More Effective Mobilization of Hg2+ from Human Serum Albumin Compared to Cd2+ by L-Cysteine at Near-Physiological Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. ‘Labeling’ of HSA with Hg2+ and Cd2+
2.3. Sample Preparation
2.4. Instrumentation
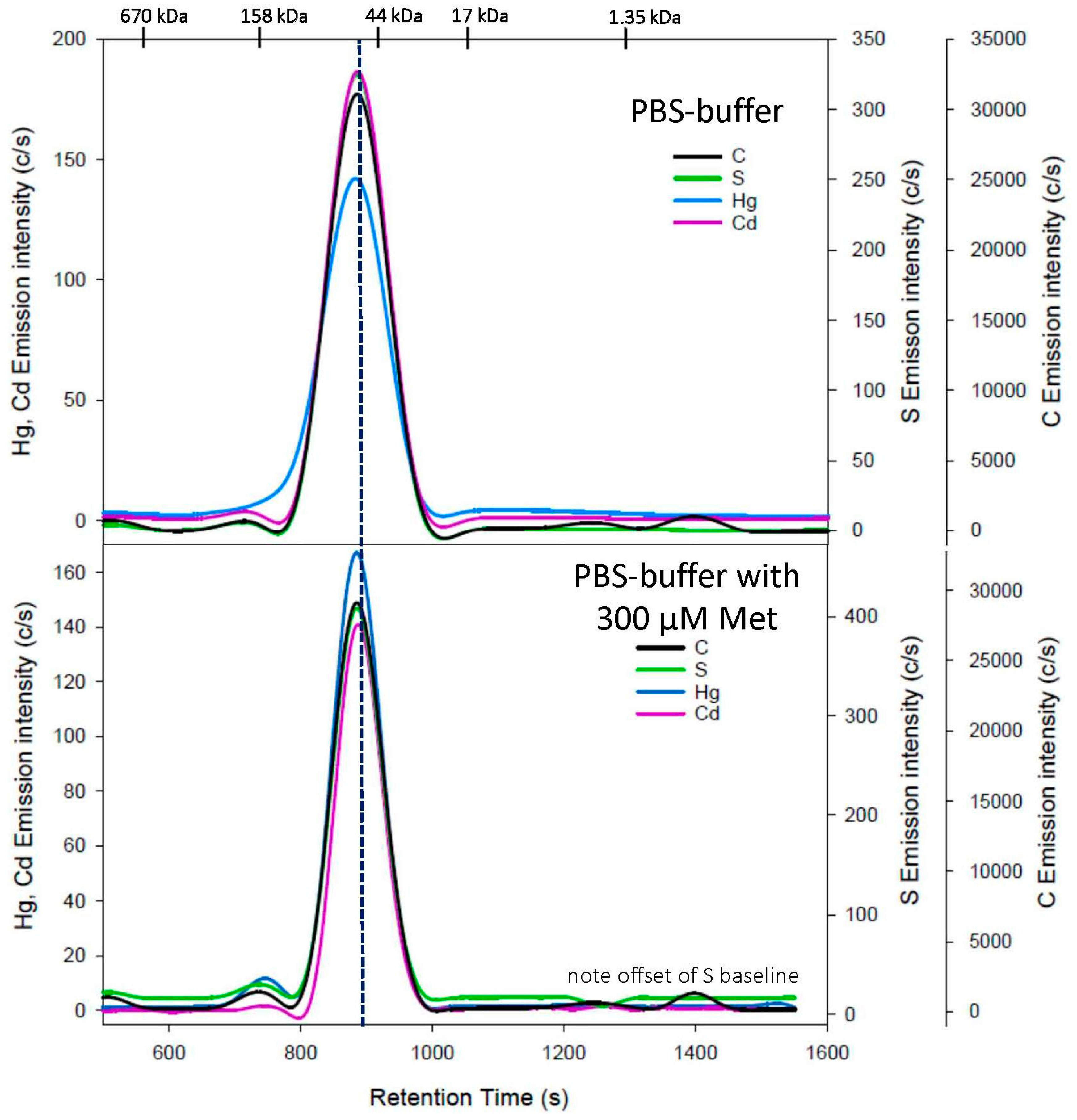
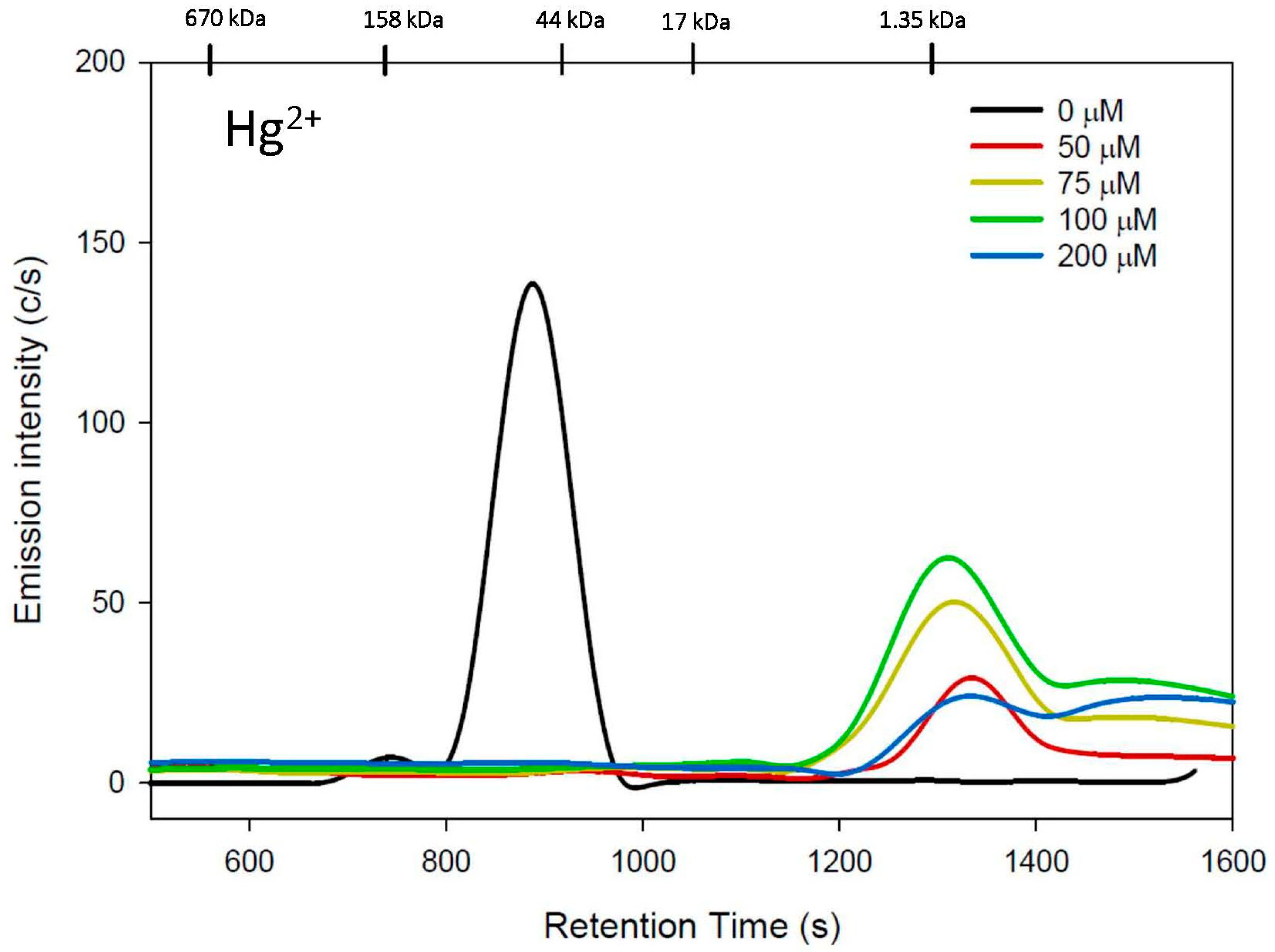
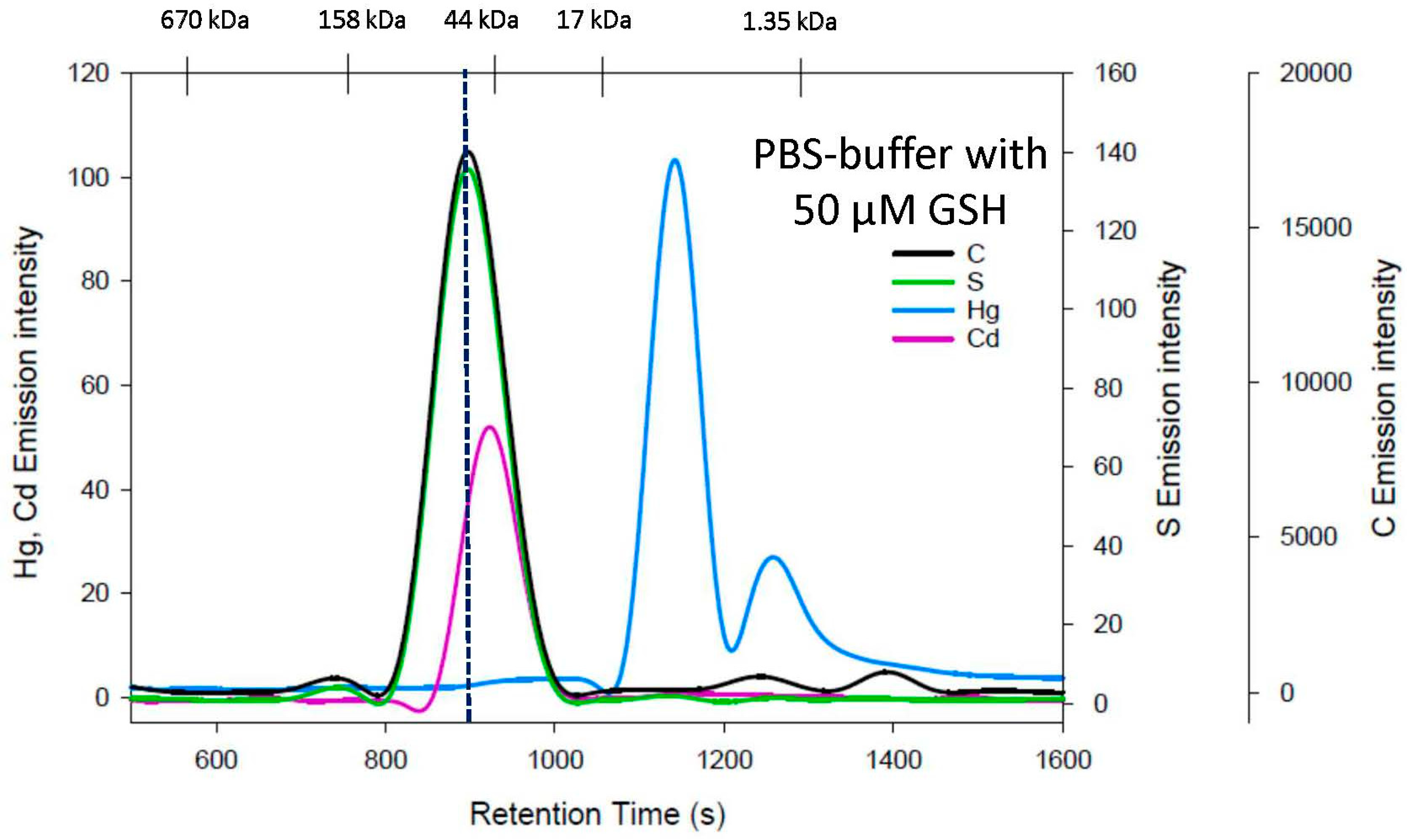
3. Results
3.1. Cys Mobile Phase
3.2. hCys Mobile Phase
3.3. GSH Mobile Phase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.; Balde, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef] [Green Version]
- Ambrose, J.L.; Gratz, L.E.; Jaffe, D.A.; Campos, T.; Flocke, F.M.; Knapp, D.J.; Stechman, D.M.; Stell, M.; Weinheimer, A.J.; Cantrell, C.A.; et al. Mercury emission ratios from coal-fired power plants in the southeastern United States during NOMADSS. Environ. Sci. Technol. 2015, 49, 10389–10397. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, T.J.; Macklin, M.G. Modeling long-term contamination in river systems from historical metal mining. Geology 2003, 31, 451–454. [Google Scholar] [CrossRef]
- Campbell, P.G.C.; Gailer, J. Effects of Non-essential Metal Releases on the Environment and Human Health. In Metal Sustainability: Global Challenges, Consequences and Prospects; Izatt, R.M., Ed.; John Wiley & Sions Ltd.: Chichester, UK, 2016; pp. 221–252. [Google Scholar]
- Yasuda, H.; Tsutsui, T. Metallomics analysis for early assessment and individualized intervention of neurodevelopmental disorders. Metallomics 2022, 14, mfac067. [Google Scholar] [CrossRef]
- Huff, J.; Lunn, R.M.; Waalkes, M.P.; Tomatis, L.; Infante, P.F. Cadmium-induced cancers in animals and humans. Int. J. Occup. Environ. Health 2007, 13, 202–212. [Google Scholar] [CrossRef] [Green Version]
- Pocsi, I.; Dockrell, M.E.; Price, R.G. Nephrotoxic biomarkers with specific indications for metalllic pollutants: Implications for environmental health. Biomarker Insights 2022, 17, 11772719221111882. [Google Scholar] [CrossRef]
- Sarpong-Kumankomah, S.; Gibson, M.A.; Gailer, J. Organ damage by toxic metals is critically determined by the bloodstream. Coord. Chem. Rev. 2018, 374, 376–386. [Google Scholar] [CrossRef]
