.-S.; Kim, D.-H.; Park, N.-Y.; Kim, S.; Kho, Y. Elevated Levels of Short Carbon-Chain PFCAs in Breast Milk among Korean Women: Current Status and Potential Challenges. Environ. Res. 2016, 148, 351–359. [Google Scholar] [CrossRef]
- Jin, H.; Mao, L.; Xie, J.; Zhao, M.; Bai, X.; Wen, J.; Shen, T.; Wu, P. Poly- and Perfluoroalkyl Substance Concentrations in Human Breast Milk and Their Associations with Postnatal Infant Growth. Sci. Total Environ. 2020, 713, 136417. [Google Scholar] [CrossRef]
- Herrick, R.L.; Buckholz, J.; Biro, F.M.; Calafat, A.M.; Ye, X.; Xie, C.; Pinney, S.M. Polyfluoroalkyl Substance Exposure in the Mid-Ohio River Valley, 1991–2012. Environ. Pollut. 2017, 228, 50–60. [Google Scholar] [CrossRef]
- Zheng, P.; Liu, Y.; An, Q.; Yang, X.; Yin, S.; Ma, L.Q.; Liu, W. Prenatal and Postnatal Exposure to Emerging and Legacy Per-/Polyfluoroalkyl Substances: Levels and Transfer in Maternal Serum, Cord Serum, and Breast Milk. Sci. Total Environ. 2022, 812, 152446. [Google Scholar] [CrossRef]
- Serrano, L.; Iribarne-Durán, L.M.; Suárez, B.; Artacho-Cordón, F.; Vela-Soria, F.; Peña-Caballero, M.; Hurtado, J.A.; Olea, N.; Fernández, M.F.; Freire, C. Concentrations of Perfluoroalkyl Substances in Donor Breast Milk in Southern Spain and Their Potential Determinants. Int. J. Hyg. Environ. Health 2021, 236, 113796. [Google Scholar] [CrossRef]
- Shabalina, I.G.; Kalinovich, A.V.; Cannon, B.; Nedergaard, J. Metabolically Inert Perfluorinated Fatty Acids Directly Activate Uncoupling Protein 1 in Brown-Fat Mitochondria. Arch. Toxicol. 2016, 90, 1117–1128. [Google Scholar] [CrossRef]
- Wang, J.; Pan, Y.; Cui, Q.; Yao, B.; Wang, J.; Dai, J. Penetration of PFASs Across the Blood Cerebrospinal Fluid Barrier and Its Determinants in Humans. Environ. Sci. Technol. 2018, 52, 13553–13561. [Google Scholar] [CrossRef]
- Cao, Y.; Ng, C. Absorption, Distribution, and Toxicity of per- and Polyfluoroalkyl Substances (PFAS) in the Brain: A Review. Environ. Sci. Process. Impacts 2021, 23, 1623–1640. [Google Scholar] [CrossRef]
- Shin, H.-M.; Bennett, D.H.; Calafat, A.M.; Tancredi, D.; Hertz-Picciotto, I. Modeled Prenatal Exposure to Per- and Polyfluoroalkyl Substances in Association with Child Autism Spectrum Disorder: A Case-Control Study. Environ. Res. 2020, 186, 109514. [Google Scholar] [CrossRef] [PubMed]
- Forns, J.; Verner, M.-A.; Iszatt, N.; Nowack, N.; Bach, C.C.; Vrijheid, M.; Costa, O.; Andiarena, A.; Sovcikova, E.; Høyer, B.B.; et al. Early Life Exposure to Perfluoroalkyl Substances (PFAS) and ADHD: A Meta-Analysis of Nine European Population-Based Studies. Environ. Health Perspect. 2020, 128, 057002. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Fu, Y.; Weng, X.; Zeng, Z.; Tan, Y.; Wu, X.; Zeng, H.; Yang, Z.; Li, Y.; Liang, H.; et al. The Association between Prenatal Per- and Polyfluoroalkyl Substances Exposure and Neurobehavioral Problems in Offspring: A Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 1668. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Yamazaki, K.; Suyama, S.; Ikeda-Araki, A.; Miyashita, C.; Ait Bamai, Y.; Kobayashi, S.; Masuda, H.; Yamaguchi, T.; Goudarzi, H.; et al. The Association between Prenatal Perfluoroalkyl Substance Exposure and Symptoms of Attention-Deficit/Hyperactivity Disorder in 8-Year-Old Children and the Mediating Role of Thyroid Hormones in the Hokkaido Study. Environ. Int. 2022, 159, 107026. [Google Scholar] [CrossRef]
