Characterization and Risk Assessment of PM2.5-Bound Polycyclic Aromatic Hydrocarbons and their Derivatives Emitted from a Typical Pesticide Factory in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Sample Collection
2.2. PAH Analysis
2.3. Health Risk Assessment
3. Results and Discussion
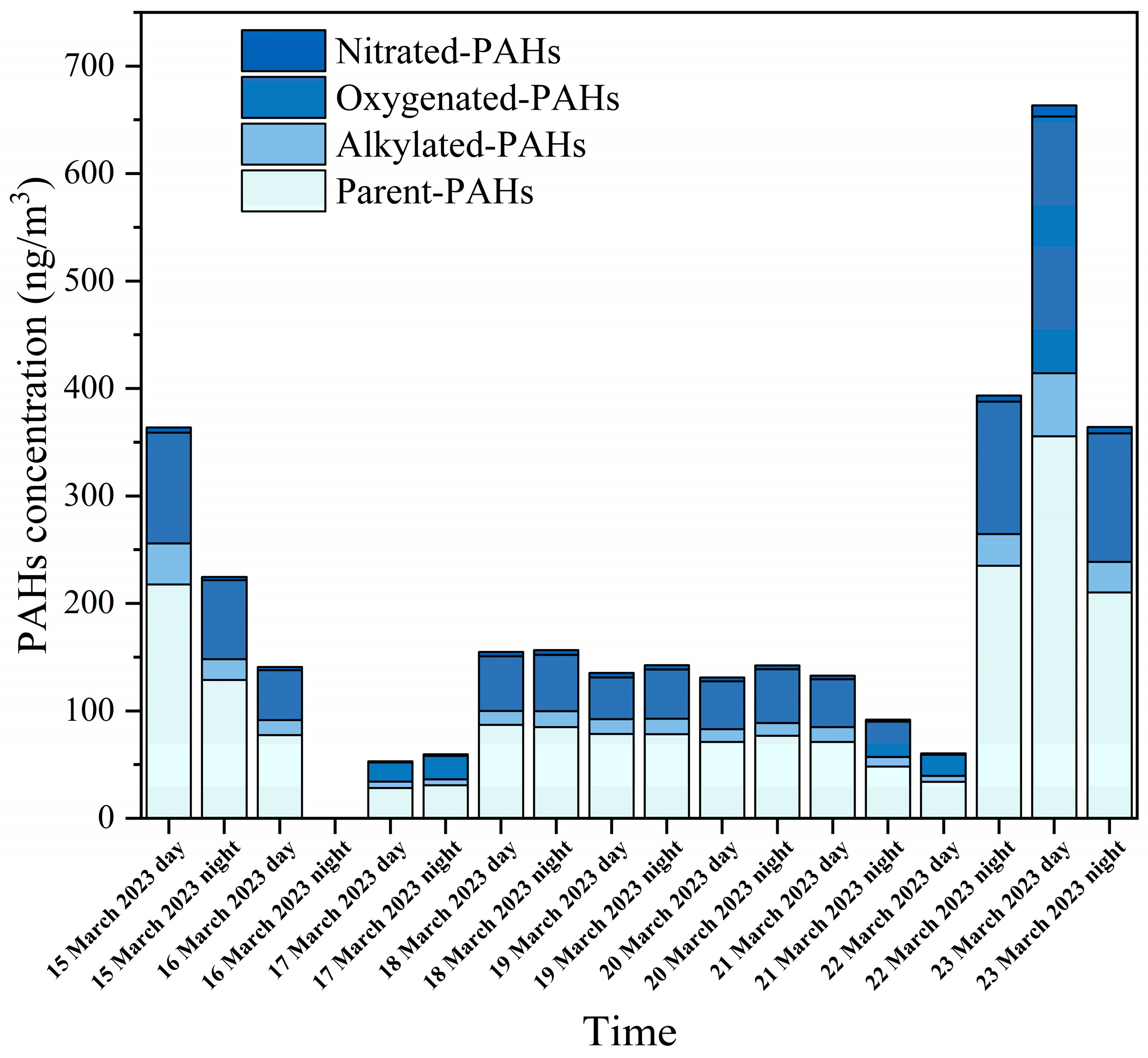
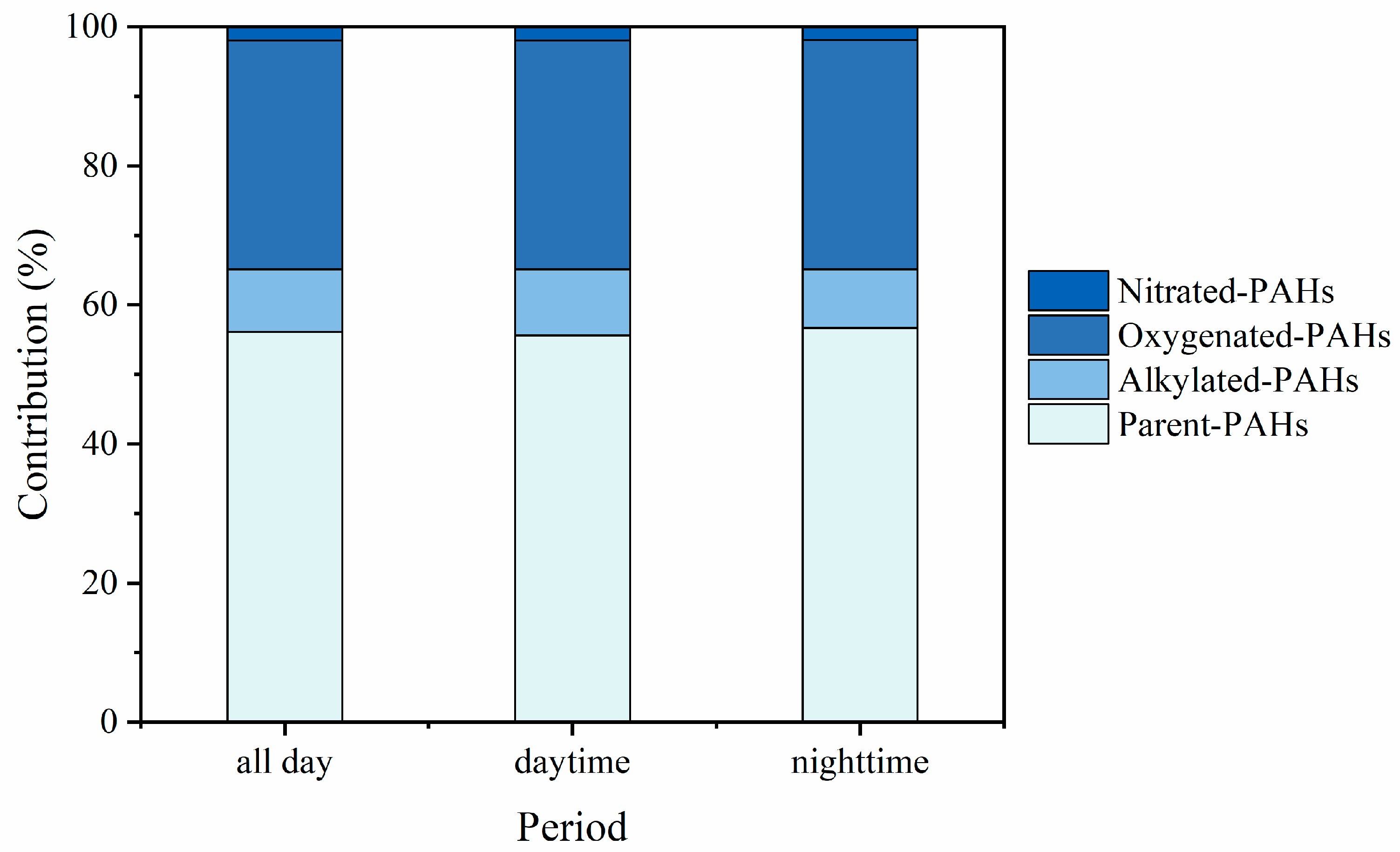
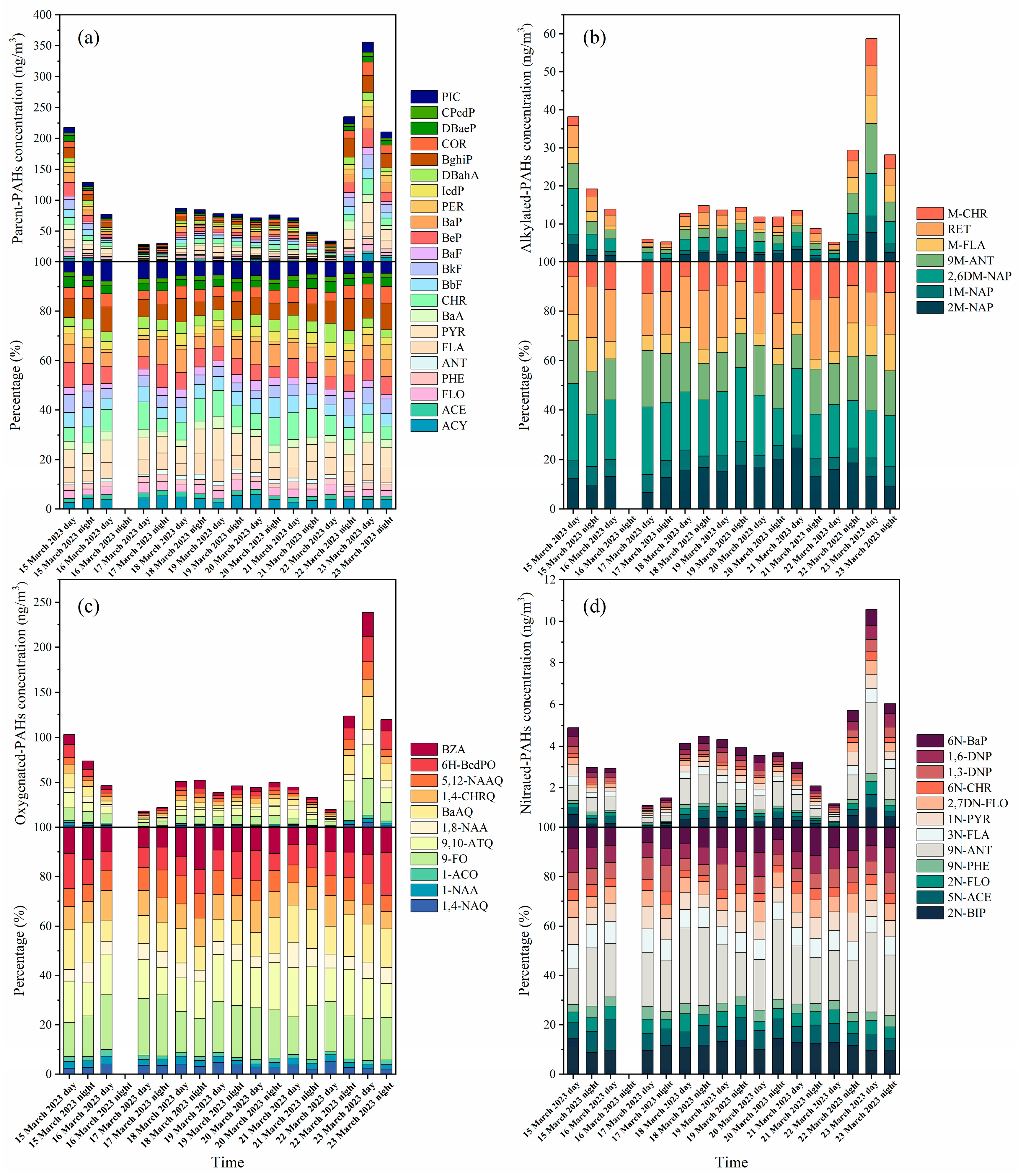
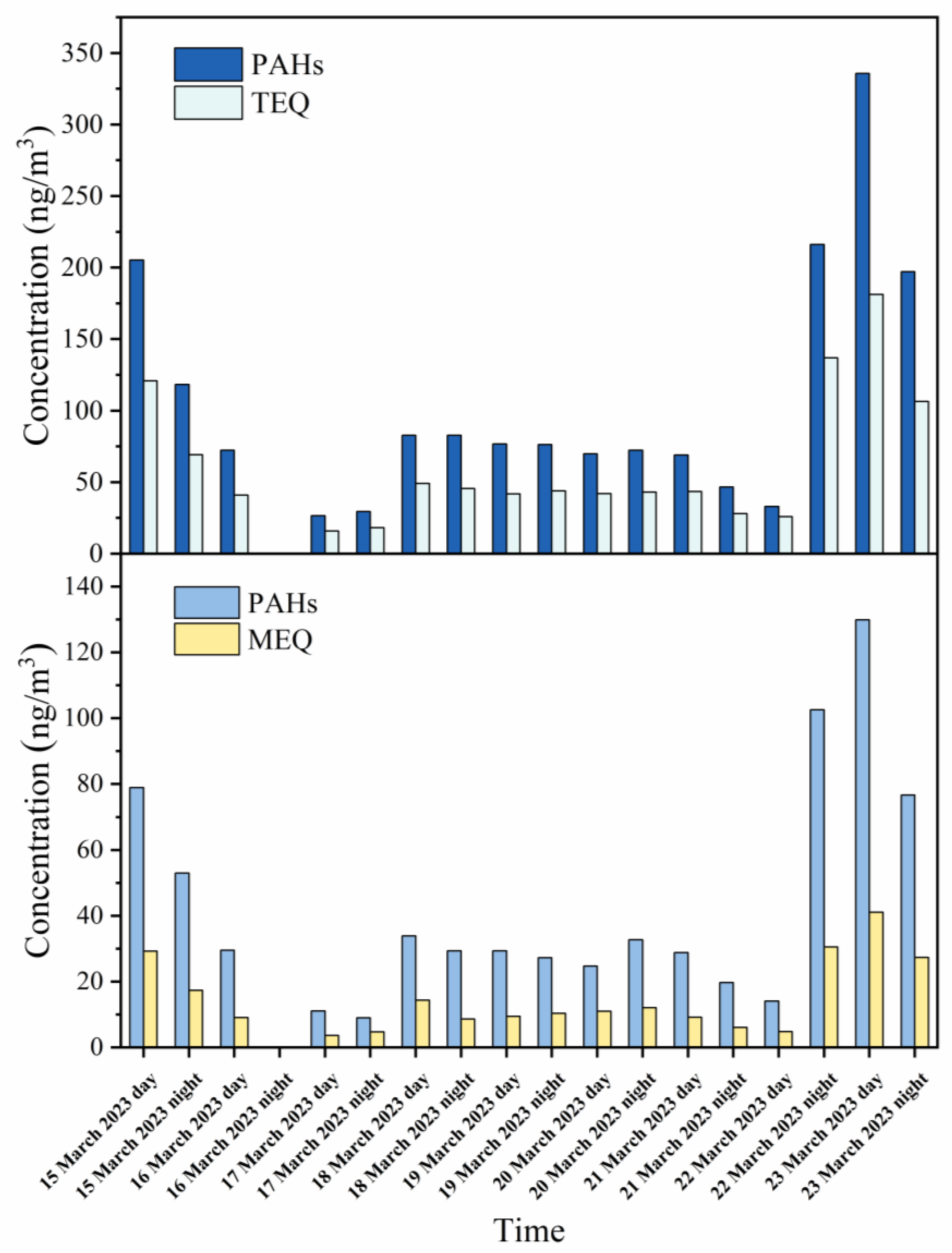
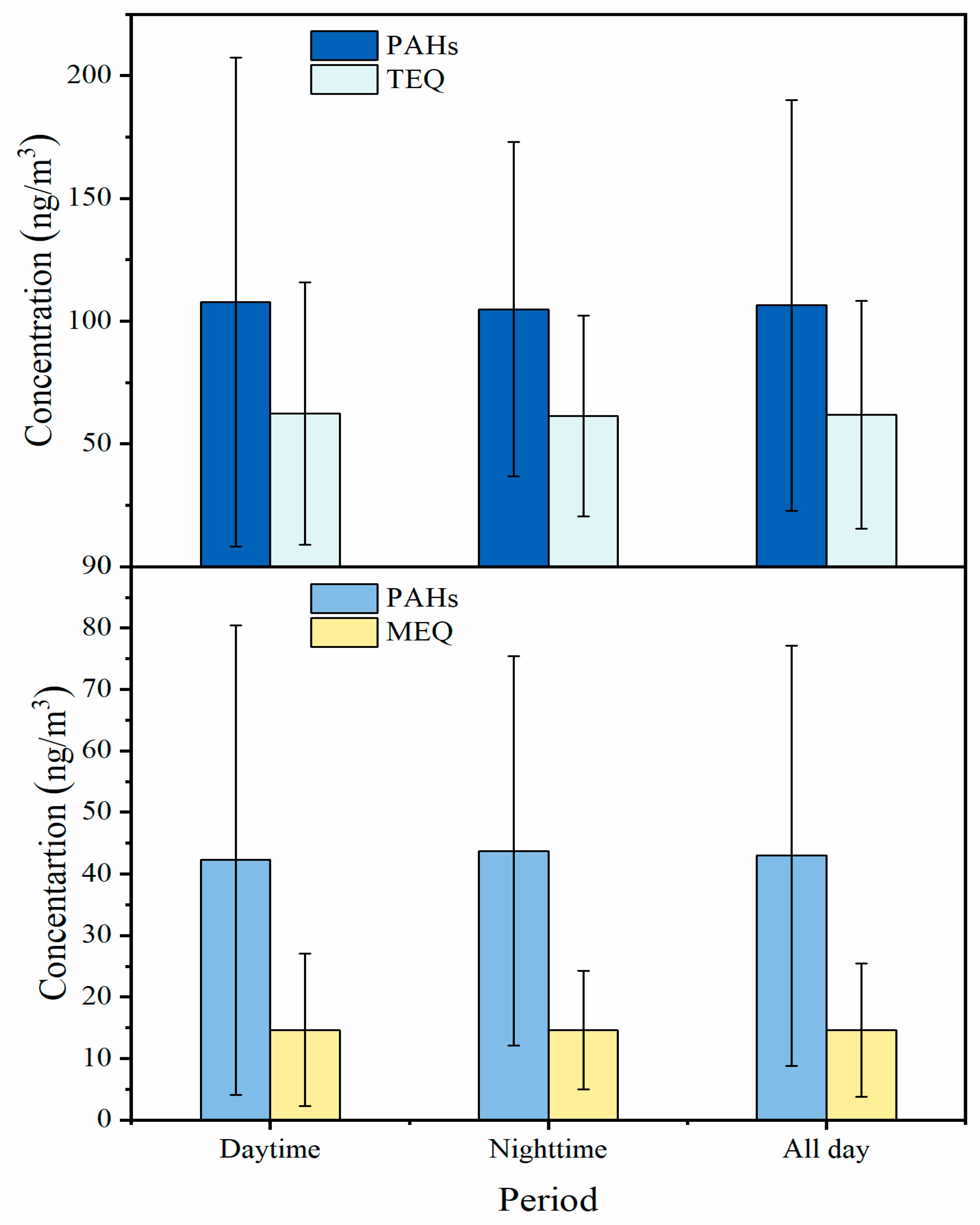
3.1. Characterization of PM2.5-Bound PAHs
3.2. Distribution of p-PAHs and Their Derivatives
3.3. Diagnostic Ratios of PAHs
3.4. Risk Assessment of PAHs from the Pesticide Factory
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, J.; Shen, Z.; Zhang, T.; Kong, S.; Zhang, H.; Zhang, Q.; Niu, X.; Huang, S.; Xu, H.; Ho, K.F.; et al. A comprehensive evalua-tion of PM2.5-bound PAHs and their derivative in winter from six megacities in China: Insight the source-dependent health risk and secondary reactions. Environ. Int. 2022, 165, 107344. [Google Scholar] [CrossRef]
- Shen, Z.; Sun, J.; Cao, J.; Zhang, L.; Zhang, Q.; Lei, Y.; Gao, J.; Huang, R.J.; Liu, S.; Huang, Y.; et al. Chemical profiles of urban fugitive dust PM2.5 samples in Northern Chinese cities. Sci. Total Environ. 2016, 569–570, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Sharma, M. Cause and Age-specific premature mortality attributable to PM2.5 Exposure: An analysis for Million-Plus Indian cities. Sci. Total Environ. 2020, 710, 135230. [Google Scholar] [CrossRef]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar]
- Li, J.; Yang, L.; Gao, Y.; Jiang, P.; Li, Y.; Zhao, T.; Zhang, J.; Wang, W. Seasonal variations of NPAHs and OPAHs in PM2.5 at heavily polluted urban and suburban sites in North China: Concentrations, molecular compositions, cancer risk assessments and sources. Ecotoxicol. Environ. Saf. 2019, 178, 58–65. [Google Scholar] [CrossRef]
- Ma, L.; Li, B.; Liu, Y.; Sun, X.; Fu, D.; Sun, S.; Thapa, S.; Geng, J.; Qi, H.; Zhang, A.; et al. Characterization, sources and risk assessment of PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) and nitrated PAHs (NPAHs) in Harbin, a cold city in Northern China. J. Clean. Prod. 2020, 264, 483. [Google Scholar] [CrossRef]
