Characterization and Polydispersity of Volcanic Ash Nanoparticles in Synthetic Lung Fluid
Abstract
:1. Introduction
2. General Background
2.1. Volcanic Ash Nanoparticles
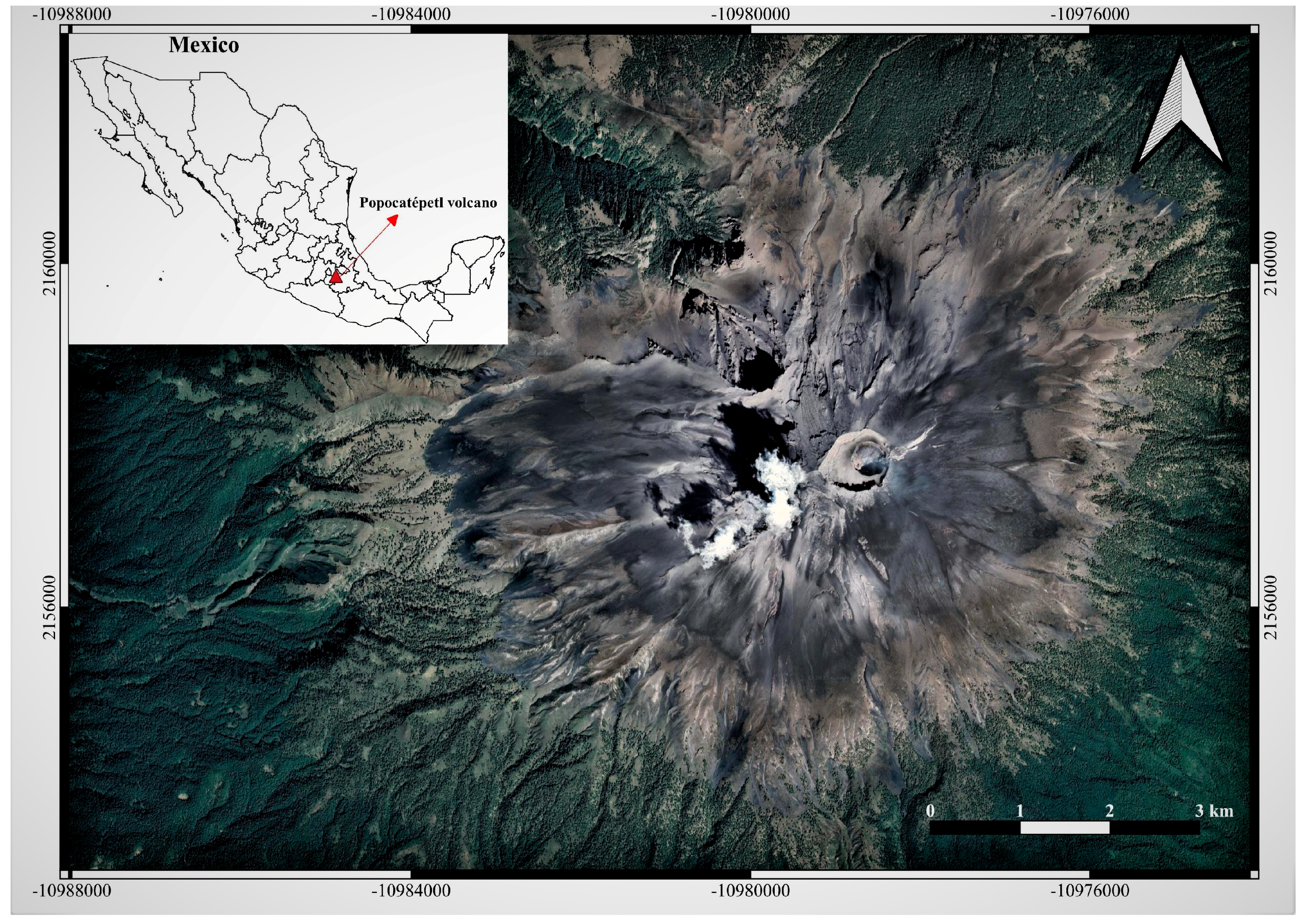
2.2. Popocatépetl Eruptive History
3. Materials and Methods
3.1. Samples of Volcanic Ash
3.2. SEM-EDS Analysis
3.3. Particle Size Analysis
4. Results and Discussion
4.1. Morphological and Chemical Analysis
4.2. Particle Size Distribution
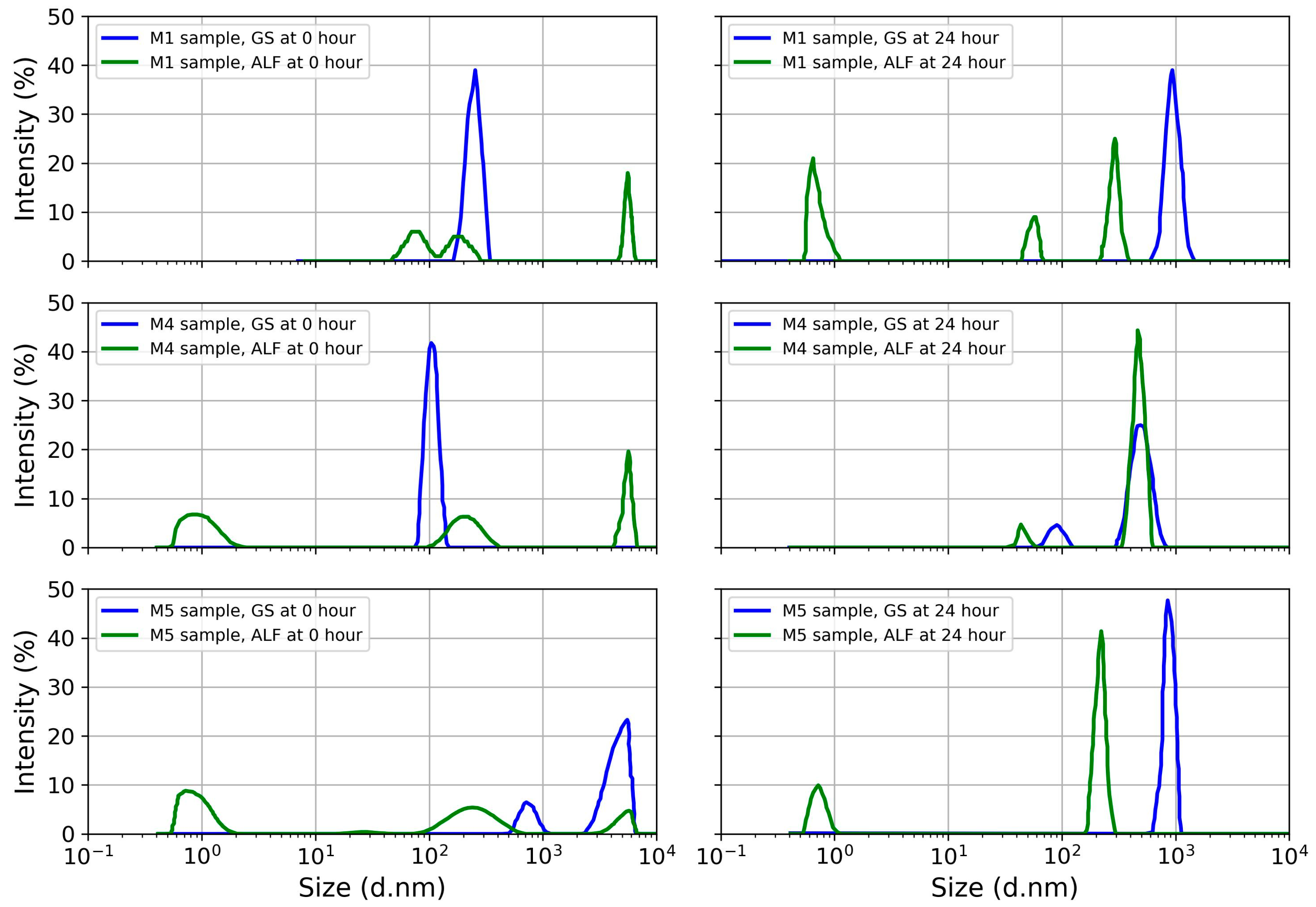
4.3. Particle Polydispersity in Simulated Lung Fluid
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakshi, S.; He, Z.L.; Harris, W.G. Natural Nanoparticles: Implications for Environment and Human Health. Crit. Rev. Environ. Sci. Technol. 2015, 45, 861–904. [Google Scholar] [CrossRef]
- Taylor, D.A. Dust in the wind. Environ. Health Perspect. 2002, 110, A80–A87. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.; Tewari, D.; Gaur, M.; Yadav, A.B.; García-Betancourt, M.L.; Abdel-Haleem, F.M.; Bechelany, M.; Barhoum, A. Nanoparticle and Nanostructure Synthesis and Controlled Growth Methods. Nanomaterials 2022, 12, 3226. [Google Scholar] [CrossRef] [PubMed]
- Ermolin, M.S.; Ivaneev, A.I.; Fedyunina, N.N.; Fedotov, P.S. Nanospeciation of metals and metalloids in volcanic ash using single particle inductively coupled plasma mass spectrometry. Chemosphere 2021, 281, 130950. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Ilyas, M.; Basheer, C.; Tariq, M.; Daud, M.; Baig, N.; Shehzad, F. Impact of nanoparticles on human and environment: Review of toxicity factors, exposures, control strategies, and future prospects. Environ. Sci. Pollut. Res. 2015, 22, 4122–4143. [Google Scholar] [CrossRef] [PubMed]
- Slezakova, K.; Morais, S.; do Carmo Pereira, M. Atmospheric Nanoparticles and Their Impacts on Public Health. In Current Topics in Public Health; Rodriguez-Morales, A.J., Ed.; IntechOpen: London, UK, 2013; pp. 503–529. [Google Scholar] [CrossRef] [Green Version]
- Sonwani, S.; Madaan, S.; Arora, J.; Suryanarayan, S.; Rangra, D.; Mongia, N.; Vats, T.; Saxena, P. Inhalation Exposure to Atmospheric Nanoparticles and Its Associated Impacts on Human Health: A Review. Front. Sustain. Cities 2021, 3, 690444. [Google Scholar] [CrossRef]
- Kumar, P.; Robins, A.; Vardoulakis, S.; Britter, R. A review of the characteristics of nanoparticles in the urban atmosphere and the prospects for developing regulatory controls. Atmos. Environ. 2010, 44, 5035–5052. [Google Scholar] [CrossRef] [Green Version]
