An In Silico Analysis of Synthetic and Natural Compounds as Inhibitors of Nitrous Oxide Reductase (N2OR) and Nitrite Reductase (NIR)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ligand Preparation
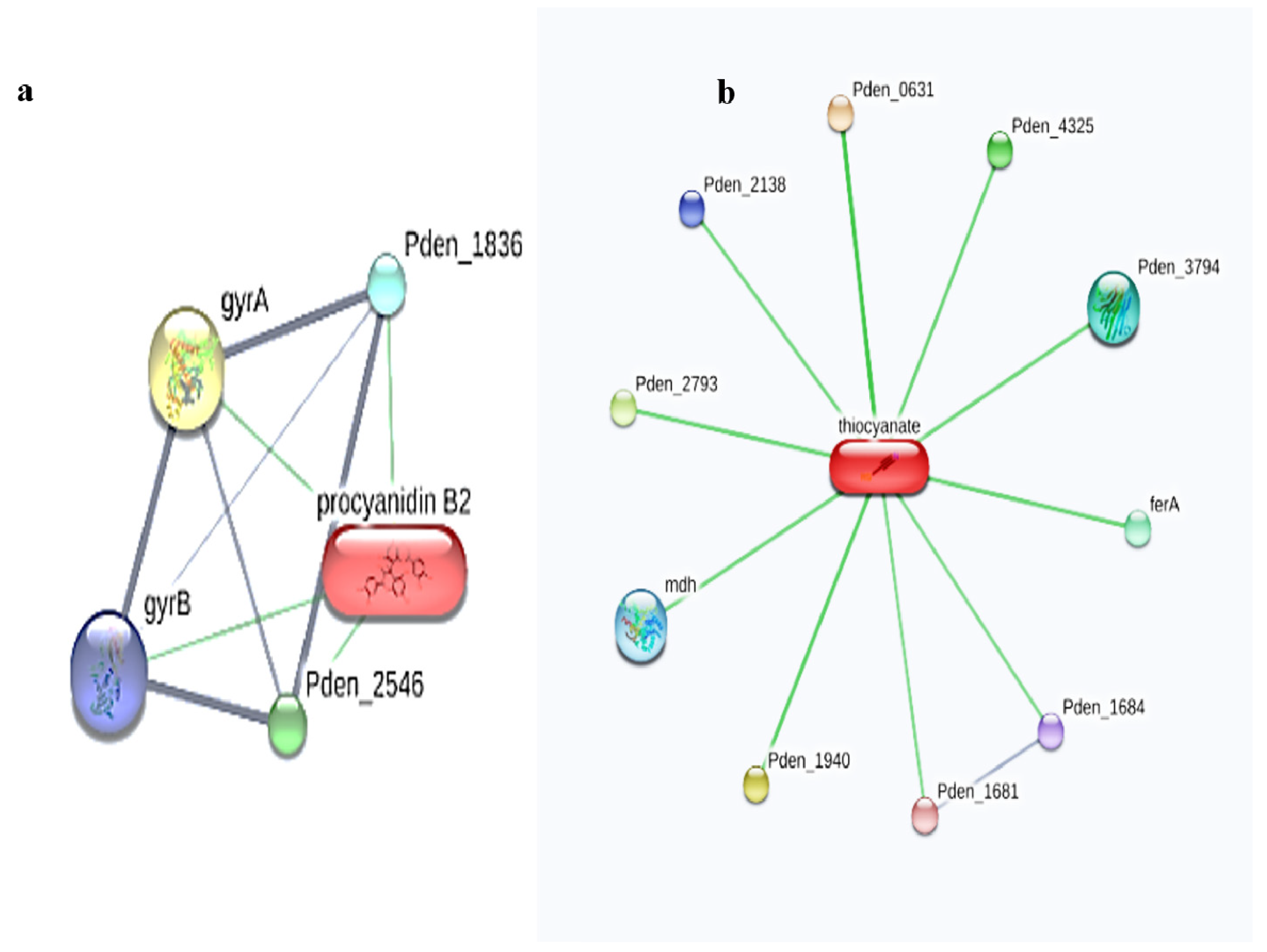
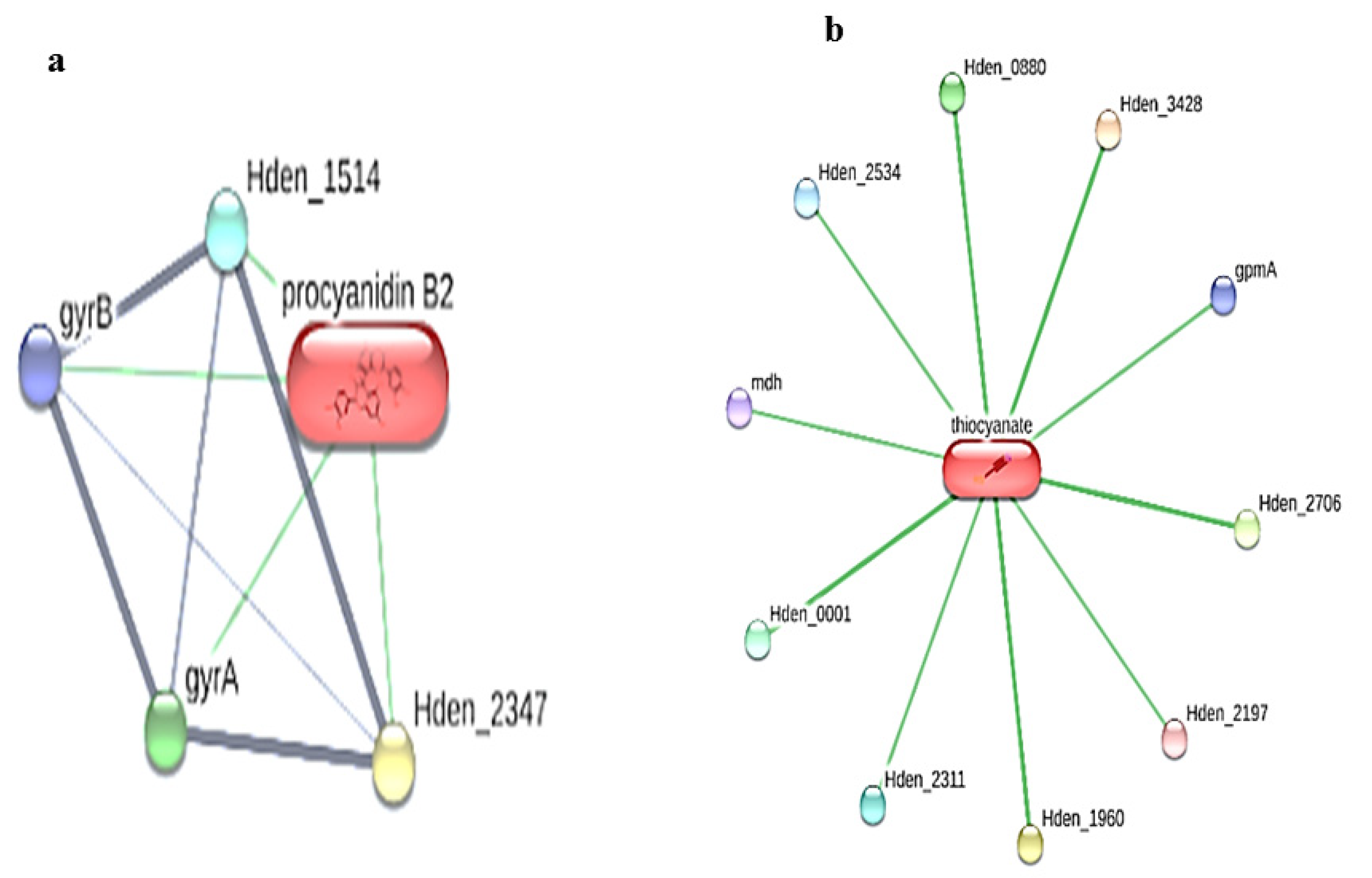
2.2. Protein Network Interaction Analysis
2.3. Prediction of Insecticide-Likeness Property
2.4. Prediction of Toxicity
2.5. Target Protein Identification and Preparation
2.6. PatchDock Study
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paredes, D.; Kuschk, P.; Mbwette, T.S.A.; Stange, F.; Müller, R.A.; Köser, H. New aspects of microbial nitrogen transformations in the context of wastewater treatment–a review. Eng. Life Sci. 2007, 7, 13–25. [Google Scholar]
- Liao, P.B.; Mayo, R.D. Salmonid hatchery water reuse systems. Aquaculture 1972, 1, 317–335. [Google Scholar]
- Easter, C. Water Chemistry Characterization and Component Performance of a Recirculating Aquaculture System Producing Hybrid Striped Bass. Master’s Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VI, USA, 1992, unpublished. [Google Scholar]
- Jones, J.R.F. Fish and River Pollution; Butterworth Inc.: London, UK, 1964. [Google Scholar]
- Throbäck, I.N.; Enwall, K.; Jarvis, Å.; Hallin, S. Reassessing PCR primers targeting nirS, nirK, and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar]
- Kiran, U.; Patra, D.D. Medicinal and aromatic plant materials as nitrification inhibitors for augmenting yield and nitrogen uptake of Japanese mint (Mentha arvensis L. Var. Piperascens). Bioresour. Technol. 2003, 86, 267–276. [Google Scholar]
- Kaleem Abbasi, M.; Manzoor, M. Effect of soil-applied calcium carbide and plant derivatives on nitrification inhibition and plant growth promotion. Int. J. Environ. Sci. Technol. 2013, 10, 961–972. [Google Scholar]
- Opoku, A.; Chaves, B.; De Neve, S. Neem seed oil: A potent nitrification inhibitor to control nitrate leaching after incorporation of crop residues. Biol. Agric. Hortic. 2014, 30, 145–152. [Google Scholar]
- Zhao, M.; Zhao, H.; Du, Q.; Shi, Y. Inhibitory effects of tropical medicinal plant extracts on urea hydrolysis and nitrification in soil: A preliminary study. HortScience 2015, 50, 744–749. [Google Scholar]
- Suescun, F.; Paulino, L.; Zagal, E.; Ovalle, C.; Muñoz, C. Plant extracts from the Mediterranean zone of Chile potentially affect soil microbial activity related to N transformations: A laboratory experiment. Acta Agric. Scand. Sect. B Soil Plant Sci. 2012, 62, 556–564. [Google Scholar]
- Mathialagan, R.; Mansor, N.; Al-Khateeb, B.; Mohamad, M.H.; Shamsuddin, M.R. Evaluation of allicin as soil urease inhibitor. Procedia Eng. 2017, 184, 449–459. [Google Scholar] [CrossRef]
- Norton, J.; Ouyang, Y. Controls and adaptive management of nitrification in agricultural soils. Front. Microbiol. 2019, 10, 1931. [Google Scholar]