- Dalsager, L.; Jensen, T.K.; Nielsen, F.; Grandjean, P.; Bilenberg, N.; Andersen, H.R. No Association between Maternal and Child PFAS Concentrations and Repeated Measures of ADHD Symptoms at Age 2½ and 5 Years in Children from the Odense Child Cohort. Neurotoxicology Teratol. 2021, 88, 107031. [Google Scholar] [CrossRef]
- Chen, M.-H.; Ha, E.-H.; Liao, H.-F.; Jeng, S.-F.; Su, Y.-N.; Wen, T.-W.; Lien, G.-W.; Chen, C.-Y.; Hsieh, W.-S.; Chen, P.-C. Perfluorinated Compound Levels in Cord Blood and Neurodevelopment at 2 Years of Age. Epidemiology 2013, 24, 800–808. [Google Scholar] [CrossRef]
- Yao, Q.; Vinturache, A.; Lei, X.; Wang, Z.; Pan, C.; Shi, R.; Yuan, T.; Gao, Y.; Tian, Y. Prenatal Exposure to Per- and Polyfluoroalkyl Substances, Fetal Thyroid Hormones, and Infant Neurodevelopment. Environ. Res. 2022, 206, 112561. [Google Scholar] [CrossRef]
- Jaspers, M.; de Winter, A.F.; Buitelaar, J.K.; Verhulst, F.C.; Reijneveld, S.A.; Hartman, C.A. Early Childhood Assessments of Community Pediatric Professionals Predict Autism Spectrum and Attention Deficit Hyperactivity Problems. J. Abnorm. Child Psychol. 2013, 41, 71–80. [Google Scholar] [CrossRef]
- Harris, M.H.; Oken, E.; Rifas-Shiman, S.L.; Calafat, A.M.; Ye, X.; Bellinger, D.C.; Webster, T.F.; White, R.F.; Sagiv, S.K. Prenatal and Childhood Exposure to Per- and Polyfluoroalkyl Substances (PFASs) and Child Cognition. Environ. Int. 2018, 115, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Viberg, H.; Lee, I.; Eriksson, P. Adult Dose-Dependent Behavioral and Cognitive Disturbances after a Single Neonatal PFHxS Dose. Toxicology 2013, 304, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, S.; Fredriksson, A.; Viberg, H. More Signs of Neurotoxicity of Surfactants and Flame Retardants—Neonatal PFOS and PBDE 99 Cause Transcriptional Alterations in Cholinergic Genes in the Mouse CNS. Environ. Toxicol. Pharmacol. 2015, 40, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, W.; Zhang, Q.; Zhao, H.; Quan, X. Effects of Developmental Perfluorooctane Sulfonate Exposure on Spatial Learning and Memory Ability of Rats and Mechanism Associated with Synaptic Plasticity. Food. Chem. Toxicol. 2015, 76, 70–76. [Google Scholar] [CrossRef]
- Starling, A.P.; Liu, C.; Shen, G.; Yang, I.V.; Kechris, K.; Borengasser, S.J.; Boyle, K.E.; Zhang, W.; Smith, H.A.; Calafat, A.M.; et al. Prenatal Exposure to Per- and Polyfluoroalkyl Substances, Umbilical Cord Blood DNA Methylation, and Cardio-Metabolic Indicators in Newborns: The Healthy Start Study. Environ. Health Perspect. 2020, 128, 127014. [Google Scholar] [CrossRef]
- Mshaty, A.; Haijima, A.; Takatsuru, Y.; Ninomiya, A.; Yajima, H.; Kokubo, M.; Khairinisa, M.A.; Miyazaki, W.; Amano, I.; Koibuchi, N. Neurotoxic Effects of Lactational Exposure to Perfluorooctane Sulfonate on Learning and Memory in Adult Male Mouse. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 145, 111710. [Google Scholar] [CrossRef]
