Occurrence, Distribution and Toxins of Benthic Cyanobacteria in German Lakes
Abstract
1. Introduction
2. Materials and Methods
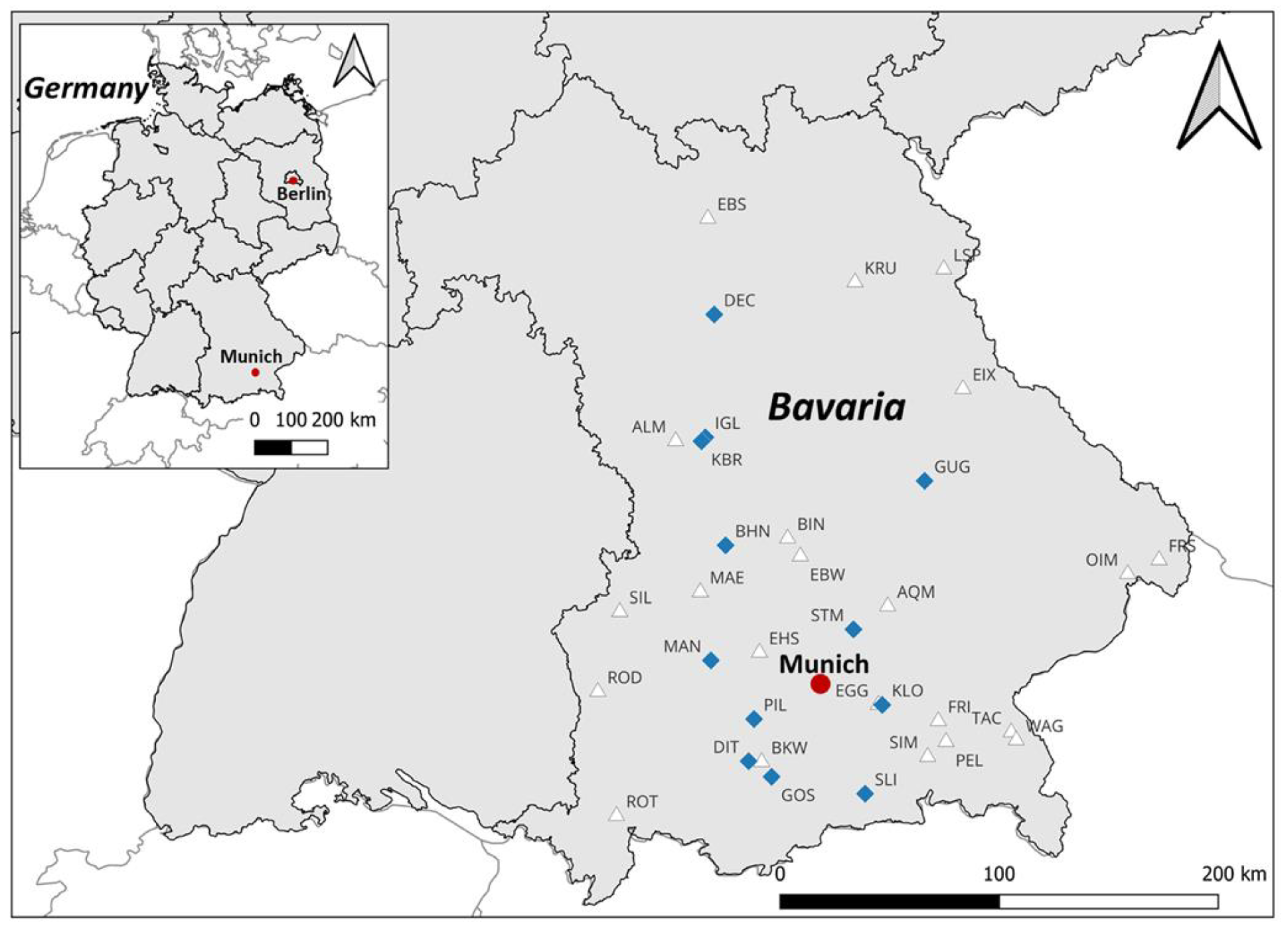
2.1. Study Site
2.2. Sampling Strategy
2.3. Microscopy
2.4. DNA Extraction and Illumina MiSeq Sequencing
Enzyme-Linked Immunosorbent Assay
2.5. Analytical Detection Method (Liquid Chromatography Coupled with Mass Spectrometry, LC-MS/MS)
2.6. Hydrochemical Analyses
3. Results
3.1. Occurrence and Distribution of Benthic Cyanobacteria in the Study Area
3.2. Community Composition
3.3. Cyanotoxins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cottingham, K.L.; Weathers, K.C.; Ewing, H.A.; Greer, M.L.; Carey, C.C. Predicting the effects of climate change on freshwater cyanobacterial blooms requires consideration of the complete cyanobacterial life cycle. J. Plankton Res. 2021, 43, 10–19. [Google Scholar] [CrossRef]
- Klawonn, I.; Nahar, N.; Walve, J.; Andersson, B.; Olofsson, M.; Svedén, J.B.; Littmann, S.; Whitehouse, M.J.; Kuypers, M.M.M.; Ploug, H. Cell-specific nitrogen-and carbon-fixation of cyanobacteria in a temperate marine system (Baltic Sea). Environ. Microbiol. 2016, 18, 4596–4609. [Google Scholar] [CrossRef]
- Sivonen, K. Cyanobacterial toxins. In Encyclopedia of Microbiology; Schaechter, M., Ed.; Elsevier: Oxford, UK, 2009; pp. 290–307. [Google Scholar]
- Svirčev, Z.; Lalić, D.; Bojadžija Savić, G.; Tokodi, N.; Drobac Backović, D.; Chen, L.; Meriluoto, J.; Codd, G.A. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef]
- Carey, C.C.; Ibelings, B.W.; Hoffmann, E.P.; Hamilton, D.P.; Brookes, J.D. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 2012, 46, 1394–1407. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Zepernick, B.N.; Wilhelm, S.W.; Bullerjahn, G.S.; Paerl, H.W. Climate change and the aquatic continuum: A cyanobacterial comeback story. Environ. Microbiol. Rep. 2023, 15, 3–12. [Google Scholar] [CrossRef]
- Mur, L.R.; Skulberg, O.M.; Utkilen, H. Cyanobacteria in the environment. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 1st ed.; Chorus, I., Bartram, J., Eds.; Taylor and Francis: Abingdon, UK, 1999. [Google Scholar]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef] [PubMed]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of current potentials and applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef]