- Hochella Jr, M.F.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, eaau8299. [Google Scholar] [CrossRef] [Green Version]
- Fedotov, P.S.; Ermolin, M.S.; Ivaneev, A.I. Study of Elemental Composition and Properties of Volcanic Ash and Urban Dust Nanoparticles. In Advances in Geochemistry, Analytical Chemistry, and Planetary Sciences; Kolotov, V.P., Bezaeva, N.S., Eds.; Springer: Cham, Switzerland, 2023; pp. 133–143. [Google Scholar] [CrossRef]
- Ermolin, M.S.; Fedotov, P.S.; Malik, N.A.; Karandashev, V.K. Nanoparticles of volcanic ash as a carrier for toxic elements on the global scale. Chemosphere 2018, 200, 16–22. [Google Scholar] [CrossRef]
- Faucher, S.; Ivaneev, A.I.; Fedotov, P.S.; Lespes, G. Characterization of volcanic ash nanoparticles and study of their fate in aqueous medium by asymmetric flow field-flow fractionation–multi-detection. Environ. Sci. Pollut. Res. 2021, 28, 31850–31860. [Google Scholar] [CrossRef]
- Mendoza-Villa, F.; Checca-Huaman, N.-R.; Ramos-Guivar, J.A. Ecotoxicological Properties of Titanium Dioxide Nanomorphologies in Daphnia magna. Nanomaterials 2023, 13, 927. [Google Scholar] [CrossRef]
- Zarria-Romero, J.Y.; Ocampo-Anticona, J.-A.; Pinotti, C.N.; Passamani, E.C.; Checca-Huaman, N.-R.; Castro-Merino, I.-L.; Pino, J.; Shiga, B.; Ramos-Guivar, J.A. Ecotoxicological properties of functionalized magnetic graphene oxide and multiwall carbon nanotube in Daphnia magna. Ceram. Int. 2023, 49, 15200–15212. [Google Scholar] [CrossRef]
- Horwell, C.J.; Baxter, P.J. The respiratory health hazards of volcanic ash: A review for volcanic risk mitigation. Bull. Volcanol. 2006, 69, 1–24. [Google Scholar] [CrossRef]
- Oberbek, P.; Kozikowski, P.; Czarnecka, K.; Sobiech, P.; Jakubiak, S.; Jankowski, T. Inhalation exposure to various nanoparticles in work environment–contextual information and results of measurements. J. Nanopart. Res. 2019, 21, 222. [Google Scholar] [CrossRef] [Green Version]
- Mc Carthy, D.; Malhotra, M.; Aoife, O.M.; Cryan, J.; O’Driscoll, C. Nanoparticles and the blood-brain barrier: Advancing from in-vitro models towards therapeutic significance. Pharm. Res. 2015, 32, 1161–1185. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of metal and metal oxide nanoparticles: A review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Barsotti, S.; Andronico, D.; Neri, A.; Del Carlo, P.; Baxter, P.J.; Aspinall, W.P.; Hincks, T. Quantitative assessment of volcanic ash hazards for health and infrastructure at Mt. Etna (Italy) by numerical simulation. J. Volcanol. Geotherm. Res. 2010, 192, 85–96. [Google Scholar] [CrossRef]