- Bridle, T.G.; Kumarathasan, P.; Gailer, J. Toxic metal species and ‘endogenous’ metalloproteins at the blood-organ interface: Analytical and bioinorganic aspects. Molecules 2021, 26, 3408. [Google Scholar] [CrossRef]
- Gailer, J. Arsenic-selenium and mercury-selenium bonds in biology. Coord. Chem. Rev. 2007, 251, 234–254. [Google Scholar] [CrossRef]
- Gibson, M.A.; Sarpong-Kumankomah, S.; Nehzati, S.; George, G.N.; Gailer, J. Remarkable differences in the biochemical fate of Cd2+, Hg2+, CH3Hg+ and thimerosal in red blood cell lysate. Metallomics 2017, 9, 1060–1072. [Google Scholar] [CrossRef]
- De Magalhanes Silva, M.; de Araujo Dantas, M.D.; de Sila Filho, R.C.; dos Santos Sales, M.V.; de Almeida Xavier, J.; Leite, A.C.R.; Goulart, M.O.F.; Grillo, L.A.M.; de Barros, W.A.; de Fatima, A.; et al. Toxicity of thimerosal in biological systems: Conformational changes in human hemoglobin, decrease of oxygen binding, increase of protein glycation and amyloid formation. Intern. J. Biol. Macromol. 2020, 154, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Bridle, T.G.; Doroudian, M.; White, W.; Gailer, J. Physiologically relevant hCys concentrations mobilize MeHg from rabbit serum albumin to form MeHg-hCys complexes. Metallomics 2022, 14, mfac010. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.Y.; Ledue, T.B.; Ritchie, R.F. Plasma Proteins. Clinical Utility and Interpretation; Dade Behring Inc.: Newark, NJ, USA, 2000. [Google Scholar]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural basis of the drug-binding specificity of human serum albumin. J. Mol. Biol. 2005, 353, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.C.; Ho, J.X. Structure of serum albumin. Adv. Prot. Sci. 1994, 45, 153–203. [Google Scholar]
- Fanali, G.; Di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.J.; Blindauer, C.A.; Berezenko, S.; Sleep, D.; Sadler, P.J. Interdomain zinc site on human albumin. Proc. Natl. Acad. Sci. USA 2003, 100, 3701–3706. [Google Scholar] [CrossRef]
- Bal, W.; Sokolowska, M.; Kurowska, E.; Faller, P. Binding of transition metal ions to albumin: Sites, affinities and rates. Biochim. Biophys. Acta 2013, 1830, 5444–5455. [Google Scholar] [CrossRef]