- Vadeboncoeur, Y.; Moore, M.V.; Stewart, S.D.; Chandra, S.; Atkins, K.S.; Baron, J.S.; Bouma-Gregson, K.; Brothers, S.; Francoeur, S.N.; Genzoli, L.; et al. Blue waters, green bottoms: Benthic filamentous algal blooms are an emerging threat to clear lakes worldwide. BioScience 2021, 71, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A.; Kelly, L.T.; Bouma-Gregson, K.; Humbert, J.; Laughinghouse, H.D.; Lazorchak, J. Toxic benthic freshwater cyanobacterial proliferations: Challenges and solutions for enhancing knowledge and improving monitoring and mitigation. Freshw. Biol. 2020, 65, 1824–1842. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.; Beattie, K.A.; Scrimgeour, C.M.; Codd, G.A. Identification of anatoxin-a in benthic cyanobacteria (blue-green algae) and in associated dog poisonings at Loch Insh, Scotland. Toxicon 1992, 30, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Gugger, M.; Lenoir, S.; Berger, C.; Ledreux, A.; Druart, J.C.; Humbert, J.F.; Guette, C.; Bernard, C. First report in a river in France of the benthic cyanobacterium Phormidium favosum producing anatoxin-a associated with dog neurotoxicosis. Toxicon 2005, 45, 919–928. [Google Scholar] [CrossRef]
- Faassen, E.; Harkema, L.; Begeman, L.; Lurling, M. First report of (homo)anatoxin-a and dog neurotoxicosis after ingestion of benthic cyanobacteria in The Netherlands. Toxicon 2012, 60, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Fastner, J.; Beulker, C.; Geiser, B.; Hoffmann, A.; Kröger, R.; Teske, K.; Hoppe, J.; Mundhenk, L.; Neurath, H.; Sagebiel, D. Fatal neurotoxicosis in dogs associated with tychoplanktic, anatoxin-a producing Tychonema sp. in mesotrophic Lake Tegel, Berlin. Toxins 2018, 10, 60. [Google Scholar] [CrossRef]
- Bauer, F.; Fastner, J.; Bartha-Dima, B.; Breuer, W.; Falkenau, A.; Mayer, C.; Raeder, U. Mass occurrence of anatoxin-a-and dihydroanatoxin-a-producing Tychonema sp. in mesotrophic reservoir Mandichosee (River Lech, Germany) as a cause of neurotoxicosis in dogs. Toxins 2020, 12, 726. [Google Scholar] [CrossRef]
- Bauer, F.; Stix, M.; Bartha-Dima, B.; Geist, J.; Raeder, U. Spatio-Temporal Monitoring of Benthic Anatoxin-a-Producing Tychonema sp. in the River Lech, Germany. Toxins 2022, 14, 357. [Google Scholar] [CrossRef]