- Covey, J.; Dominelli, L.; Horwell, C.J.; Rachmawati, L.; Martin-Del Pozzo, A.L.; Armienta, M.A.; Nugroho, F.; Ogawa, R. Carers’ perceptions of harm and the protective measures taken to safeguard children’s health against inhalation of volcanic ash: A comparative study across Indonesia, Japan and Mexico. Int. J. Disaster Risk Reduct. 2021, 59, 102194. [Google Scholar] [CrossRef]
- Lähde, A.; Gudmundsdottir, S.S.; Joutsensaari, J.; Tapper, U.; Ruusunen, J.; Ihalainen, M.; Karhunen, T.; Torvela, T.; Jokiniemi, J.; Jarvinen, K.; et al. In Vitro evaluation of pulmonary deposition of airborne volcanic ash. Atmos. Environ. 2013, 70, 18–27. [Google Scholar] [CrossRef]
- Damby, D.E.; Murphy, F.A.; Horwell, C.J.; Raftis, J.; Donaldson, K. The in vitro respiratory toxicity if cristobalite-bearing volcanic ash. Environ. Res. 2016, 145, 74–84. [Google Scholar] [CrossRef] [Green Version]
- Trejos, E.M.; Silva, L.F.O.; Hower, J.C.; Flores, E.M.M.; González, C.M.; Pachón, J.E.; Aristizábal, B.H. Volcanic emissions and atmospheric pollution: A study of nanoparticles. Geosci. Front. 2021, 12, 746–755. [Google Scholar] [CrossRef]
- Beckett, K.P.; Freer-Smith, P.H.; Taylor, G. Particulate pollution capture by urban trees: Effect of species and windspeed. Glob. Chang. Biol. 2000, 6, 995–1003. [Google Scholar] [CrossRef]
- Ammann, M.; Burtscher, H. Characterization of ultrafine aerosol particles in Mt. Etna emissions. Bull. Volcanol. 1990, 52, 577–583. [Google Scholar] [CrossRef]
- Oberbeck, V.R.; Farlow, N.H.; Fong, W.; Snetsinger, K.G.; Ferry, G.V.; Hayes, D.M. Mount St. Helens aerosol evolution. Geophys. Res. Lett. 1982, 9, 1089–1092. [Google Scholar] [CrossRef] [Green Version]
- Gudmundsson, G. Respiratory health effects of volcanic ash with special reference to Iceland. A review. Clin. Respir. J. 2011, 5, 2–9. [Google Scholar] [CrossRef]
- Horwell, C.J. Grain-size analysis of volcanic ash for the rapid assessment of respiratory health hazard. J. Environ. Manag. 2007, 9, 1107–1115. [Google Scholar] [CrossRef]
- Aguilera, C.; Viteri, M.; Seqqat, R.; Navarrette, L.A.; Toulkeridis, T.; Ruano, A.; Torres-Arias, M. Biological Impact of Exposure to Extremely Fine-Grained Volcanic Ash. J. Nanotechnol. 2018, 2018, 7543859. [Google Scholar] [CrossRef] [Green Version]