- Li, Y.; Yan, X.-P.; Chen, C.H.; Xia, Y.-L.; Jiang, Y. Human serum albumin-mercurial species interactions. J. Proteome Res. 2007, 6, 2277–2286. [Google Scholar] [CrossRef]
- Song, S.; Li, Y.; Liu, Q.S.; Wang, H.; Li, P.; Shi, J.; Hu, L.G.; Zhang, H.; Liu, Y.; Li, K.; et al. Interaction of mercury ion (Hg2+) with blood and cytotoxicity attenuation by serum albumin binding. J. Haz. Mater. 2021, 412, 125158. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Kendall, M.D.; Osborn, D. Cadmium and mercury nephrotoxicity. Nature 1983, 304, 633–635. [Google Scholar] [CrossRef]
- Bridges, C.C.; Zalups, R.K. Mechanisms involved in the transport of mercuric ions in target tissues. Arch. Toxicol. 2017, 91, 63–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katrusiak, A.E.; Paterson, P.G.; Kamencic, H.; Shoker, A.; Lyon, A.W. Pre-column derivatization high-performance liquid chromatographic method for determination of cysteine, cysteinyl-glycine, homocysteine and glutathione in plasma and cell extracts. J. Chromatogr. B 2001, 758, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Williams, M. Total plasma homocysteine determination using ion-exchange chromatography. J. Liq. Chromatogr. 2000, 23, 3143–3153. [Google Scholar] [CrossRef]
- Wang, W.; Rusin, O.; Xu, X.; Kim, K.K.; Escobedo, J.O.; Fakayode, S.O.; Fletcher, K.A.; Lowry, M.; Schowalter, C.M.; Lawrence, C.M.; et al. Detection of homocysteine and cysteine. J. Am. Chem. Soc. 2005, 127, 15949–15958. [Google Scholar] [CrossRef] [Green Version]
- Jalilehvand, F.; Leung, B.O.; Izadifard, M.; Damian, E. Mercury (II) cysteine complexes in alkaline solution. Inorg. Chem. 2006, 45, 66–73. [Google Scholar] [CrossRef]
- Ajsuvakova, O.P.; Tinkov, A.A.; Aschner, M.; Rocha, J.B.T.; Michalke, B.; Skalnaya, M.G.; Skaklny, A.V.; Butnariu, M.; Dadar, M.; Sarac, I.; et al. Sylfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020, 417, 213343. [Google Scholar] [CrossRef]
- Cheesman, B.V.; Arnold, A.P.; Rabenstein, D.L. Nuclear magnetic resonance studies on the solution chemistry of metal complexes. 25. Hg(thiol)3 complexes and Hg(II)-thiol ligand exchange kinetics. J. Am. Chem. Soc. 1988, 110, 6359–6364. [Google Scholar] [CrossRef]