- Mez, K.; Hanselmann, K.; Preisig, H.R. Environmental conditions in high mountain lakes containing toxic benthic cyanobacteria. Hydrobiologia 1998, 368, 1–15. [Google Scholar] [CrossRef]
- Zwirglmaier, K.; Keiz, K.; Engel, M.; Geist, J.; Raeder, U. Seasonal and spatial patterns of microbial diversity along a trophic gradient in the interconnected lakes of the Osterseen Lake District, Bavaria. Front. Microbiol. 2015, 6, 1168. [Google Scholar]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, 590–596. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar]
- Krienitz, L.; Dadheech, P.K.; Fastner, J.; Kotut, K. The rise of potentially toxin producing cyanobacteria in Lake Naivasha, Great African Rift Valley, Kenya. Harmful Algae 2013, 27, 42–51. [Google Scholar] [CrossRef]
- Deutsche Einheitsverfahren zur Wasser-, Abwasser-und Schlammuntersuchung; Wasserchemische Gesellschaft in der Gesellschaft Deutscher Chemiker; Wiley-VCH: Weinheim, Germany, 2013.
- Donahue, W.F.; Turner, M.A.; Findlay, D.L.; Leavitt, P.R. The role of solar radiation in structuring the shallow benthic communities of boreal forest lakes. Limnol. Oceanogr. 2003, 48, 31–47. [Google Scholar] [CrossRef]
- Timoshkin, O.A. Groundwater contamination by sewage causes benthic algal outbreaks in the littoral zone of Lake Baikal (East Siberia). J. Great Lakes Res. 2018, 44, 230–244. [Google Scholar]
- Naranjo, R.C.; Niswonger, R.G.; Smith, D.; Rosenberry, D.; Chandra, S. Links between hydrology and seasonal variations of nutrients and periphyton in a large oligotrophic subalpine lake. J. Hydrol. 2019, 568, 877–890. [Google Scholar] [CrossRef]
- Wood, S.A.; Depree, C.; Brown, L.; McAllister, T.; Hawes, I. Entrapped sediments as a source of phosphorus in epilithic cyanobacterial proliferations in low nutrient rivers. PLoS ONE 2015, 10, e0141063. [Google Scholar] [CrossRef]
- Padisák, J.; Soróczki-Pintér, É.; Rezner, Z. Sinking properties of some phytoplankton shapes and the relation of form resistance to morphological diversity of plankton—An experimental study. Hydrobiologia 2003, 500, 243–257. [Google Scholar]
- Belykh, O.I.; Sorokovikova, E.G. Autotrophic picoplankton in Lake Baikal: Abundance, dynamics, and distribution. Aquatic. Ecosyst. Health Manag. 2003, 6, 251–261. [Google Scholar]