- Zhao, T.; Yang, L.; Huang, Q.; Zhang, Y.; Bie, S.; Li, J.; Zhang, W.; Duan, S.; Gao, H.; Wang, W. PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) and their derivatives (nitrated-PAHs and oxygenated-PAHs) in a road tunnel located in Qingdao, China: Characteristics, sources and emission factors. Sci. Total Environ. 2020, 720, 137521. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Z.; Sun, J.; Zhang, L.; Zhang, B.; Zou, H.; Zhang, T.; Hang Ho, S.S.; Chang, X.; Xu, H.; et al. Parent, alkylated, oxygenated and nitrated polycyclic aromatic hydrocarbons in PM2.5 emitted from residential biomass burning and coal combustion: A novel database of 14 heating scenarios. Environ. Pollut. 2021, 268, 115881. [Google Scholar] [CrossRef]
- Li, H.; Zeng, X.; Zhang, D.; Chen, P.; Yu, Z.; Sheng, G.; Fu, J.; Peng, P. Occurrence and carcinogenic potential of airborne polycyclic aromatic hydrocarbons in some large-scale enclosed/semi-enclosed vehicle parking areas. J. Hazard Mater. 2014, 274, 279–286. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Yang, H.; Li, R.; Yu, Q. Seasonal variation and potential source regions of PM2.5-bound PAHs in the megacity Beijing, China: Impact of regional transport. Environ. Pollut. 2017, 231, 329–338. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, H.; Xu, X.; Zhuang, G.; Zhao, C. A wintertime study of polycyclic aromatic hydrocarbons in PM2.5 and PM2.5−10 in Beijing: Assessment of energy structure conversion. J. Hazard. Mater. 2008, 157, 47–56. [Google Scholar] [CrossRef]
- Samburova, V.; Zielinska, B.; Khlystov, A. Do 16 Polycyclic Aromatic Hydrocarbons Represent PAH Air Toxicity? Toxics 2017, 5, 17. [Google Scholar] [CrossRef]
- Durant, J.L.; Busby, W.F., Jr.; Lafleur, A.L.; Penman, B.W.; Crespi, C.L. Human cell mutagenicity of oxygenated, nitrated and unsubstituted polycyclic aromatic hydrocarbons associated with urban aerosols. Mutat. Res. Genet. Toxicol. 1996, 371, 123–157. [Google Scholar] [CrossRef]