- Reardon, A.J.F.; Karathra, J.; Ribbenstedt, A.; Benskin, J.P.; MacDonald, A.M.; Kinniburgh, D.W.; Hamilton, T.J.; Fouad, K.; Martin, J.W. Neurodevelopmental and Metabolomic Responses from Prenatal Coexposure to Perfluorooctanesulfonate (PFOS) and Methylmercury (MeHg) in Sprague-Dawley Rats. Chem. Res. Toxicol. 2019, 32, 1656–1669. [Google Scholar] [CrossRef]
- Ninomiya, A.; Mshaty, A.; Haijima, A.; Yajima, H.; Kokubo, M.; Khairinisa, M.A.; Ariyani, W.; Fujiwara, Y.; Ishii, S.; Hosoi, N.; et al. The Neurotoxic Effect of Lactational PFOS Exposure on Cerebellar Functional Development in Male Mice. Food Chem. Toxicol. 2022, 159, 112751. [Google Scholar] [CrossRef]
- Gaballah, S.; Swank, A.; Sobus, J.R.; Howey, X.M.; Schmid, J.; Catron, T.; McCord, J.; Hines, E.; Strynar, M.; Tal, T. Evaluation of Developmental Toxicity, Developmental Neurotoxicity, and Tissue Dose in Zebrafish Exposed to GenX and Other PFAS. Environ. Health Perspect. 2020, 128, 47005. [Google Scholar] [CrossRef]
- Min, H.; Dong, J.; Wang, Y.; Wang, Y.; Teng, W.; Xi, Q.; Chen, J. Maternal Hypothyroxinemia-Induced Neurodevelopmental Impairments in the Progeny. Mol. Neurobiol. 2016, 53, 1613–1624. [Google Scholar] [CrossRef]
- Kato, S.; Itoh, S.; Yuasa, M.; Baba, T.; Miyashita, C.; Sasaki, S.; Nakajima, S.; Uno, A.; Nakazawa, H.; Iwasaki, Y.; et al. Association of Perfluorinated Chemical Exposure in Utero with Maternal and Infant Thyroid Hormone Levels in the Sapporo Cohort of Hokkaido Study on the Environment and Children’s Health. Environ. Health Prev. Med. 2016, 21, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Preston, E.V.; Webster, T.F.; Henn, B.C.; McClean, M.D.; Gennings, C.; Oken, E.; Rifas-Shiman, S.L.; Pearce, E.N.; Calafat, A.M.; Fleisch, A.F.; et al. Prenatal Exposure to Per- and Polyfluoroalkyl Substances and Maternal and Neonatal Thyroid Function in the Project Viva Cohort: A Mixtures Approach. Environ. Int. 2020, 139, 105728. [Google Scholar] [CrossRef] [PubMed]
- Reardon, A.J.F.; Khodayari Moez, E.; Dinu, I.; Goruk, S.; Field, C.J.; Kinniburgh, D.W.; MacDonald, A.M.; Martin, J.W. APrON Study Longitudinal Analysis Reveals Early-Pregnancy Associations between Perfluoroalkyl Sulfonates and Thyroid Hormone Status in a Canadian Prospective Birth Cohort. Environ. Int. 2019, 129, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liang, J.; Gao, A. Contact to Perfluoroalkyl Substances and Thyroid Health Effects: A Meta-Analysis Directing on Pregnancy. Chemosphere 2023, 315, 137748. [Google Scholar] [CrossRef]
- Zuñiga, L.F.F.; Muñoz, Y.S.; Pustovrh, M.C. Thyroid Hormones: Metabolism and Transportation in the Fetoplacental Unit. Mol. Reprod. Dev. 2022, 89, 526–539. [Google Scholar] [CrossRef]
- Conti, A.; Strazzeri, C.; Rhoden, K.J. Perfluorooctane Sulfonic Acid, a Persistent Organic Pollutant, Inhibits Iodide Accumulation by Thyroid Follicular Cells in Vitro. Mol. Cell. Endocrinol. 2020, 515, 110922. [Google Scholar] [CrossRef]