- Subbarao, G.V.; Rondon, M.; Ito, O.; Ishikawa, T.; Rao, I.M.; Nakahara, K.; Lascano, C.; Berry, W.L. Biological nitrification inhibition (BNI)—Is it a widespread phenomenon? Plant Soil 2007, 294, 5–18. [Google Scholar]
- Zakir, H.A.; Subbarao, G.V.; Pearse, S.J.; Gopalakrishnan, S.; Ito, O.; Ishikawa, T.; Kawano, N.; Nakahara, K.; Yoshihashi, T.; Ono, H.; et al. Detection, isolation and characterization of a root-exuded compound, methyl 3-(4-hydroxyphenyl) propionate, responsible for biological nitrification inhibition by sorghum (Sorghum bicolor). New Phytol. 2008, 180, 442–451. [Google Scholar]
- Erickson, A.J.; Ramsewak, R.S.; Smucker, A.J.; Nair, M.G. Nitrification inhibitors from the roots of Leucaena leucocephala. J. Agric. Food Chem. 2000, 48, 6174–6177. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, G.V.; Nakahara, K.; Hurtado, M.D.; Ono, H.; Moreta, D.E.; Salcedo, A.F.; Yoshihashi, A.T.; Ishikawa, T.; Ishitani, M.; Ohnishi-Kameyama, M.; et al. Evidence for biological nitrification inhibition in Brachiaria pastures. Proc. Natl. Acad. Sci. USA 2009, 106, 17302–17307. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lu, Y.; Yu, F.; Kronzucker, H.J.; Shi, W. Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency. New Phytol. 2016, 212, 646–656. [Google Scholar] [PubMed] [Green Version]
- Subbarao, G.V.; Sahrawat, K.L.; Nakahara, K.; Ishikawa, T.; Kishii, M.; Rao, I.M.; Hash, C.T.; George, T.S.; Rao, P.S.; Nardi, P.; et al. Biological Nitrification inhibition—A novel strategy to regulate nitrification in agricultural systems. Adv. Agron. 2012, 114, 249–302. [Google Scholar]
- Nardi, P.; Laanbroek, H.J.; Nicol, G.W.; Renella, G.; Cardinale, M.; Pietramellara, G.; Weckwerth, W.; Trinchera, A.; Ghatak, A.; Nannipieri, P. Biological nitrification inhibition in the rhizosphere: Determining interactions and impact on microbially mediated processes and potential applications. FEMS Microbiol. Rev. 2020, 44, 874–908. [Google Scholar] [PubMed]
- Frimpong, K.A.; Yawson, D.O.; Adu, M.O. Nitrous oxide emissions from soils amended with polyphenols and cowpea residues. West Afr. J. Appl. Ecol. 2014, 22, 69–85. [Google Scholar]
- Chaves, B.; De Neve, S.; del Carmen Lillo Cabrera, M.; Boeckx, P.; Van Cleemput, O.; Hofman, G. The effect of mixing organic biological waste materials and high-N crop residues on the short-time N 2 O emission from horticultural soil in model experiments. Biol. Fertil. Soils 2005, 41, 411–418. [Google Scholar]
- Adamczyk, S.; Kiikkilä, O.; Kitunen, V.; Smolander, A. Potential response of soil processes to diterpenes, triterpenes, and tannins: Nitrification, growth of microorganisms and precipitation of proteins. Appl. Soil. Ecol. 2013, 67, 47–52. [Google Scholar]