- Huang, W.; Huang, B.; Bi, X.; Lin, Q.; Liu, M.; Ren, Z.; Zhang, G.; Wang, X.; Sheng, G.; Fu, J. Emission of PAHs, NPAHs and OPAHs from residential honeycomb coal briquette combustion. Energy Fuels 2014, 28, 636–642. [Google Scholar] [CrossRef]
- Wei, C.; Han, Y.; Bandowe, B.A.; Cao, J.; Huang, R.J.; Ni, H.; Tian, J.; Wilcke, W. Occurrence, gas/particle partitioning and carcinogenic risk of polycyclic aromatic hydrocarbons and their oxygen and nitrogen containing derivatives in Xi’an, central China. Sci. Total Environ. 2015, 505, 814–822. [Google Scholar] [CrossRef]
- Jariyasopit, N.; Harner, T.; Wu, D.; Williams, A.; Halappanavar, S.; Su, K. Mapping Indicators of Toxicity for Polycyclic Aromatic Compounds in the Atmosphere of the Athabasca Oil Sands Region. Environ. Sci. Technol. 2016, 50, 11282–11291. [Google Scholar] [CrossRef]
- Fu, P.P.; Herreno-Saenz, D.; Von Tungeln, L.S.; Lay, J.O.; Wu, Y.-S.; Lai, J.S.; Evans, F.E. DNA Adducts and Carcinogenicity of Nitro-Polycyclic Aromatic Hydrocarbons. Environ. Health Perspect. 1994, 102, 177–183. [Google Scholar]
- Chung, M.Y.; Lazaro, R.A.; Lim, D.; Jackson, J.; Lyon, J.; Rendulic, D.; Hasson, A.S. Aerosol-Borne Quinones and Reactive Oxygen Species Generation by Particulate Matter Extracts. Environ. Sci. Technol. 2006, 40, 4880–4886. [Google Scholar] [CrossRef]
- Zhang, B.; Peng, Z.; Lv, J.; Peng, Q.; He, K.; Xu, H.; Sun, J.; Shen, Z. Gas Particle Partitioning of PAHs Emissions from Typical Solid Fuel Combustions as Well as Their Health Risk Assessment in Rural Guanzhong Plain, China. Toxics 2023, 11, 80. [Google Scholar] [CrossRef]
- Zimmermann, K.; Jariyasopit, N.; Massey Simonich, S.L.; Tao, S.; Atkinson, R.; Arey, J. Formation of nitro-PAHs from the heterogeneous reaction of ambient particle-bound PAHs with N2O5/NO3/NO2. Environ. Sci. Technol. 2013, 47, 8434–8442. [Google Scholar] [CrossRef]
- Chen, H.-L.; Wu, Y.-C.; Chen, M.-R.; Chou, J.-S.; Zheng, S.-K.; Hou, J.-Z. Risk Assessment of PAH Exposure Involving Metal Working Fluids in Fastener Manufacturing Industries. Aerosol Air Qual. Res. 2016, 16, 3212–3221. [Google Scholar] [CrossRef]