- Coperchini, F.; Awwad, O.; Rotondi, M.; Santini, F.; Imbriani, M.; Chiovato, L. Thyroid Disruption by Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoate (PFOA). J. Endocrinol. Investig. 2017, 40, 105–121. [Google Scholar] [CrossRef]
- Gutshall, D.M.; Pilcher, G.D.; Langley, A.E. Mechanism of the Serum Thyroid Hormone Lowering Effect of Perfluoro-n-Decanoic Acid (PFDA) in Rats. J. Toxicol. Environ. Health 1989, 28, 53–65. [Google Scholar] [CrossRef]
- Marchese, M.J.; Li, S.; Liu, B.; Zhang, J.J.; Feng, L. Perfluoroalkyl Substance Exposure and the BDNF Pathway in the Placental Trophoblast. Front. Endocrinol. 2021, 12, 694885. [Google Scholar] [CrossRef]
- Yu, G.; Luo, F.; Nian, M.; Li, S.; Liu, B.; Feng, L.; Zhang, J. Exposure to Perfluoroalkyl Substances During Pregnancy and Fetal BDNF Level: A Prospective Cohort Study. Front. Endocrinol. 2021, 12, 653095. [Google Scholar] [CrossRef]
- Wang, F.; Liu, W.; Jin, Y.; Dai, J.; Zhao, H.; Xie, Q.; Liu, X.; Yu, W.; Ma, J. Interaction of PFOS and BDE-47 Co-Exposure on Thyroid Hormone Levels and TH-Related Gene and Protein Expression in Developing Rat Brains. Toxicol. Sci. 2011, 121, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Wan Ibrahim, W.N.; Tofighi, R.; Onishchenko, N.; Rebellato, P.; Bose, R.; Uhlén, P.; Ceccatelli, S. Perfluorooctane Sulfonate Induces Neuronal and Oligodendrocytic Differentiation in Neural Stem Cells and Alters the Expression of PPARγ in Vitro and in Vivo. Toxicol. Appl. Pharmacol. 2013, 269, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Q.; Liu, C.; Li, C.; Li, Y.; Li, S.; Liu, X.; Shao, J. Evaluation of PFOS-Mediated Neurotoxicity in Rat Primary Neurons and Astrocytes Cultured Separately or in Co-Culture. Toxicol. In Vitro 2017, 38, 77–90. [Google Scholar] [CrossRef]
- Liu, X.; Jin, Y.; Liu, W.; Wang, F.; Hao, S. Possible Mechanism of Perfluorooctane Sulfonate and Perfluorooctanoate on the Release of Calcium Ion from Calcium Stores in Primary Cultures of Rat Hippocampal Neurons. Toxicol. In Vitro 2011, 25, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Wielsøe, M.; Long, M.; Ghisari, M.; Bonefeld-Jørgensen, E.C. Perfluoroalkylated Substances (PFAS) Affect Oxidative Stress Biomarkers in Vitro. Chemosphere 2015, 129, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.-C.; Zhu, B.-Q.; Wang, Y.-Q.; He, Q.-Z. ROS-Triggered Autophagy Is Involved in PFOS-Induced Apoptosis of Human Embryo Liver L-02 Cells. Biomed. Res. Int. 2021, 2021, 6625952. [Google Scholar] [CrossRef]
- Lee, H.-G.; Lee, Y.J.; Yang, J.-H. Perfluorooctane Sulfonate Induces Apoptosis of Cerebellar Granule Cells via a ROS-Dependent Protein Kinase C Signaling Pathway. Neurotoxicology 2012, 33, 314–320. [Google Scholar] [CrossRef]
- Liu, X.; Liu, W.; Jin, Y.; Yu, W.; Liu, L.; Yu, H. Effects of Subchronic Perfluorooctane Sulfonate Exposure of Rats on Calcium-Dependent Signaling Molecules in the Brain Tissue. Arch. Toxicol. 2010, 84, 471–479. [Google Scholar] [CrossRef]
- Sim, K.H.; Lee, Y.J. Perfluorohexane Sulfonate Induces Memory Impairment and Downregulation of Neuroproteins via NMDA Receptor-Mediated PKC-ERK/AMPK Signaling Pathway. Chemosphere 2022, 288 Pt 1, 132503. [Google Scholar] [CrossRef]