- Sauder, L.A.; Ross, A.A.; Neufeld, J.D. Nitric oxide scavengers differentially inhibit ammonia oxidation in ammonia-oxidizing archaea and bacteria. FEMS Microbiol. Lett. 2016, 363, fnw052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girardi, J.P.; Korz, S.; Muñoz, K.; Jamin, J.; Schmitz, D.; Rösch, V.; Riess, K.; Schützenmeister, K.; Jungkunst, H.F.; Brunn, M. Nitrification inhibition by polyphenols from invasive Fallopia japonica under copper stress. J. Plant Nutr. Soil. Sci. 2022, 185, 923–934. [Google Scholar]
- Gao, J.; Zhao, G. Potentials of using dietary plant secondary metabolites to mitigate nitrous oxide emissions from the excreta of cattle: Impacts, mechanisms, and perspectives. Anim. Nutr. 2022, 9, 327–334. [Google Scholar]
- Han, F.; Zhang, M.; Liu, Z.; Shang, H.; Li, Q.; Zhou, W. Dynamic characteristics of microbial community and soluble microbial products in partial nitrification biofilm system developed from marine sediments treating high salinity wastewater. J. Environ. Manag. 2021, 290, 112586. [Google Scholar]
- Radhakrishnan, N.; Prabhakaran, V.S.; Wadaan, M.A.; Baabbad, A.; Vinayagam, R.; Kang, S.G. STITCH, Physicochemical, ADMET, and In Silico Analysis of Selected Mikania Constituents as Anti-Inflammatory Agents. Processes 2023, 11, 1722. [Google Scholar]
- Szklarczyk, D.; Santos, A.; Von Mering, C.; Jensen, L.J.; Bork, P.; Kuhn, M. STITCH 5: Augmenting protein–chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 2016, 44, D380–D384. [Google Scholar] [PubMed] [Green Version]
- Tice, C.M. Selecting the right compounds for screening: Does Lipinski’s Rule of 5 for pharmaceuticals apply to agrochemicals? Pest Manag. Sci. Former. Pestic. Sci. 2001, 57, 3–16. [Google Scholar]
- Avram, S.; Funar-Timofei, S.; Borota, A.; Chennamaneni, S.R.; Manchala, A.K.; Muresan, S. Quantitative estimation of pesticide-likeness for agrochemical discovery. J. Cheminformatics 2014, 6, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, S.; Prabhakaran, V.S.; Narayanaswamy, R. In Silico analysis of Cissus rotundifolia constituents as human neutrophil elastase (HNE), matrix metalloproteinases (MMP 2 and MMP 9), and tyrosinase inhibitors. Appl. Biochem. Biotechnol. 2022, 194, 232–245. [Google Scholar] [CrossRef]
- Moreira-Filho, J.T.; Braga, R.C.; Lemos, J.M.; Alves, V.M.; Borba, J.V.; Costa, W.S.; Kleinstreuer, N.; Muratov, E.N.; Andrade, C.H.; Neves, B.J. BeetoxAI: An artificial intelligence-based web app to assess the acute toxicity of chemicals to honey bees. Artif. Intell. Life Sci. 2021, 1, 100013. [Google Scholar]
- Crisan, L.; Funar-Timofei, S.; Borota, A. Homology Modeling and Molecular Docking Approaches for the Proposal of Novel Insecticides against the African Malaria Mosquito (Anopheles gambiae). Molecules 2022, 27, 3846. [Google Scholar] [CrossRef]