- Stewart, C.; Damby, D.E.; Horwell, C.J.; Elias, T.; Ilynskaya, E.; Tomasek, I.; Longo, B.M.; Schmidt, A.; Carlsen, H.K.; Mason, E.; et al. Volcanic air pollution and human health: Recent advances and future directions. Bull. Volcanol. 2022, 84, 11. [Google Scholar] [CrossRef]
- Tomašek, I.; Damby, D.E.; Stewart, C.; Horwell, C.J.; Plumlee, G.; Ottley, C.J.; Delmelle, P.; Morman, S.; El Yazidi, S.; Claeys, P.; et al. Development of a simulated lung fluid leaching method to assess the release of potentially toxic elements from volcanic ash. Chemosphere 2021, 278, 130303. [Google Scholar] [CrossRef]
- Capra, L.; Poblete, M.A.; Alvarado, R. The 1997 and 2001 lahars of Popocatépetl volcano (Central Mexico): Textural and sedimentological constrains on their origin and hazards. J. Volcanol. Geotherm. Res. 2004, 131, 352–369. [Google Scholar] [CrossRef]
- Schaaf, P.; Stimac, J.; Siebe, C.; Macías, J.L. Geochemical Evidence for Mantle Origin and Crustal Processes in Volcanic Rocks from Popocatépetl and Surrounding Monogenetic Volcanoes, Central Mexico. J. Petrol. 2005, 46, 1243–1282. [Google Scholar] [CrossRef]
- De la Cruz-Reyna, S.; Tilling, R.I.; Valdés-González, C. Challenges in Responding to a Sustained, Continuing Volcanic Crisis: The Case of Popocatépetl Volcano, Mexico, 1994-Present. In Observing the Volcano World. Advances in Volcanology; Fearnley, C.J., Bird, D.K., Haynes, K., McGuire, W.J., Jolly, G., Eds.; Springer: Cham, Switzerland, 2017; pp. 235–252. [Google Scholar] [CrossRef] [Green Version]
- Martin-Del Pozzo, A.L. Precursors to eruptions of Popocatépetl Volcano, Mexico. Geofís. Int. 2012, 51, 87–107. Available online: https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0016-71692012000100007 (accessed on 15 April 2023). [CrossRef]
- Stremme, W.; Ortega, I.; Siebe, C.; Grutter, M. Gas composition of Popocatépetl Volcano between 2007 and 2008: FTIR spectroscopic measurements of an explosive event and during quiescent degassing. Earth Planet. Sci. Lett. 2011, 301, 502–510. [Google Scholar] [CrossRef]
- Witter, J.B.; Kress, V.C.; Newhall, C.G. Volcán Popocatépetl, Mexico. Petrology, magma mixing, and immediate sources of volatiles for the 1994–present eruption. J. Petrol. 2005, 46, 2337–2366. [Google Scholar] [CrossRef] [Green Version]