- Sovago, I.; Narnagy, K. Cadmium(II) complexes of amino acids and peptides. In Cadmium: From Toxicity to Essentiality; Sigel, A.S.H., Sigel, R.K.O., Eds.; Springer Science+Business Media Dordrecht: New York, NY, USA, 2013; Volume 11, pp. 275–302. [Google Scholar]
- Jalilehvand, F.; Leung, B.O.; Mah, V. Cadmium(II) complex formation with cysteine and penicillamine. Inorg. Chem. 2009, 48, 5758–5771. [Google Scholar] [CrossRef] [Green Version]
- Gautam, A.; Gomez, A.; Mendoza Rengifo, E.; George, G.N.; Pickering, I.J.; Gailer, J. Structural characterization of toxicologically relevant Cd2+-L-Cysteine complexes. Toxics 2023, 11, 294. [Google Scholar] [CrossRef]
- Manley, S.A.; Byrns, S.; Lyon, A.W.; Brown, P.; Gailer, J. Simultaneous Cu-, Fe-, and Zn-specific detection of metalloproteins contained in rabbit plasma by size-exclusion chromatography-inductively coupled plasma atomic emission spectroscopy. J. Biol. Inorg. Chem. 2009, 14, 61–74. [Google Scholar] [CrossRef]
- Bjorklund, G.; Dadar, M.; Mutter, J.; Aaseth, J. The toxicology of mercury: Current research and emerging trends. Environ. Res. 2017, 159, 545–554. [Google Scholar] [CrossRef]
- Merino, J.J.; Parmigiani-Izquierdo, J.M.; Gasca, A.T.; Cabana-Munoz, M.E. The long-term algae extract (Chlorella and Fucus sp) and aminosulphurate supplementation modulate SOD-1 activity and decrease heavy metals (Hg++, Sn) levels in patients with long-term dental titanium and amalgam fillings restorations. Antioxidants 2019, 8, 101. [Google Scholar] [CrossRef] [Green Version]
- Morris, T.T.; Keir, J.L.A.; Boshart, S.J.; Lobanov, V.P.; Ruhland, A.M.A.; Bahl, N.; Gailer, J. Mobilization of Cd from human serum albumin by small molecular weight thiols. J. Chromatogr. B 2014, 958, 16–21. [Google Scholar] [CrossRef]
- Sagmeister, P.; Gibson, M.A.; McDade, K.H.; Gailer, J. Physiologically relevant plasma D, L-homocysteine concentrations mobilize Cd from human serum albumin. J. Chromatogr. B 2016, 1027, 181–186. [Google Scholar] [CrossRef]