- Schneidman-Duhovny, D.; Inbar, Y.; Nussinov, R.; Wolfson, H.J. PatchDock and SymmDock: Servers for rigid and symmetric docking. Nucleic Acids Res. 2005, 33, W363–W367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantarel, A.A.; Pommier, T.; Desclos-Theveniau, M.; Diquélou, S.; Dumont, M.; Grassein, F.; Kastl, E.M.; Grigulis, K.; Laîné, P.; Lavorel, S.; et al. Using plant traits to explain plant–microbe relationships involved in nitrogen acquisition. Ecology 2015, 96, 788–799. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Arango, J.; Masahiro, K.; Hooper, A.M.; Yoshihashi, T.; Ando, Y.; Nakahara, K.; Deshpande, S.; Ortiz-Monasterio, I.; Ishitani, M.; et al. Genetic mitigation strategies to tackle agricultural GHG emissions: The case for biological nitrification inhibition technology. Plant Sci. 2017, 262, 165–168. [Google Scholar] [PubMed]
- Upadhyay, R.K.; Tewari, S.K.; Patra, D.D. Natural nitrification inhibitors for higher nitrogen use efficiency, crop yield, and for curtailing global warming. J. Trop. Agric. 2011, 49, 19–24. [Google Scholar]
- Sahrawat, K.L. Nitrification inhibitors, with emphasis on natural products, and the persistence of fertilizer nitrogen in the soil. In Nitrogen Economy in Tropical Soils: Proceedings of the International Symposium on Nitrogen Economy in Tropical Soils, Held in Trinidad, WI, USA, 9–14 January 1994; Springer: Dordrecht, The Netherlands, 1996; pp. 379–388. [Google Scholar]
- Sahrawat, K.L.; Mukherjee, S.K. Nitrification inhibitors: Studies with karanjin, a furanolflavonoid from karanja (Pongamia glabra) seeds. Plant Soil 1977, 47, 27–36. [Google Scholar]
- Jain, J.M.; Narayanasamy, G.; Sarkar, M.C.; Datta, M.N. An evaluation of nitrification retardation property of Citrullus colosynthis cake and its influence on yield and N uptake by wheat. J. Indian Soc. Soil Sci. 1980, 28, 480–484. [Google Scholar]
- Nawarathna, K.K.; Dandeniya, W.S.; Dharmakeerthi, R.S.; Weerasinghe, P. Evaluating the nitrification inhibition potential of selected botanicals and their non-target effects. Can. J. Soil Sci. 2021, 102, 489–504. [Google Scholar] [CrossRef]
- Prasad, R.; Power, J.F. Nitrification inhibitors for agriculture, health, and the environment. Adv. Agron. 1995, 54, 233–281. [Google Scholar]
- Ram, M.; Patra, D.D.; Singh, D.V. Effect of nitrification inhibitors on herb and essential oil yield of Japanese mint on sandy soil. Fertil. Res. 1995, 44, 17–21. [Google Scholar] [CrossRef]
- Patra, D.D.; Anwar, M.; Chand, S.; Kiran, U.; Rajput, D.K.; Kumar, S. Nimin and Mentha spicata oil as nitrification inhibitors for optimum yield of Japanese mint. Commun. Soil Sci. Plant Anal. 2002, 33, 451–460. [Google Scholar] [CrossRef]
- Haile, W.; Mala, T.; Osotsapar, Y.; Verasan, V. Nitrification inhibiting ability of Ethiopian medicinal herbs as affected by soil types. Kamphaengsaen Acadamic J. 2006, 4, 61–73. [Google Scholar]
- Pande, P.; Chand, S.; Pandey, A.; Patra, D.D. Effect of sole and conjoint application of iron and manganese on herb yield, nutrient uptake, oil quality vis-a-vis their optimal level in spearmint (Mentha spicata Linn. emend. Nathh. cv. ‘Arka’). Indian J. Nat. Prod. Resour. 2011, 2, 242–249. [Google Scholar]