- Ruber, J.; Bauer, F.R.; Millard, A.D.; Raeder, U.; Geist, J.; Zwirglmaier, K. Synechococcus diversity along a trophic gradient in the Osterseen Lake District, Bavaria. Microbiology 2016, 162, 2053–2063. [Google Scholar]
- Sorokovikova, E.; Belykh, O.; Krasnopeev, A.; Potapov, S.; Tikhonova, I.; Khanaev, I.; Kabilov, M.; Baturina, O.; Podlesnaya, G.; Timoshkin, O. First data on cyanobacterial biodiversity in benthic biofilms during mass mortality of endemic sponges in Lake Baikal. J. Great Lakes Res. 2020, 46, 75–84. [Google Scholar] [CrossRef]
- Belykh, O.I.; Fedorova, G.A.; Kuzmin, A.V.; Tikhonova, I.V.; Timoshkin, O.A.; Sorokovikova, E.G. Microcystins in cyanobacterial biofilms from the littoral zone of Lake Baikal. Mosc. Univ. Biol. Sci. Bull. 2017, 72, 225–231. [Google Scholar] [CrossRef]
- Richardson, L.L.; Sekar, R.; Myers, J.L.; Gantar, M.; Voss, J.D.; Kaczmarsky, L.; Remily, E.R.; Boyer, G.L.; Zimba, P.V. The presence of the cyanobacterial toxin microcystin in black band disease of corals. FEMS Microbiol. Lett. 2007, 272, 182–187. [Google Scholar] [CrossRef]
- Wood, S.A.; Heath, M.W.; Holland, P.T.; Munday, R.; McGregor, G.B.; Ryan, K.G. Identification of a benthic microcystin-producing filamentous cyanobacterium (Oscillatoriales) associated with a dog poisoning in New Zealand. Toxicon 2010, 55, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Shalygin, S.; Shalygina, R.; Johansen, J.R.; Pietrasiak, N.; Berrendero Gómez, E.; Bohunická, M.; Mares, J.; Sheil, C.A. Cyanomargarita gen. nov. (Nostocales, Cyanobacteria): Convergent evolution resulting in a cryptic genus. J. Phycol. 2017, 53, 762–777. [Google Scholar] [CrossRef]
- Aboal, M.; Puig, M.A. Microcystin production in Rivularia colonies of calcareous streams from Mediterranean Spanish basins. Algol. Stud. 2009, 130, 39. [Google Scholar] [CrossRef]
- Smith, F.M.; Wood, S.A.; Wilks, T.; Kelly, D.; Broady, P.A.; Williamson, W.; Graw, S. Survey of Scytonema (Cyanobacteria) and associated saxitoxins in the littoral zone of recreational lakes in Canterbury, New Zealand. Phycologia 2012, 51, 542–551. [Google Scholar] [CrossRef]