- Jahromi, E.Z.; White, W.; Wu, Q.; Yamdagni, R.; Gailer, J. Remarkable effect of mobile phase buffer on the SEC-ICP-AES derived Cu, Fe and Zn-metalloproteome pattern of rabbit blood plasma. Metallomics 2010, 2, 460–468. [Google Scholar] [CrossRef]
- Taylor, N.J.; Carty, A.J. Nature of Hg2+-L-cysteine complexes implicated in mercury biochemistry. J. Am. Chem. Soc. 1977, 99, 6143–6145. [Google Scholar] [CrossRef]
- Watton, S.P.; Wright, J.G.; MacDonnel, F.M.; Bryson, J.W.; Sabat, M.; O’Halloran, T.V. Trigonal mercuric complex of an aliphatic thiolate: A spectroscopic and structural model for the receptor site in the Hg(II) biosensor MerR. J. Am. Chem. Soc. 1990, 112, 2824–2826. [Google Scholar] [CrossRef]
- Barrie, P.J.; Gyani, A.; Motevalli, M.; O’Brien, P. Solid-state 113Cd NMR studies on cadmium complexes with glycine, L-alanine, and L-cysteine. Inorg. Chem. 1993, 32, 3862–3867. [Google Scholar] [CrossRef]
- Mills, N.L.; Donaldson, K.; Hadoke, P.W.; Boon, N.A.; MacNee, W.; Cassee, F.R.; Sandstroem, T.; Blomberg, A.; Newby, D.E. Adverse cardionvascular effects of air pollution. Nat. Clin. Pract. Card. 2009, 6, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Wong, C.-S.; Chung, C.-J.; Wu, M.-Y.; Huang, Y.-L.; Ao, P.-L.; Lin, Y.-F.; Lin, Y.C.; Shiue, H.-S.; Su, C.-T.; et al. The association between plasma selenium and chronic kidney disease related to lead, cadmium and arsenic exposure in a Taiwanese population. J. Haz. Mater. 2019, 375, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Remelli, M.; Nurchi, V.M.; Lachowicz, J.I.; Medici, S.; Zoroddu, M.A.; Peana, M. Competition between Cd(II) and other divalent transition metal ions during complex formation with amino acids, peptides, and chelating agents. Coord. Chem. Rev. 2016, 327–328, 55–69. [Google Scholar] [CrossRef]
- Gomez-Ariza, J.L.; Jahromi, E.Z.; Gonzalez-Fernandez, M.; Garcia-Barrera, T.; Gailer, J. Liquid chromatography-inductively coupled plasma-based metallomic approaches to probe health-relevant interactions between xenobiotics and mammalian organisms. Metallomics 2011, 3, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Gailer, J. Linking molecular targets of Cd in the bloodstream to organ-based adverse health effects. J. Inorg Biochem. 2021, 216, 111279. [Google Scholar] [CrossRef] [PubMed]