- Schiavo, B.; Stremme, W.; Grutter, M.; Campion, R.; Guarin, C.A.; Rivera, C.; Inguaggiato, S. Characterization of a UV camera system for SO2 measurements from Popocatépetl Volcano. J. Volcanol. Geotherm. Res. 2019, 370, 82–94. [Google Scholar] [CrossRef]
- Taquet, N.; Stremme, W.; Grutter, M.; Bezanilla, A.; Schiavo, B.; Rivera, C.; Campion, C.; Boulesteix, T.; Torres, A.N.; Perena, R.E.; et al. Variability in the gas composition of the Popocatepetl volcanic plume. Front. Earth Sci. 2019, 7, 114. [Google Scholar] [CrossRef] [Green Version]
- Schiavo, B.; Morton-Bermea, O.; Salgado-Martinez Arellano, J.; Hernández-Álvarez, E. Estimates of mercury flux and temporal variability of Hg/SO2 ratio in the plume of Popocatépetl volcano (Mexico). J. S. Am. Earth Sci. 2020, 101, 102614. [Google Scholar] [CrossRef]
- Schiavo, B.; Morton-Bermea, O.; Salgado-Martinez, E.; Hernández-Álvares, E. Evaluation of possible impact on human health of atmospheric mercury emanations from the Popocatépetl volcano. Environ. Geochem. Health 2020, 42, 3717–3729. [Google Scholar] [CrossRef]
- Armienta, M.A.; Martin Del Pozzo, A.L.; Espinasa, R.; Cruz, O.; Ceniceros, N.; Aguayo, A.; Butron, A.M. Geochemistry of Ash Leachates During the 1994–1996 Activity of Popocatepetl Volcano. Appl. Geochem. 1998, 13, 841–850. [Google Scholar] [CrossRef]
- Armienta, M.A.; De la Cruz-Reyna, S.; Morton, O.; Cruz, O.; Ceniceros, N. Chemical variations of tephra-fall deposit leachates for three eruptions from Popocatépetl volcano. J. Volcanol. Geotherm. Res. 2002, 113, 61–80. [Google Scholar] [CrossRef]
- Armienta, M.A.; De la Cruz-Reyna, S.; Cruz, O.; Ceniceros, N.; Aguayo, A.; Marin, M. Fluoride in ash leachates: Environmental implications at Popocatépetl volcano, central Mexico. Nat. Hazards Earth Syst. Sci. 2011, 11, 1949–1956. [Google Scholar] [CrossRef]
- Meza-Figueroa, D.; Barboza-Flores, M.; Romero, F.M.; Acosta-Elias, M.; Hernández-Mendiola, E.; Maldonado-Escalante, F.; Pérez-Segura, E.; González-Grijalva, B.; Meza-Montenegro, M.; García-Rico, L.; et al. Metal bioaccessibility, particle size distribution and polydispersity of playground dust in synthetic lysosomal fluids. Sci. Total Environ. 2020, 713, 136481. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Monhemius, A.J.; Plant, J.A. Platinum, palladium and rhodium release from vehicle exhaust catalysts and road dust exposed to simulated lung fluids. Ecotoxicol. Environ. Saf. 2008, 71, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Kastury, F.; Smith, E.; Juhasz, A.L. A critical review of approaches and limitations of inhalation bioavailability and bioaccessibility of metal(loid)s from ambient particulate matter or dust. Sci. Total Environ. 2017, 574, 1054–1074. [Google Scholar] [CrossRef]