- Belykh, O.I.; Tikhonova, I.V.; Kuzmin, A.V.; Sorokovikova, E.G.; Fedorova, G.A.; Khanaev, I.V.; Sherbakova, T.A.; Timoshkin, O.A. First detection of benthic cyanobacteria in Lake Baikal producing paralytic shellfish toxins. Toxicon 2016, 121, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Méjean, A.; Mann, S.; Maldiney, T.; Vassiliadis, G.; Lequin, O.; Ploux, O. Evidence that biosynthesis of the neurotoxic alkaloids anatoxin-a and homoanatoxin-a in the cyanobacterium Oscillatoria PCC 6506 occurs on a modular polyketide synthase initiated by l-proline. J. Am. Chem. Soc. 2009, 131, 7512–7513. [Google Scholar] [CrossRef]
- Wood, S.A.; Biessy, L.; Puddick, J. Anatoxins are consistently released into the water of streams with Microcoleus autumnalis-dominated (cyanobacteria) proliferations. Harmful Algae 2018, 80, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Puddick, J.; van Ginkel, R.; Page, C.D.; Murray, J.S.; Greenhough, H.E.; Bowater, J.; Selwood, A.I.; Wood, S.A.; Prinsep, M.R.; Truman, P. Acute toxicity of dihydroanatoxin-a from Microcoleus autumnalis in comparison to anatoxin-a. Chemosphere 2021, 263, 127937. [Google Scholar] [PubMed]
- Hamill, K.D. Toxicity in benthic freshwater cyanobacteria (blue-green algae): First observations in New Zealand. N. Z. J. Mar. Freshw 2001, 35, 1057–1059. [Google Scholar]
- Mazmouz, R.; Chapuis-Hugon, F.; Mann, S.; Pichon, V.; Méjean, A.; Ploux, O. Biosynthesis of cylindrospermopsin and 7-epicylindrospermopsin in Oscillatoria sp. strain PCC 6506: Identification of the cyr gene cluster and toxin analysis. Appl. Environ. Microbiol. 2010, 76, 4943–4949. [Google Scholar]
- Radhakrishnan, B.; Prasanna, R.; Jaiswal, P.; Nayak, S.; Dureja, P. Modulation of biocidal activity of Calothrix sp. and Anabaena sp. by environmental factors. Biologia 2009, 64, 881–889. [Google Scholar]
- Stoyneva-Gärtner, M.P.; Descy, J.P.; Latli, A.; Uzunov, B.A.; Pavlova, V.T.; Bratanova, Z.; Babica, P.; Marsalek, B.; Meriluoto, J.; Spoof, L. Assessment of cyanoprokaryote blooms and of cyanotoxins in Bulgaria in a 15-years period (2000–2015). Adv. Oceanogr. Limnol. 2017, 8. [Google Scholar] [CrossRef][Green Version]
- Ahmed, W.A.; El-Semary, N.A.; Abd El-Hameed, O.M.; El Tawill, G.; Ibrahim, D.M. Bioactivity and cytotoxic effect of cyanobacterial toxin against hepatocellular carcinoma. J. Cancer Sci. Ther. 2017, 9, 505–511. [Google Scholar]
- Rangel, M.; Brunetti, R.L.; Garcia, A.N.; Cambui, C.C.N.; Conserva, G.A.A.; Neves, A.C.; Sant’Anna, E.L.; Carvalho, L.R. Acute effects of three Geitlerinema spp. (cyanobacteria) extracts administrated in mice: Symptoms and histopathological aspects. Phytochem. Rev. 2013, 12, 543–553. [Google Scholar]