- Blazka, M.E.; Shaikh, Z.A. Cadmium and mercury accumulation in rat hepatocytes: Interactions with other metal ions. Toxicol. Appl. Pharmacol. 1992, 113, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.T.; Sunaga, H.; Kobayashi, E.; Shimojo, N. Mercaptoalbumin as a selective cadmium-binding protein in rat serum. Toxicol. Appl. Pharmacol. 1986, 86, 466–473. [Google Scholar] [CrossRef]
- Zalups, R.K. Early aspects of the intrarenal distribution of mercury after the administration of mercuric chloride. Toxicology 1993, 79, 215–228. [Google Scholar] [CrossRef]
- Li, Y.L.; Huang, Y.S.; He, B.; Liu, R.Z.; Qu, G.B.; Yin, Y.G.; Shi, J.B.; Hu, L.G.; Jiang, G.B. Cadmium-binding proteins in human blood plasma. Ecotox. Environ. Safe. 2020, 188, 7. [Google Scholar] [CrossRef]
- Sadler, P.J.; Viles, J.H. 1H and 113Cd NMR investigation of Cd2+ and Zn2+ binding sites on serum albumin: Competition with Ca2+, Ni2+, Cu2+, and Zn2+. Inorg. Chem. 1996, 35, 4490–4496. [Google Scholar] [CrossRef]
- Goumakos, W.; Laussac, J.P.; Sarkar, B. Binding of cadmium(II) and zinc(II) to human and dog serum albumins—An equilibrium dialysis and CD-113-NMR study. Biochem. Cell Biol. 1991, 69, 809–820. [Google Scholar] [CrossRef]
- Yun, Z.; Li, L.; Liu, L.; He, B.; Zhao, X.; Jiang, G. Characterization of mercury-containing protein in human plasma. Metallomics 2013, 5, 821–827. [Google Scholar] [CrossRef]
- Shin, S.-Y.; Fauman, E.B.; Petersen, A.-K.; Krumsiek, J.; Santos, R.; Huang, J.; Arnold, M.; Erte, I.; Forgetta, V.; Yang, T.-P.; et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 2014, 46, 543–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalups, R.K. Evidence for basolateral uptake of cadmium in the kidneys of rats. Toxicol. Appl. Pharmacol. 2000, 164, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, D.J.; Smith, J.C. Effects of coadministered low-molecular weight thiol compounds on short-term distribution of methylmercury in the rat. Toxicol. Appl. Pharmacol. 1982, 62, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Keith, R.L.; McGuinness, S.J.; Gandolfi, A.J.; Lowe, T.P.; Chen, Q.; Fernando, Q. Interaction of metals during their uptake and accumulation in rabbit renal cortical slices. Environ. Health Perspect. 1995, 103 (Suppl. S1), 77–80. [Google Scholar] [PubMed] [Green Version]