- Sanchez-Soberon, F.; van Drooge, B.L.; Rovira, J.; Grimalt, J.O.; Nadal, M.; Domingo, J.L.; Schuhmacher, M. Size-distribution of airborne polycyclic aromatic hydrocarbons and other organic source markers in the surroundings of a cement plant powered with alternative fuels. Sci. Total Environ. 2016, 550, 1057–1064. [Google Scholar] [CrossRef]
- Wang, N.; Shi, M.; Wu, S.; Guo, X.; Zhang, X.; Ni, N.; Sha, S.; Zhang, H. Study on Volatile Organic Compound (VOC) Emission Control and Reduction Potential in the Pesticide Industry in China. Atmosphere 2022, 13, 1241. [Google Scholar] [CrossRef]
- Masih, A.; Lall, A.S.; Taneja, A.; Singhvi, R. Exposure levels and health risk assessment of ambient BTX at urban and rural environments of a terai region of northern India. Environ. Pollut. 2018, 242, 1678–1683. [Google Scholar] [CrossRef]
- Bari, M.A.; Kindzierski, W.B. Concentrations, sources and human health risk of inhalation exposure to air toxics in Edmonton, Canada. Chemosphere 2017, 173, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; He, H.; Sun, C.; Wang, R.; Cai, H. Distribution characteristics of PAHS in a brownfield in Changzhou. Environ. Chem. 2011, 30, 1456–1461. (In Chinese) [Google Scholar]
- Jungers, G.; Portet-Koltalo, F.; Cosme, J.; Seralini, G.E. Petroleum in Pesticides: A Need to Change Regulatory Toxicology. Toxics 2022, 10, 670. [Google Scholar] [CrossRef]
- Cao, J.-J.; Wang, Q.-Y.; Chow, J.C.; Watson, J.G.; Tie, X.-X.; Shen, Z.-X.; Wang, P.; An, Z.-S. Impacts of aerosol compositions on visibility impairment in Xi’an, China. Atmos. Environ. 2012, 59, 559–566. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Q.; Cao, J.; Zhang, L.; Lei, Y.; Huang, Y.; Huang, R.J.; Gao, J.; Zhao, Z.; Zhu, C.; et al. Optical properties and possible sources of brown carbon in PM2.5 over Xi’an, China. Atmos. Environ. 2017, 150, 322–330. [Google Scholar] [CrossRef]
- Ho, S.S.; Yu, J.Z.; Chow, J.C.; Zielinska, B.; Watson, J.G.; Sit, E.H.; Schauer, J.J. Evaluation of an in-injection port thermal desorption-gas chromatography/mass spectrometry method for analysis of non-polar organic compounds in ambient aerosol samples. J. Chromatogr. A 2008, 1200, 217–227. [Google Scholar] [CrossRef]