- Rangel, M.; Martins, J.C.; Garcia, A.N.; Conserva, G.A.; Costa-Neves, A.; Sant’Anna, C.L.; De Carvalho, L.R. Analysis of the toxicity and histopathology induced by the oral administration of Pseudanabaena galeata and Geitlerinema splendidum (cyanobacteria) extracts to mice. Mar. Drugs 2014, 12, 508–524. [Google Scholar]
- Gantar, M.; Sekar, R.; Richardson, L.L. Cyanotoxins from black band disease of corals and from other coral reef environments. Microb. Ecol. 2009, 58, 856–864. [Google Scholar]
- Izaguirre, G.; Taylor, W.D. A guide to geosmin-and MIB-producing cyanobacteria in the United States. Water Sci. Technol. 2004, 49, 19–24. [Google Scholar]
- Melo, N.; Wolff, G.H.; Costa-da-Silva, A.L.; Arribas, R.; Triana, M.F.; Gugger, M.; Riffel, J.A.; DeGennaro, M.; Stensmyr, M.C. Geosmin attracts Aedes aegypti mosquitoes to oviposition sites. Curr. Biol. 2020, 30, 127–134. [Google Scholar] [PubMed]
- Zhang, M.; Zhang, Y.; Yang, Z.; Wei, L.; Yang, W.; Chen, C.; Kong, F. Spatial and seasonal shifts in bloom-forming cyanobacteria in Lake Chaohu: Patterns and driving factors. Phycol. Res. 2016, 64, 44–55. [Google Scholar]
- Izaguirre, G.; Taylor, W.D. Geosmin and 2-methylisoborneol production in a major aqueduct system. Water Sci. Technol. 1995, 31, 41–48. [Google Scholar]
- Bates, H.A.; Rapoport, H. Synthesis of anatoxin-a via intramolecular cyclization of iminium salts. J. Am. Chem. Soc. 1979, 101, 1259–1265. [Google Scholar]
- Wonnacott, S.; Jackman, S.; Swanson, K.L.; Rapoport, H.; Albuquerque, E.X. Nicotinic pharmacology of anatoxin analogs. Side chain structure-activity relationships at neuronal nicotinic ligand binding sites. J. Pharmacol. Exp. Ther. 1991, 259, 387–391. [Google Scholar] [PubMed]
- Wood, S.A.; Smith, F.M.; Heath, M.W.; Palfroy, T.; Gaw, S.; Young, R.G.; Ryan, K.G. Within-mat variability in anatoxin-a and homoanatoxin-a production among benthic Phormidium (cyanobacteria) strains. Toxins 2012, 4, 900–912. [Google Scholar]
- Christensen, V.G.; Khan, E. Freshwater neurotoxins and concerns for human, animal, and ecosystem health: A review of anatoxin-a and saxitoxin. Sci. Total Environ. 2020, 736, 139515. [Google Scholar]