- Gopalakrishnan, S.; Subbarao, G.V.; Nakahara, K.; Yoshihashi, T.; Ito, O.; Maeda, I.; Ono, H.; Yoshida, M. Nitrification inhibitors from the root tissues of Brachiaria humidicola, a tropical grass. J. Agric. Food Chem. 2007, 55, 1385–1388. [Google Scholar] [CrossRef]
- Ghoneim, A. Examination of nitrification inhibition by sorghum (Sorghum bicolor) in soil around its roots. Proc. Int. Acad. Ecol. Environ. Sci. 2014, 4, 30. [Google Scholar]
- Kristjansson, J.K.; Hollocher, T.C. First practical assay for soluble nitrous oxide reductase of denitrifying bacteria and partial kinetic characterization. J. Biol. Chem. 1980, 255, 704–707. [Google Scholar] [CrossRef]
- Kuypers, M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Galland, W.; Piola, F.; Mathieu, C.; Bouladra, L.; Simon, L.; Haichar, F.E.Z. Does biological denitrification inhibition (BDI) in the field induce an increase in plant growth and nutrition in Apium graveolens L. grown for a long period? Microorganisms 2020, 8, 1204. [Google Scholar] [CrossRef]
- Heger, A.; Becker, J.N.; Navas, L.K.V.; Eschenbach, A. Factors controlling soil organic carbon stocks in hardwood floodplain forests of the lower middle Elbe River. Geoderma 2021, 404, 115389. [Google Scholar] [CrossRef]
- Barrena, I.; Menéndez, S.; Correa-Galeote, D.; Vega-Mas, I.; Bedmar, E.J.; González-Murua, C.; Estavillo, J.M. Soil water content modulates the effect of the nitrification inhibitor 3, 4-dimethyl pyrazole phosphate (DMPP) on nitrifying and denitrifying bacteria. Geoderma 2017, 303, 1–8. [Google Scholar] [CrossRef]
- Berks, B.C.; Baratta, D.; Richardson, D.J.; Ferguson, S.J. Purification and characterization of a nitrous oxide reductase from Thiosphaera pantotropha. FEBS J. 1993, 212, 467–476. [Google Scholar]
- Bardon, C.; Poly, F.; el ZaharHaichar, F.; Le Roux, X.; Simon, L.; Meiffren, G.; Comte, G.; Rouifed, S.; Piola, F. Biological denitrification inhibition (BDI) with procyanidins induces modification of root traits, growth and N status in Fallopia x bohemica. Soil Biol. Biochem. 2017, 107, 41–49. [Google Scholar] [CrossRef]
- Bardon, C.; Poly, F.; Piola, F.; Pancton, M.; Comte, G.; Meiffren, G.; Haichar, F.E. Mechanism of biological denitrification inhibition: Procyanidins induce an allosteric transition of the membrane-bound nitrate reductase through membrane alteration. FEMS Microbiol. Ecol. 2016, 92, fiw034. [Google Scholar] [CrossRef] [Green Version]
- Rahmadani, K.; Manguntungi, B.; Arwansyah, A.; Jumadi, O.; Khizbullah, M.A.; Hidayat, A.; Ayunda, N.G.; Faiz, M.; Vanggy, L.R.; Septiawati, E. Efficiency of nitrification inhibitor on designing nitrogen fertilizer by neem compounds based on molecular docking. Trends Sci. 2023, 20, 6395. [Google Scholar] [CrossRef]