- Guo, X.; Chen, F.; Lu, J.; Zhang, W. Seasonal variation, sources, and risk assessment of PM2.5-bound PAHs in Nantong, China: A pre- and post-COVID-19 case study. Front. Environ. Sci. 2022, 10, 947705. [Google Scholar] [CrossRef]
- Peng, C.; Chen, W.; Liao, X.; Wang, M.; Ouyang, Z.; Jiao, W.; Bai, Y. Polycyclic aromatic hydrocarbons in urban soils of Beijing: Status, sources, distribution and potential risk. Environ. Pollut. 2011, 159, 802–808. [Google Scholar] [CrossRef]
- Chen, S.C.; Liao, C.M. Health risk assessment on human exposed to environmental polycyclic aromatic hydrocarbons pollution sources. Sci. Total Environ. 2006, 366, 112–123. [Google Scholar] [CrossRef] [PubMed]
- An, Y.Q.; Hao, H.Y.; Jin, H.; Yuan, S.H.; Pei, X.K.; Yang, N.J.; Liu, Y.G. Pollution level and health risk assessment of polycyclic aromatic hydrocarbons in PM2.5 of four cities in Hebei Province. Occup. Health 2018, 34, 3129–3133. (In Chinese) [Google Scholar]
- Wang, W.; Huang, M.J.; Kang, Y.; Wang, H.S.; Leung, A.O.; Cheung, K.C.; Wong, M.H. Polycyclic aromatic hydrocarbons (PAHs) in urban surface dust of Guangzhou, China: Status, sources and human health risk assessment. Sci. Total Environ. 2011, 409, 4519–4527. [Google Scholar] [CrossRef]
- Ferreira-Baptista, L.; De Miguel, E. Geochemistry and risk assessment of street dust in Luanda, Angola: A tropical urban environment. Atmos. Environ. 2005, 39, 4501–4512. [Google Scholar] [CrossRef]
- Gamo, M.; Oka, T.; Nakanishi, J. A Method Evaluating Population Risks from Chemical Exposure: A Case Study Concerning Prohibition of Chlordane Use in Japan. Regul. Toxicol. Pharmacol. 1995, 21, 151–157. [Google Scholar] [CrossRef]
- He, K.; Xu, H.; Feng, R.; Shen, Z.; Li, Y.; Zhang, Y.; Sun, J.; Zhang, Q.; Zhang, T.; Yang, L.; et al. Characteristics of indoor and personal exposure to particulate organic compounds emitted from domestic solid fuel combustion in rural areas of northwest China. Atmos. Res. 2021, 248, 105181. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, Y.; Qiu, X.; Li, R.; Fang, Y.; Wang, J.; Zhu, Y.; Hu, D. Sources, transformation, and health implications of PAHs and their nitrated, hydroxylated, and oxygenated derivatives in PM2.5 in Beijing. J. Geophys. Res. Atmos. 2015, 120, 7219–7228. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, Y.; Qiu, X.; Lin, Y.; Cao, J.; Hu, D. A quantitative assessment of source contributions to fine particulate matter (PM2.5)-bound polycyclic aromatic hydrocarbons (PAHs) and their nitrated and hydroxylated derivatives in Hong Kong. Environ. Pollut. 2016, 219, 742–749. [Google Scholar] [CrossRef]
- Luo, M.; Ji, Y.; Ren, Y.; Gao, F.; Zhang, H.; Zhang, L.; Yu, Y.; Li, H. Characteristics and Health Risk Assessment of PM2.5-Bound PAHs during Heavy Air Pollution Episodes in Winter in Urban Area of Beijing, China. Atmosphere 2021, 12, 323. [Google Scholar] [CrossRef]
- Khalili, N.R.; Scheff, P.A.; Holsen, T.M. PAH source fingerprints for coke ovens, diesel and, gasoline engines, highway tunnels, and wood combustion emissions. Atmos. Environ. 1995, 29, 533–542. [Google Scholar] [CrossRef]
- Wang, R.; Liu, G.; Sun, R.; Yousaf, B.; Wang, J.; Liu, R.; Zhang, H. Emission characteristics for gaseous- and size-segregated particulate PAHs in coal combustion flue gas from circulating fluidized bed (CFB) boiler. Environ. Pollut. 2018, 238, 581–589. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, X.; Huo, R.; Shi, Z.; Sun, Y.; Feng, Y.; Harrison, R.M. Organic compound source profiles of PM2.5 from traffic emissions, coal combustion, industrial processes and dust. Chemosphere 2021, 278, 130429. [Google Scholar] [CrossRef]
- Nisbet, I.C.; Lagoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Jung, K.H.; Yan, B.; Chillrud, S.N.; Perera, F.P.; Whyatt, R.; Camann, D.; Kinney, P.L.; Miller, R.L. Assessment of benzo(a)pyrene-equivalent carcinogenicity and mutagenicity of residential indoor versus outdoor polycyclic aromatic hydrocarbons exposing young children in New York City. Int. J. Environ. Res. Public Health 2010, 7, 1889–1900. [Google Scholar] [CrossRef]