- Petroff, R.L.; Cavalcante, R.G.; Langen, E.S.; Dolinoy, D.C.; Padmanabhan, V.; Goodrich, J.M. Mediation Effects of DNA Methylation and Hydroxymethylation on Birth Outcomes after Prenatal Per- and Polyfluoroalkyl Substances (PFAS) Exposure in the Michigan Mother-Infant Pairs Cohort. Clin. Epigenetics 2023, 15, 49. [Google Scholar] [CrossRef]
- Guo, X.-X.; He, Q.-Z.; Li, W.; Long, D.-X.; Pan, X.-Y.; Chen, C.; Zeng, H.-C. Brain-Derived Neurotrophic Factor Mediated Perfluorooctane Sulfonate Induced-Neurotoxicity via Epigenetics Regulation in SK-N-SH Cells. Int. J. Mol. Sci. 2017, 18, 893. [Google Scholar] [CrossRef] [PubMed]
| Type/Characteristic | Structural Formula | CAS | Molecular Weight (g/mol) | pKa | Solubility |
|---|---|---|---|---|---|
| perfluorobutanoic acid (PFBA) | 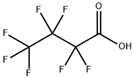 | 375-22-4 | 214.04 | pK1:0.17 (25 °C) | Chloroform: soluble; Methanol: soluble |
| perfluorobutane sulfonic acid (PFBS) |  | 375-73-5 | 300.1 | −3.57 ± 0.50 (Predicted) | Soluble in water |
| perfluorodecanoicacid (PFDA) |  | 335-76-2 | 514.08 | 0.52 ± 0.10 (Predicted) | methanol: soluble 10% |
| Perfluoroheptanoate (PFHpA) |  | 120885-29-2 | 363.05 | - | - |
| perfluorohexanoic acid (PFHxA) |  | 307-24-4 | 314.05 | 0.42 ± 0.10 (Predicted) | water: insoluble |
| perfluorohexane sulfonic acid (PFHxS) |  | 355-46-4 | 400.11 | −3.34 ± 0.50 (Predicted) | DMSO (Slightly), Methanol (Slightly) |
| perfluorononan-1-oic acid (PFNA) | 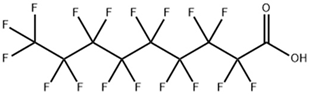 | 375-95-1 | 464.08 | 0.52 ± 0.10 (Predicted) | Acetone (Slightly), DMSO (Slightly), Methanol (Slightly) |
| perfluorooctanoic acid (PFOA) | 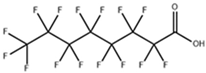 | 335-67-1 | 414.07 | 0.50 ± 0.10 (Predicted) | Water: 3.4 g/L |
| perfluorooctane sulfonic acid and its derivatives (PFOS) | 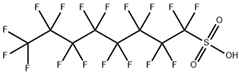 | 1763-23-1 | 500.13 | −3.27 ± 0.50 (Predicted) | Ethanol: 10 mg/mL |
| perfluoroundecanoic acid (PFUnA) | 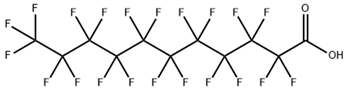 | 2058-94-8 | 564.09 | 0.52 ± 0.10 (Predicted) | DMSO (Slightly), Methanol (Slightly) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuchen, H.-Y.; Wang, J.-Y.; Liu, X.-S.; Shi, Y.-W. Research Progress on Neurodevelopmental Toxicity in Offspring after Indirect Exposure to PFASs in Early Life. Toxics 2023, 11, 571. https://doi.org/10.3390/toxics11070571
Zhuchen H-Y, Wang J-Y, Liu X-S, Shi Y-W. Research Progress on Neurodevelopmental Toxicity in Offspring after Indirect Exposure to PFASs in Early Life. Toxics. 2023; 11(7):571. https://doi.org/10.3390/toxics11070571
Chicago/Turabian StyleZhuchen, Huai-Yu, Jie-Yu Wang, Xiao-Shan Liu, and Yan-Wei Shi. 2023. "Research Progress on Neurodevelopmental Toxicity in Offspring after Indirect Exposure to PFASs in Early Life" Toxics 11, no. 7: 571. https://doi.org/10.3390/toxics11070571
APA StyleZhuchen, H.-Y., Wang, J.-Y., Liu, X.-S., & Shi, Y.-W. (2023). Research Progress on Neurodevelopmental Toxicity in Offspring after Indirect Exposure to PFASs in Early Life. Toxics, 11(7), 571. https://doi.org/10.3390/toxics11070571