- Boogerd, F.C.; Appeldoorn, K.J.; Stouthamer, A.H. Effects of electron transport inhibitors and uncouplers on denitrification in Paracoccus denitrificans. FEMS Microbiol. Lett. 1983, 20, 455–460. [Google Scholar] [CrossRef]
- Matsuoka, M.; Kumar, A.; Muddassar, M.; Matsuyama, A.; Yoshida, M.; Zhang, K.Y. Discovery of fungal denitrification inhibitors by targeting copper nitrite reductase from Fusarium oxysporum. J. Chem. Inf. Model. 2017, 57, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Martineau, C.; Villeneuve, C.; Mauffrey, F.; Villemur, R. Complete genome sequence of Hyphomicrobium nitrativorans strain NL23, a denitrifying bacterium isolated from biofilm of a methanol-fed denitrification system treating seawater at the Montreal Biodome. Genome Announc. 2014, 2, e01165-13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brittain, T.; Blackmore, R.; Greenwood, C.; Thomson, A.J. Bacterial nitrite-reducing enzymes. FEBS J. 1992, 209, 793–802. [Google Scholar] [CrossRef] [PubMed]
| Ligand Name | Structure |
|---|---|
| Ammonia | NH3 |
| Arabinoxylan | 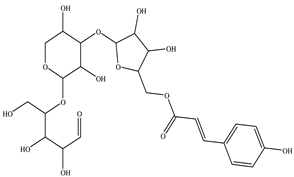 |
| Anthracene−1−carbonyl azide |  |
| 2−Anthracene carboxylic acid azide | 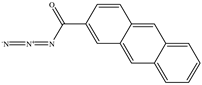 |
| Benzyl azide |  |
| 4−Chloro−5−dimethylamino−2−phenyl−3− (2H)−pyridazinone | 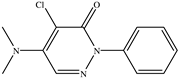 |
| Dicyandiamide |  |
| 3,5−Dimethylpyrazole |  |
| 3, 4−Dimethylpyrazole phosphate | 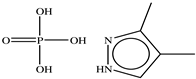 |
| Ethyl azide |  |
| 5−Iodonaphthyl−1−azide | 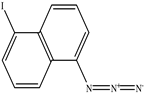 |
| Methyl azide | 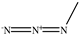 |
| 1−Naphthyl azide |  |
| Nicotinoylazide |  |
| 2−Nitrophenyl azide |  |
| Phenyl azide |  |
| Procyanidin A1 |  |
| Procyanidin A2 | 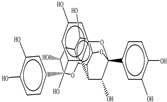 |
| Procyanidin B1 | 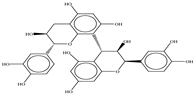 |
| Procyanidin B2 | 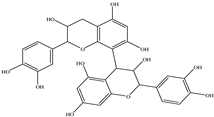 |
| Thiocyanate | 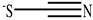 |
| Vitamin C | 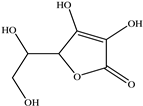 |
| Ligand | MW1 | MLogP2 | HBD3 | HBA4 | RB5 | TICE Rule Violation (for Insecticide Property) |
|---|---|---|---|---|---|---|
| Ammonia | 17.031 | ND7 | 1 | 1 | 0 | ND7 |
| Arabinoxylan | 560.50 | −4.03 | 8 | 15 | 13 | Yes |
| Anthracene-1-carbonyl azide | 247.75 | 3.26 | 0 | 4 | 2 | No |
| 2-Anthracene carboxylic acid azide | 247.25 | 3.26 | 0 | 4 | 2 | No |
| Benzyl azide | 133.15 | 1.18 | 0 | 3 | 2 | No |
| 4-Chloro-5-dimethylamino-2-phenyl-3-(2H)-pyridazinone | 249.70 | 2.37 | 0 | 2 | 2 | No |
| Dicyandiamide | 84.08 | −1.51 | 2 | 2 | 0 | Yes |
| 3,5-Dimethylpyrazole | 96.13 | 0.35 | 1 | 1 | 0 | No |
| 3,4-Dimethylpyrazole phosphate | 194.13 | −1.50 | 4 | 5 | 0 | Yes |
| Ethyl azide | 71.08 | −0.66 | 0 | 3 | 1 | Yes |
| 5-Iodonaphthyl-1-azide | 295.08 | 3.04 | 0 | 3 | 1 | No |
| Methyl azide | 57.05 | −1.30 | 0 | 3 | 0 | Yes |