- Schiavo, B.; Meza-Figueroa, D.; Pedroza-Montero, M.; Vidal-Solano, J.; González-Grijalva, B.; Navarro-Espinoza, S.; Romero, F.; Hernández, E.; Gutiérrez-Ruiz, M.E.; Ceniceros-Gómez, A.E. In Vitro assessment oral and respiratory bioaccessibility of Mn in school dust: Insight of seasonality in a semiarid environment. Appl. Geochem. 2021, 134, 105102. [Google Scholar] [CrossRef]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Characterization of Nanomaterials: Tools and Challenges. In Nanomaterials for Food Applications, 1st ed.; López Rubio, A., Fabra Rovira, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–353. [Google Scholar] [CrossRef]
- Liu, E.J.; Cashman, K.V.; Rust, A.C. Optimising shape analysis to quantify volcanic ash morphology. GeoResJ 2015, 8, 14–30. [Google Scholar] [CrossRef] [Green Version]
- Shoji, D.; Noguchi, R.; Otsuki, S.; Hino, H. Classification of volcanic ash particles using a convolutional neural network and probability. Sci. Rep. 2018, 8, 8111. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Vecino, M.C.; Rossi, E.; Freret-Lorgeril, V.; Fries, A.; Gabellini, P.; Lemus, J.; Pollastri, S.; Poulidis, A.P.; Iguchi, M.; Bonadonna, C. Aerodynamic characteristics and genesis of aggregates at Sakurajima Volcano, Japan. Sci. Rep. 2022, 12, 2044. [Google Scholar] [CrossRef]
- Tiwari, S.; Pipal, A.S.; Hopke, P.K.; Bisht, D.S.; Srivastava, A.K.; Tiwari, S.; Saxena, P.N.; Khan, A.H.; Pervez, S. Study of the carbonaceous aerosol and morphological analysis of fine particles along with their mixing state in Delhi, India: A case study. Environ. Sci. Pollut. Res. 2021, 22, 10744–10757. [Google Scholar] [CrossRef]
- Leikauf, G.D.; Kim, S.-H.; Jang, A.S. Mechanisms of ultrafine particle-induced respiratory health effects. Exp. Mol. Med. 2020, 52, 329–337. [Google Scholar] [CrossRef]
- Paredes-Mariño, J.; Scheu, B.; Montanaro, C.; Arciniega-Ceballos, A.; Dingwell, D.B.; Perugini, D. Volcanic ash generation: Effects of componentry, particle size and conduit geometry on size-reduction process. Earth Planet. Sci. Lett. 2019, 514, 13–27. [Google Scholar] [CrossRef]