- Ventafridda, V.; Tamburini, M.; Caraceni, A.; De Conno, F.; Naldi, F. A validation study of the WHO method for cancer pain relief. Cancer 1987, 59, 850–856. [Google Scholar] [CrossRef]
- Huang, B.; Liu, M.; Bi, X.; Chaemfa, C.; Ren, Z.; Wang, X.; Sheng, G.; Fu, J. Phase distribution, sources and risk assessment of PAHs, NPAHs and OPAHs in a rural site of Pearl River Delta region, China. Atmos. Pollut. Res. 2014, 5, 210–218. [Google Scholar] [CrossRef]
- Wang, C.; Meng, Z.; Yao, P.; Zhang, L.; Wang, Z.; Lv, Y.; Tian, Y.; Feng, Y. Sources-specific carcinogenicity and mutagenicity of PM2.5-bound PAHs in Beijing, China: Variations of contributions under diverse anthropogenic activities. Ecotoxicol. Environ. Saf. 2019, 183, 109552. [Google Scholar] [CrossRef]
- Liao, C.M.; Chiang, K.C. Probabilistic risk assessment for personal exposure to carcinogenic polycyclic aromatic hydrocarbons in Taiwanese temples. Chemosphere 2006, 63, 1610–1619. [Google Scholar] [CrossRef]
- Iwegbue, C.M.A.; Ogbuta, A.A.; Tesi, G.O.; Ossai, C.J.; Olisah, C.; Nwajei, G.E.; Martincigh, B.S. Spatial distribution of polycyclic aromatic hydrocarbons in dust and soils from informal trade sites in southern Nigeria: Implications for risk and source analysis. Chemosphere 2023, 315, 137624. [Google Scholar] [CrossRef] [PubMed]
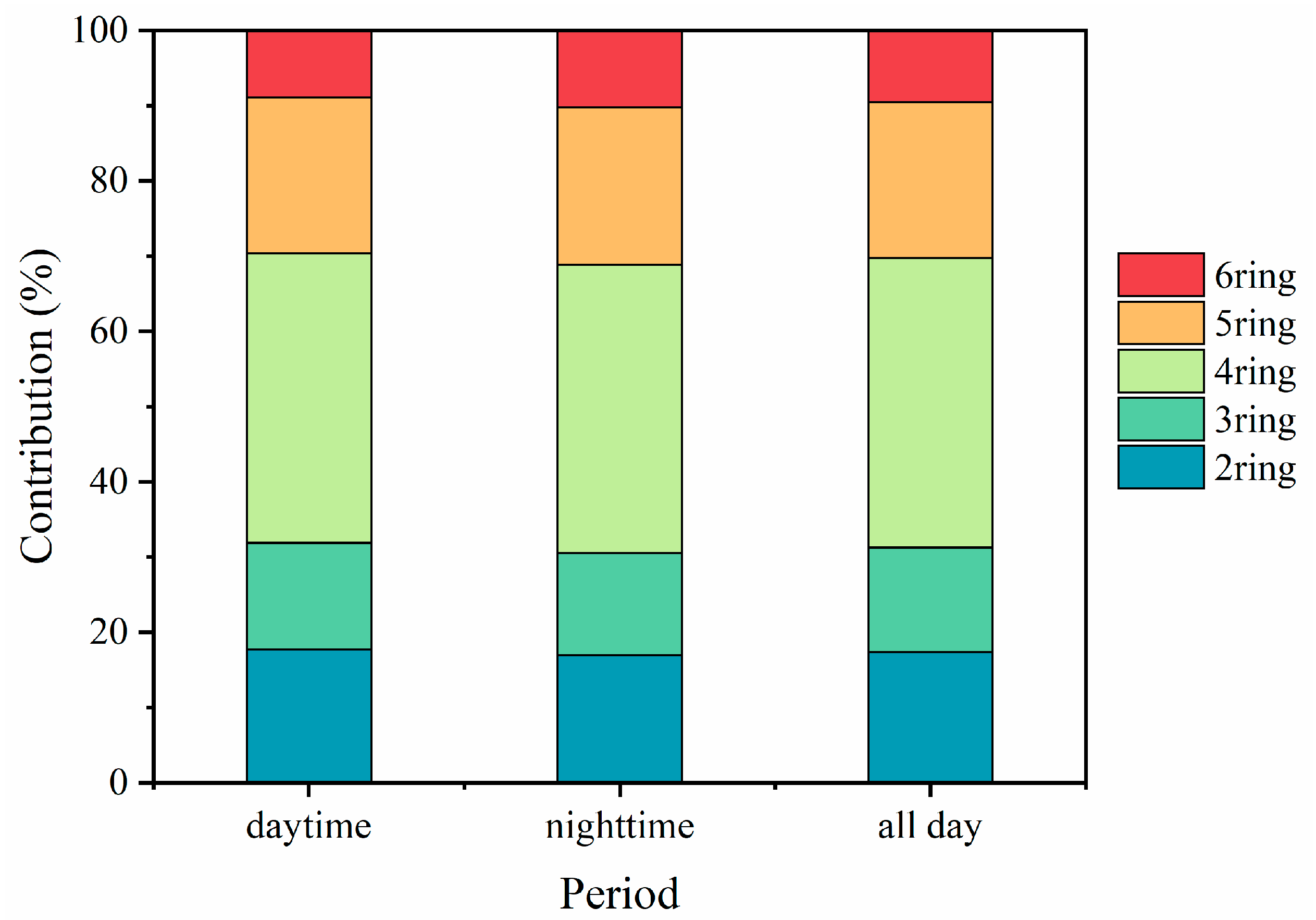
| Diagnostic Ratios | Daytime | Nighttime | Sources in the Reference |
|---|---|---|---|
| ANT/(ANT + PHE) | 0.37 ± 0.08 | 0.35 ± 0.11 | Pyrogenic sources: >0.1 [1] |
| BaA/(BaA + CHR) | 0.29 ± 0.05 | 0.32 ± 0.11 | Mixed source: 0.20–0.35 [1] |
| FLO/(FLO + PYR) | 0.29 ± 0.05 | 0.29 ± 0.05 | Petroleum combustion: 0.40–0.50 [1] |
| IcdP/(IcdP + BghiP) | 0.32 ± 0.06 | 0.29 ± 0.07 | Petroleum combustion: 0.20–0.50 [41] |
| FLA/(FLA + PYR) | 0.42 ± 0.05 | 0.45 ± 0.04 | Coal combustion: 0.40–0.50 [6] |
| BaP/(BaP + CHR) | 0.46 ± 0.09 | 0.48 ± 0.11 | Diesel emission: 0.50 [7] |
| BbF/BkF | 1.27 ± 0.34 | 1.22 ± 0.37 | Diesel emission: >0.5 [7] |
| BaP/BghiP | 0.96 ± 0.25 | 0.94 ± 0.38 | Coal combustion: >0.9 [6] |
| PYR/BaP | 1.24 ± 0.39 | 1.15 ± 0.46 | Gasoline: ~1 [7] |
| p-PAHs | a-PAHs | o-PAHs | n-PAHs | ΣPAHs | ||
|---|---|---|---|---|---|---|