- Pushie, M.J.; Pickering, I.J.; Korbas, M.; Hackett, M.J.; George, G.N. Elemental and chemically specific X-ray fluorescence imaging of biological systems. Chem. Rev. 2014, 114, 8499–8541. [Google Scholar] [CrossRef] [Green Version]
- James, A.K.; Dolgova, N.V.; Nehzati, S.; Korbas, M.; Cotelesage, J.J.H.; Sokaras, D.; Kroll, T.; O’Donoghue, J.L.; Watson, G.E.; Myers, G.J.; et al. Molecular fates of organometallic mercury in human brain. ACS Chem. Neurosci. 2022, 13, 1756–1768. [Google Scholar] [CrossRef]
- Zalups, R.K. Molecular interactions with mercury in the kidney. Pharmacol. Rev. 2000, 52, 113–143. [Google Scholar]
- Rana, S.V.S. Metals and apoptosis: Recent developments. J. Trace Elem. Med. Biol. 2008, 22, 262–284. [Google Scholar] [CrossRef]
- Jarup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [Green Version]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Tchounwou, P.B.; Ayensu, W.K.; Ninashvili, N.; Sutton, D. Environmental exposure to mercury and its toxicopathologic implications for public health. Environ. Toxicol. 2003, 18, 149–175. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Sly, J.L.; Ruchirawat, M.; Silva, E.R.; Huo, X.; Diaz-Barriga, F.; Zar, H.J.; King, M.; Ha, E.H.; Asante, K.A.; et al. Health consequences of environmental exposures: Changing global patterns of exposure and disease. Ann. Glob. Health 2016, 82, 10–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossignol, D.A.; Genius, S.J.; Frye, R.E. Environmental toxicants and autism spetrum disorders: A systematic review. Transl. Psychiatry 2014, 4, e360. [Google Scholar]
- Cooper, G.S.; Miller, F.W.; Germolec, D.R. Occupational exposure and autoimmune diseases. Intern. Immunopharmacol. 2002, 2, 303–313. [Google Scholar] [CrossRef]
- Greenberg, M.I.; Vearrier, D. Metal fume fever and polymer fume fever. Clin. Toxicol. 2015, 53, 195–203. [Google Scholar] [CrossRef] [PubMed]