- Brown, R.J.; Bonadonna, C.; Durant, A.J. A review of volcanic ash aggregation. Phys Chem Earth 2012, 45–46, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Beckett, F.; Rossi, E.; Devenish, B.; Witham, C.; Bonadonna, C. Modelling the size distribution of aggregated volcanic ash and implications for operational atmospheric dispersion modelling. Atmos. Chem. Phys. 2022, 22, 3409–3431. [Google Scholar] [CrossRef]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle Aggregation: Challenges to Understanding Transport and Reactivity in the Environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef] [Green Version]
- Konduru, N.V.; Molina, R.M.; Swami, A.; Damiani, F.; Pyrgiotakis, G.; Lin, P.; Andreozzi, P.; Donaghey, T.C.; Demokritou, P.; Krol, S.; et al. Protein corona: Implications for nanoparticle interactions with pulmonary cells. Part. Fibre Toxicol. 2017, 14, 42. [Google Scholar] [CrossRef] [Green Version]
- Kendall, M.; Ding, P.; Kendall, K. Particle and nanoparticle interactions with fibrinogen: The importance of aggregation in nanotoxicology. Nanotoxicology 2010, 5, 55–65. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Semmler-Behnke, M.; Seitz, J.; Scymczak, W.; Wenk, A.; Mayer, P.; Takenaka, S.; Oberdörster, G. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal. Toxicol. 2009, 21, 55–60. [Google Scholar] [CrossRef]
- Kendall, M.; Tetley, T.D.; Wigzell, E.; Hutton, B.; Nieuwenhuijsen, M.; Luckham, P. Lung lining liquid modifies PM2.5 in favor of particle aggregation: A protective mechanism. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L109–L114. [Google Scholar] [CrossRef]
- Oberdorster, G. Significance of particle parameters in the evaluation of exposure-dose-response relationships of inhaled particles. Inhal. Toxicol. 1996, 8, 73–89. [Google Scholar] [CrossRef]
- Horie, M.; Tabei, Y. Role of oxidative stress in nanoparticles toxicity. Free Radic. Res. 2021, 55, 331–342. [Google Scholar] [CrossRef]
- Dzherayan, T.G.; Ermolin, M.S.; Vanifatova, N.G. Effectiveness of the Simultaneous Application of Capillary Zone Electrophoresis and Static Light Scattering in the Study of Volcanic Ash Nano- and Submicroparticles. J. Anal. Chem. 2020, 75, 67–72. [Google Scholar] [CrossRef]



| Sample | M1 | M2 | M3 | M4 | M5 |
|---|---|---|---|---|---|
| Element | |||||
| Al | x | x | x | x | x |
| As | x | ||||
| Ba | x | ||||
| Br | x | x | |||
| Ca | x | x | x | x | x |
| Cu | x | x | |||
| Fe | x | x | x | x | x |
| K | x | x | x | x | x |
| Mg | x | x | x | x | x |
| Na | x | x | x | x | x |
| O | x | x | x | x | x |