| 1-Naphthyl azide | 169.18 | 2.20 | 0 | 3 | 1 | No |
| Nicotinoylazide | 148.12 | −0.25 | 0 | 5 | 2 | Yes |
| 2-Nitrophenyl azide | 164.12 | 0.88 | 0 | 5 | 2 | No |
| Phenyl azide | 119.12 | 1.09 | 0 | 3 | 1 | No |
| Procyanidin A1 | 592.50 | −0.34 | 10 | 13 | 2 | Yes |
| Procyanidin A2 | 576.50 | 0.14 | 9 | 12 | 3 | Yes |
| Procyanidin B1 | 578.52 | −0.26 | 10 | 12 | 3 | Yes |
| Procyanidin B2 | 578.52 | −0.26 | 10 | 12 | 3 | Yes |
| Thiocyanate | 58.08 | −1.01 | 0 | 1 | 0 | Yes |
| Vitamin C | 176.12 | −2.60 | 4 | 6 | 2 | Yes |
| Ligand Name | Toxicity | ||
|---|---|---|---|
| Bee | Protozoa * | Fish ** | |
| Ammonia | ND | −2.328 | 2.911 |
| Arabinoxylan | Non-toxic | 0.285 | 8.481 |
| Anthracene-1-carbonyl azide | Non-toxic | 0.327 | −0.326 |
| 2-Anthracene carboxylic acid azide | Non-toxic | 0.336 | −0.326 |
| Benzyl azide | Non-toxic | 0.233 | 1.445 |
| 4-Chloro-5-dimethylamino-2-phenyl-3-(2H)-pyridazinone | Non-toxic | 0.763 | 1.09 |
| Dicyandiamide | Non-toxic | −0.162 | 3.549 |
| 3,5-Dimethylpyrazole | Non-toxic | −0.477 | 2.706 |
| 3,4-Dimethylpyrazole phosphate | Non-toxic | 0.126 | 3.096 |
| Ethyl azide | Non-toxic | −0.851 | 2.349 |
| 5-Iodonaphthyl-1-azide | Non-toxic | 0.506 | −0.67 |
| Methyl azide | Non-toxic | −1.056 | 2.514 |
| 1-Naphthyl azide | Non-toxic | 0.399 | 0.31 |
| Nicotinoylazide | Non-toxic | 0.074 | 1.924 |
| 2-Nitrophenyl azide | Non-toxic | 0.593 | 0.971 |
| Phenyl azide | Non-toxic | 0.084 | 1.48 |
| Procyanidin A1 | Non-toxic | 0.285 | 8.966 |
| Procyanidin A2 | Non-toxic | 0.285 | 7.542 |
| Procyanidin B1 | Non-toxic | 0.285 | 8.151 |
| Procyanidin B2 | Non-toxic | 0.285 | 8.704 |
| Thiocyanate | Non-toxic | −0.823 | 2.537 |
| Vitamin C | Non-toxic | 0.285 | 4.386 |
| Lgand | −ACE▪ (kcal/mol) | Interaction of Amino Acid (A.A) Residue | Bond Distance (in A°) |
|---|---|---|---|
| Ammonia | 15.83 | Phe498 | 2.52 |
| Arabinoxylan | 188.05 | Arg45 | 2.70 |
| Lys114 | 2.28 | ||
| Thr525 | 3.18 | ||
| Glu538 | 2.83 & 3.37 | ||
| Trp563 | 2.18 | ||
| Anthracene-1-carbonyl azide | 61.89 | Ser72 | 3.44 |
| 2-Anthracene carboxylic acid azide | 47.71 | Asn102 | 2.73 |
| Thr103 | 3.34 | ||
| Glu120 | 3.07 | ||
| Benzyl azide | 120.82 | No interactions | - |
| 4-Chloro-5-dimethylamino-2-phenyl-3-(2H)-pyridazinone | 58.25 | Glu120 | 3.12 |
| Dicyandiamide | 75.65 | No interactions | - |
| 3,5-Dimethylpyrazole | 78.91 | No interactions | - |
| 3,4-Dimethylpyrazole phosphate | 48.32 | Thr55 | 2.67 & 2.76 |
| Thr60 | 3.26 | ||
| Glu538 | 3.36 | ||
| Ethyl azide | 56.82 | No interactions | - |
| 5-Iodonaphthyl-1-azide | 63.94 | Ser72 | 2.81 |
| Glu120 | 2.02 | ||
| Methyl azide | 28.18 | Asn227 | 3.14 |
| Asp300 | 3.12 & 3.40 | ||
| Tyr304 | 2.88 | ||
| 1-Naphthyl azide | 60.80 | Leu151 | 3.60 |
| Nicotinoyl azide | 130.54 | Thr544 | 2.82 |
| 2-Nitrophenyl azide | 125.37 | Thr55 | 2.68 |
| Thr60 | 3.07 & 3.08 | ||
| Thr544 | 3.07 | ||