- Sukenik, A.; Kaplan, A. Cyanobacterial harmful algal blooms in aquatic ecosystems: A comprehensive outlook on current and emerging mitigation and control approaches. Microorganisms 2021, 9, 1472. [Google Scholar]
- Gupta, V.; Ratha, S.K.; Sood, A.; Chaudhary, V.; Prasanna, R. New insights into the biodiversity and applications of cyanobacteria (blue-green algae)—Prospects and challenges. Algal Res. 2013, 2, 79–97. [Google Scholar]
- Drobac, D.; Tokodi, N.; Kiprovski, B.; Malenčić, D.; Važić, T.; Nybom, S.; Meriluoto, J.; Svirčev, Z. Microcystin accumulation and potential effects on antioxidant capacity of leaves and fruits of Capsicum annuum. J. Toxicol. Environ. Health Part A 2017, 80, 145–154. [Google Scholar]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; Taylor and Francis: Abingdon, UK, 2021; pp. 1–858. [Google Scholar]
- Bauer, F.; Schmalz, C.W. Occurrence, Distribution and Toxins of Benthic Cyanobacteria in German Lakes; Open Scientific Framework, 2023. [Google Scholar] [CrossRef]

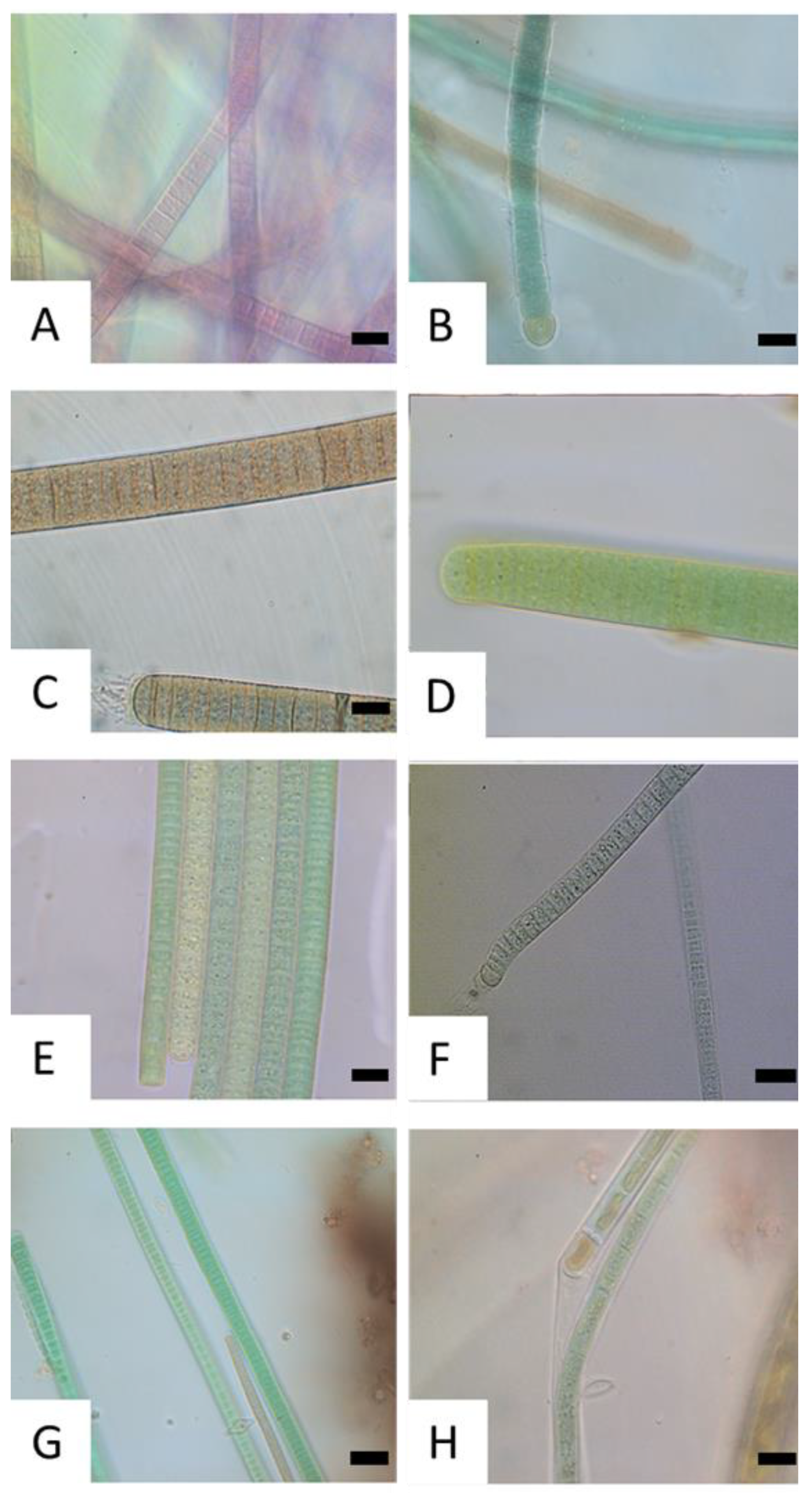



| Taxon ** | BHN | STM | PIL | SLI | DEC | DIT | GOS | GUG | IGL | KBR | KLO | MAN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anabaena | - | - | - | - | - | + | - | - | - | - | - | - |
| Aphanizomenon | - | - | + | - | - | - | - | - | - | - | - | - |
| Caenarcaniphilales ** | - | - | - | - | - | - | - | - | - | + | - | - |
| Calothrix | - | - | + | - | + | - | - | - | - | - | + | - |
| Candidatus obscuribacter | - | - | - | - | + | - | - | - | - | - | - | - |
| Cyanobium | + | - | + | + | + * | + | + | + | + | + * | + | + |
| Cyanomargarita | - | - | - | + * | - | - | - | - | - | - | - | - |
| Cyanothece | - | - | - | - | - | - | - | - | - | + | - | - |
| Dactylothamnos | - | - | - | - | - | - | - | - | - | - | + | - |
| Geitlerinema | - | - | - | - | - | - | - | - | - | - | - | + |
| Geminocystis | - | - | - | - | - | - | - | - | - | + | - | - |
| Gloeobacter | - | - | + | + | - | - | - | - | - | - | - | - |
| Kamptonema | - | + * | - | - | - | + | + | - | - | - | - | - |
| Leptolyngbya | - | - | - | - | + | - | - | - | + | - | + * | - |
| Leptolyngbyaceae ** | - | - | + | + | + | - | - | - | + * | + | + | - |
| Limnothrix | - | - | - | - | + | - | - | - | - | - | - | - |
| Microcoleus | - | - | - | - | - | + | - | - | - | - | + | - |
| Microcystaceae ** | - | - | - | - | - | - | - | - | - | + | - | - |
| Microcystis | - | - | + | - | - | - | - | - | - | - | - | - |