| S | x | x | |||
| Si | x | x | x | x | x |
| Sr | x | x | x | x | |
| Ti | x | x | x | x |
| Sample | Number of Particles | PM1 | PM2.5 | PM5 | PM10 | PM20 | Equivalent Circle (μm) | * Circularity |
|---|---|---|---|---|---|---|---|---|
| M1 | 694 | 64.7 | 10.4 | 17.3 | 5.9 | 1.7 | 2.4 | 0.67 |
| M2 | 229 | 7.8 | 27.9 | 43.7 | 14.4 | 6.1 | 3.9 | 0.72 |
| M3 | 858 | 71.8 | 13.7 | 12.0 | 1.4 | 1.1 | 1.6 | 0.43 |
| M4 | 303 | 53.8 | 27.7 | 13.5 | 3.9 | 0.9 | 2.1 | 0.53 |
| M5 | 377 | 33.4 | 20.2 | 29.2 | 11.7 | 5.6 | 3.7 | 0.51 |
| GS | ALF | |||
|---|---|---|---|---|
| Time | 0 h | 24 h | 0 h | 24 h |
| M1 | ||||
| Polydispersity index | 0.77 | 0.65 | 1 | 1 |
| Hydrodynamic diameter (d.nm) | 240 | 963.6 | 79, 184.9, and 5560 | 0.65, 55.3, and 291.9 |
| M4 | ||||
| Polydispersity index | - | 0.76 | 0.95 | 1 |
| Hydrodynamic diameter (d.nm) | 109.7 | 89.7 and 498.1 | 0.91, 209.4 and 5367 | 45.3 and 468.7 |
| M5 | ||||
| Polydispersity index | 0.35 | 0.89 | 0.31 | 1 |
| Hydrodynamic diameter (d.nm) | 749.3 and 4516 | 864.9 | 0.72, 251.7, and 4857 | 0.71 and 216.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiavo, B.; Morton-Bermea, O.; Meza-Figueroa, D.; Acosta-Elías, M.; González-Grijalva, B.; Armienta-Hernández, M.A.; Inguaggiato, C.; Valera-Fernández, D. Characterization and Polydispersity of Volcanic Ash Nanoparticles in Synthetic Lung Fluid. Toxics 2023, 11, 624. https://doi.org/10.3390/toxics11070624
Schiavo B, Morton-Bermea O, Meza-Figueroa D, Acosta-Elías M, González-Grijalva B, Armienta-Hernández MA, Inguaggiato C, Valera-Fernández D. Characterization and Polydispersity of Volcanic Ash Nanoparticles in Synthetic Lung Fluid. Toxics. 2023; 11(7):624. https://doi.org/10.3390/toxics11070624
Chicago/Turabian StyleSchiavo, Benedetto, Ofelia Morton-Bermea, Diana Meza-Figueroa, Mónica Acosta-Elías, Belem González-Grijalva, Maria Aurora Armienta-Hernández, Claudio Inguaggiato, and Daisy Valera-Fernández. 2023. "Characterization and Polydispersity of Volcanic Ash Nanoparticles in Synthetic Lung Fluid" Toxics 11, no. 7: 624. https://doi.org/10.3390/toxics11070624
APA StyleSchiavo, B., Morton-Bermea, O., Meza-Figueroa, D., Acosta-Elías, M., González-Grijalva, B., Armienta-Hernández, M. A., Inguaggiato, C., & Valera-Fernández, D. (2023). Characterization and Polydispersity of Volcanic Ash Nanoparticles in Synthetic Lung Fluid. Toxics, 11(7), 624. https://doi.org/10.3390/toxics11070624