| Male adult | Inhalation | 5.70 × 10−8 | 2.62 × 10−12 | 2.05 × 10−9 | 1.75 × 10−9 | 6.08 × 10−8 |
| Ingestion | 7.86 × 10−4 | 3.62 × 10−8 | 2.83 × 10−5 | 2.42 × 10−5 | 8.38 × 10−4 | |
| Dermal | 1.40 × 10−3 | 6.43 × 10−8 | 5.03 × 10−5 | 4.29 × 10−5 | 1.49 × 10−3 | |
| ILCR | 2.18 × 10−3 | 1.00 × 10−7 | 7.86 × 10−5 | 6.71 × 10−5 | 2.33 × 10−3 | |
| Female adult | Inhalation | 5.00 × 10−8 | 2.30 × 10−12 | 1.80 × 10−9 | 1.54 × 10−9 | 5.34 × 10−8 |
| Ingestion | 8.54 × 10−4 | 3.93 × 10−8 | 3.08 × 10−5 | 2.63 × 10−5 | 9.11 × 10−4 | |
| Dermal | 1.52 × 10−3 | 6.99 × 10−8 | 5.47 × 10−5 | 4.67 × 10−5 | 1.62 × 10−3 | |
| ILCR | 2.37 × 10−3 | 1.09 × 10−7 | 8.55 × 10−5 | 7.29 × 10−5 | 2.53 × 10−3 |
| p-PAHs | a-PAHs | o-PAHs | n-PAHs | ΣPAHs | |
|---|---|---|---|---|---|
| Male adult | 11,169 | 0.51 | 403 | 343 | 11,915 |
| Female adult | 12,141 | 0.56 | 438 | 373 | 12,952 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Wu, S.; Gong, X.; Ding, T.; Lei, Y.; Sun, J.; Shen, Z. Characterization and Risk Assessment of PM2.5-Bound Polycyclic Aromatic Hydrocarbons and their Derivatives Emitted from a Typical Pesticide Factory in China. Toxics 2023, 11, 637. https://doi.org/10.3390/toxics11070637
Wang D, Wu S, Gong X, Ding T, Lei Y, Sun J, Shen Z. Characterization and Risk Assessment of PM2.5-Bound Polycyclic Aromatic Hydrocarbons and their Derivatives Emitted from a Typical Pesticide Factory in China. Toxics. 2023; 11(7):637. https://doi.org/10.3390/toxics11070637
Chicago/Turabian StyleWang, Diwei, Shengmin Wu, Xuesong Gong, Tao Ding, Yali Lei, Jian Sun, and Zhenxing Shen. 2023. "Characterization and Risk Assessment of PM2.5-Bound Polycyclic Aromatic Hydrocarbons and their Derivatives Emitted from a Typical Pesticide Factory in China" Toxics 11, no. 7: 637. https://doi.org/10.3390/toxics11070637
APA StyleWang, D., Wu, S., Gong, X., Ding, T., Lei, Y., Sun, J., & Shen, Z. (2023). Characterization and Risk Assessment of PM2.5-Bound Polycyclic Aromatic Hydrocarbons and their Derivatives Emitted from a Typical Pesticide Factory in China. Toxics, 11(7), 637. https://doi.org/10.3390/toxics11070637