| Mobile Phase | Concentration (µM) | Mercury | Cadmium | ||
|---|---|---|---|---|---|
| Retention Time (s) * | Total Recovery (%) | Retention Time (s) * | Total Recovery (%) | ||
| Cys | 0 | 880 ± 2 | 100 | 887 ± 2 | 100 |
| 50 | 1210 ± 3 + 1304 ± 2 | 66 ± 9 | (543 ± 2) 925 ± 0 + 1051 ± 2 1155 ± 9 1322 ± 2 | 54 ± 6 | |
| 75 | 1225 ± 2 + 1299 ± 3 | 71 ± 17 | (555 ± 3) 954 ± 6 1069 ± 3 + 1319 ± 1 | 75 ± 11 | |
| 100 | 1237 ± 2 | 73 ± 16 | 1084 ± 3 + 1351 ± 1 | 62 ± 9 | |
| 200 | 1262 ± 4 | 67 ± 4 | 1138 ± 41+ 1299 ± 2 | 51 ± 9 | |
| Met | 300 | 744 ± 2 886 ± 1 + | 72 ± 21 | 727 ± 2 889 ± 2 + | 61 ± 2 |
| h-Cys | 0 | 743 ± 1 886 ± 2 + | 100 | 732 ± 2 888 ± 2 + | 100 |
| 50 | 1337 ± 3 | 40 ± 10 | 538 ± 6 928 ± 2 + | 27 ± 8 | |
| 75 | 1336 ± 26 + 1504 ± 14 | 86 ± 21 | 539 ± 1 + 800 ± 135 | 9 ± 6 | |
| 100 | 1329 ± 25 + 1501 ± 16 | 93 ± 16 | 545 ± 1 + 803 ± 130 | 5 ± 3 | |
| 200 | 1358 ± 34 + 1545 ± 18 + | 57 ± 11 | 640 ± 22 | 20 ± 6 | |
| Tris buffer | 0 | 709 # 858 +# | 100 | 698 # 862 +# | 100 |
| 200 (hCys) | 1340 ± 15 + 1501 ± 27 | 71 ± 15 | 660 ± 47 | 4 ± 1 | |
| GSH | 0 | 904 ± 3 | 100 | 900 ± 1 | 100 |
| 50 | 1143 +# 1258 # | 113 # | 924 +# | 50 # | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautam, A.; Gailer, J. More Effective Mobilization of Hg2+ from Human Serum Albumin Compared to Cd2+ by L-Cysteine at Near-Physiological Conditions. Toxics 2023, 11, 599. https://doi.org/10.3390/toxics11070599
Gautam A, Gailer J. More Effective Mobilization of Hg2+ from Human Serum Albumin Compared to Cd2+ by L-Cysteine at Near-Physiological Conditions. Toxics. 2023; 11(7):599. https://doi.org/10.3390/toxics11070599
Chicago/Turabian StyleGautam, Astha, and Jürgen Gailer. 2023. "More Effective Mobilization of Hg2+ from Human Serum Albumin Compared to Cd2+ by L-Cysteine at Near-Physiological Conditions" Toxics 11, no. 7: 599. https://doi.org/10.3390/toxics11070599
APA StyleGautam, A., & Gailer, J. (2023). More Effective Mobilization of Hg2+ from Human Serum Albumin Compared to Cd2+ by L-Cysteine at Near-Physiological Conditions. Toxics, 11(7), 599. https://doi.org/10.3390/toxics11070599