| Phenyl azide | 111.39 | No interactions | - |
| Procyanidin A1 | 95.81 | Ser72 | 3.38 |
| Thr103 | 2.64 | ||
| Lys125 | 3.34 | ||
| Leu151 | 2.26 | ||
| Asn153 | 2.78 & 3.58 | ||
| Thr862 | 3.39 | ||
| Procyanidin A2 | 101.08 | Ser72 | 3.28 |
| Asn123 | 2.59 | ||
| Ala124 | 3.40 | ||
| Gly126 | 3.16 | ||
| Asn244 | 3.09 | ||
| Procyanidin B1 | 1.85 | Ser72 | 3.55 |
| His73 | 2.86 | ||
| Asn123 | 3.01 | ||
| Asn244 | 3.38 | ||
| Procyanidin B2 | 83.73 | Thr103 | 3.28 |
| Ile121 | 2.82 | ||
| Arg861 | 2.50 | ||
| Thr862 | 2.50 & 3.15 | ||
| Thiocyanate | 105.42 | Arg254 | 3.54 |
| Vitamin C | 57.47 | Thr55 | 2.48 |
| Thr60 | 3.27 & 3.49 |
| Ligand | −ACE▪ (kcal/mol) | Interaction of Amino Acid (A.A) Residue | Bond Distance (in A°) |
|---|---|---|---|
| Ammonia | 15.20 | No interactions | - |
| Arabinoxylan | 169.95 | Thr336 | 1.96 & 3.54 |
| Anthracene-1-carbonyl azide | 104.61 | Thr336 | 2.58 |
| 2-Anthracene carboxylic acid azide | 126.37 | Ala136 | 3.14 |
| Benzyl azide | 151.70 | No interactions | - |
| 4-Chloro-5-dimethylamino-2-phenyl-3-(2H)-pyridazinone | 203.06 | Pro26 | 2.38 |
| Dicyandiamide | 50.91 | Asp179 | 3.14 |
| 3,5-Dimethylpyrazole | 107.94 | No interactions | - |
| 3,4-Dimethylpyrazole phosphate | 118.81 | Leu421 | 2.78 |
| Ethyl azide | 57.42 | Asp178 | 2.63 |
| Asp179 | 2.95 & 3.43 | ||
| 5-Iodonaphthyl-1-azide | 180.56 | Leu421 | 3.30 |
| Methyl azide | 63.85 | Leu421 | 2.85, 3.03 & 3.16 |
| 1-Naphthyl azide | 153.91 | No interactions | - |
| Nicotinoyl azide | 144.25 | Leu421 | 3.13 |
| 2-Nitrophenyl azide | 156.39 | Leu421 | 2.95 & 3.11 |
| Phenyl azide | 131.36 | No interactions | - |
| Procyanidin A1 | 211.30 | Thr336 | 2.57 |
| Arg337 | 3.55 | ||
| Procyanidin A2 | 207.69 | Thr336 | 2.39 |
| Procyanidin B1 | 250.92 | Asp178 | 2.92 |
| Procyanidin B2 | 240.34 | Pro26 | 2.41 |
| Val28 | 2.69 | ||
| Thiocyanate | 76.65 | Leu421 | 3.55 |
| Vitamin C | 112.51 | No interactions | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanaswamy, R.; Prabhakaran, V.-S.; Al-Ansari, M.M.; Al-Humaid, L.A.; Tiwari, P. An In Silico Analysis of Synthetic and Natural Compounds as Inhibitors of Nitrous Oxide Reductase (N2OR) and Nitrite Reductase (NIR). Toxics 2023, 11, 660. https://doi.org/10.3390/toxics11080660
Narayanaswamy R, Prabhakaran V-S, Al-Ansari MM, Al-Humaid LA, Tiwari P. An In Silico Analysis of Synthetic and Natural Compounds as Inhibitors of Nitrous Oxide Reductase (N2OR) and Nitrite Reductase (NIR). Toxics. 2023; 11(8):660. https://doi.org/10.3390/toxics11080660
Chicago/Turabian StyleNarayanaswamy, Radhakrishnan, Vasantha-Srinivasan Prabhakaran, Mysoon M. Al-Ansari, Latifah A. Al-Humaid, and Pragya Tiwari. 2023. "An In Silico Analysis of Synthetic and Natural Compounds as Inhibitors of Nitrous Oxide Reductase (N2OR) and Nitrite Reductase (NIR)" Toxics 11, no. 8: 660. https://doi.org/10.3390/toxics11080660