| Neolyngbya | - | - | - | - | - | - | + | + * | - | - | - | - |
| Nodosilinea | - | - | - | - | + | - | - | - | - | + | + | - |
| Oscillatoria | + | - | - | - | - | + * | + * | - | - | - | - | - |
| Phormidiaceae ** | - | - | - | - | - | - | - | + | - | - | - | - |
| Phormidium | + | - | - | - | - | - | - | - | - | - | - | - |
| Planktothrix | - | + | - | - | - | - | - | + | - | + | - | - |
| Pleurocapsa | - | - | + * | - | - | - | - | - | - | - | - | - |
| Prochlotothrix | + | - | - | - | - | - | - | - | - | - | - | - |
| Pseudanabaena | + | - | - | - | + | - | - | - | - | + | - | + |
| Pseudanabaenaceae ** | - | - | + | + | - | - | - | - | - | - | - | - |
| Scytolyngbya | - | - | - | - | - | - | - | - | - | - | + | - |
| Scytonema | - | - | - | - | - | - | - | - | - | - | + | - |
| Sericytochromatia | - | - | - | - | - | - | - | - | - | + | - | - |
| Snowella | - | - | - | - | - | + | - | - | - | - | - | - |
| Synechococcus | - | - | - | + | - | - | - | - | - | - | - | - |
| Synechocystis | - | - | + | - | - | - | - | - | - | - | - | - |
| Tenebriella | + | - | - | - | - | - | + | - | - | - | - | - |
| Tychonema | - | - | - | - | - | + | + | - | - | + | - | + * |
| Other | + * | + | + | + | + | + | + | + | + | + | + | + |
| Total number of genera | 6 | 3 | 9 | 6 | 8 | 7 | 6 | 4 | 3 | 11 | 9 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, F.; Wolfschlaeger, I.; Geist, J.; Fastner, J.; Schmalz, C.W.; Raeder, U. Occurrence, Distribution and Toxins of Benthic Cyanobacteria in German Lakes. Toxics 2023, 11, 643. https://doi.org/10.3390/toxics11080643
Bauer F, Wolfschlaeger I, Geist J, Fastner J, Schmalz CW, Raeder U. Occurrence, Distribution and Toxins of Benthic Cyanobacteria in German Lakes. Toxics. 2023; 11(8):643. https://doi.org/10.3390/toxics11080643
Chicago/Turabian StyleBauer, Franziska, Immanuel Wolfschlaeger, Juergen Geist, Jutta Fastner, Carina Wiena Schmalz, and Uta Raeder. 2023. "Occurrence, Distribution and Toxins of Benthic Cyanobacteria in German Lakes" Toxics 11, no. 8: 643. https://doi.org/10.3390/toxics11080643
APA StyleBauer, F., Wolfschlaeger, I., Geist, J., Fastner, J., Schmalz, C. W., & Raeder, U. (2023). Occurrence, Distribution and Toxins of Benthic Cyanobacteria in German Lakes. Toxics, 11(8), 643. https://doi.org/10.3390/toxics11080643








