Genotoxicity of Occupational Pesticide Exposures among Agricultural Workers in Arab Countries: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Review Objectives
2.2. Identification and Management of Studies
2.3. Assessment of Study Eligibility
2.4. Risk of Bias (RoB) in Individual Studies
2.5. Data Extraction
2.6. Data Synthesis
3. Results
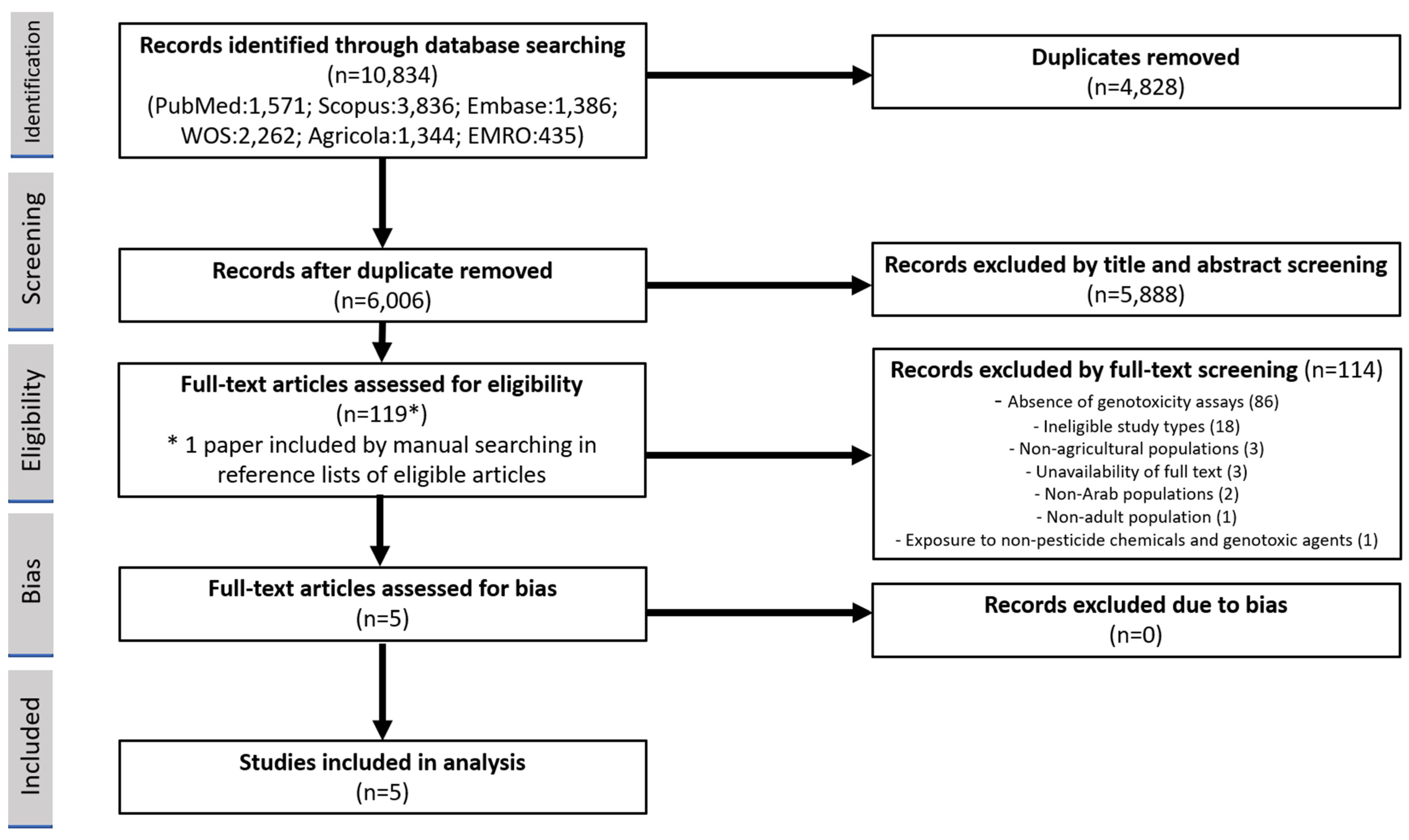
3.1. Identification of Eligible Studies
3.2. Summary of Results of Included Studies
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food Safety: Pesticides European Commission: Directorate-General for Health and Food Safety. 2021. Available online: https://ec.europa.eu/food/plants/pesticides_en (accessed on 15 May 2023).
- California Department of Pesticide Regulation. Actively Registered Active Ingredients (AI) by Common Name. Product/Label Database Queries & Lists; California Department of Pesticide Regulation: Sacramento, CA, USA, 2023. [Google Scholar]
- Bolognesi, C.; Holland, N. The use of the lymphocyte cytokinesis-block micronucleus assay for monitoring pesticide-exposed populations. Mutat. Res./Rev. Mutat. Res. 2016, 770, 183–203. [Google Scholar] [PubMed]
- Clendennen, S.K.; Boaz, N.W. Chapter 14—Betaine amphoteric surfactants—Synthesis, properties, and applications. In Biobased Surfactants, 2nd ed.; Hayes, D.G., Solaiman, D.K.Y., Ashby, R.D., Eds.; AOCS Press: Urbana, IL, USA, 2019; pp. 447–469. [Google Scholar]
- Mustafa, I.F.; Hussein, M.Z. Synthesis and Technology of Nanoemulsion-Based Pesticide Formulation. Nanomaterials 2020, 10, 1608. [Google Scholar] [PubMed]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [PubMed]
- Jeyaratnam, J. Acute pesticide poisoning: A major global health problem. World Health Stat. Q. 1990, 43, 139–144. [Google Scholar]
- Iyaniwura, T. Non-target and environmental hazards of pesticides. Rev. Environ. Health 1991, 9, 161–176. [Google Scholar]
- Bolognesi, C. Genotoxicity of pesticides: A review of human biomonitoring studies. Mutat. Res. 2003, 543, 251–272. [Google Scholar]
- WHO-JMPR Toxicological Monographs-Pesticide Residues in Food. 2022. Available online: https://inchem.org/pages/jmpr.html (accessed on 4 April 2023).
- Bastos, P.L.; Bastos, A.; Gurgel, A.D.M.; Gurgel, I.G.D. Carcinogenicity and mutagenicity of malathion and its two analogues: A systematic review. Ciênc. Saúde Coletiva 2020, 25, 3273–3298. [Google Scholar]
- Feulefack, J.; Khan, A.; Forastiere, F.; Sergi, C.M. Parental Pesticide Exposure and Childhood Brain Cancer: A Systematic Review and Meta-Analysis Confirming the IARC/WHO Monographs on Some Organophosphate Insecticides and Herbicides. Children 2021, 8, 1096. [Google Scholar]
- Nagy, K.; Duca, R.C.; Lovas, S.; Creta, M.; Scheepers, P.T.; Godderis, L.; Ádám, B. Systematic review of comparative studies assessing the toxicity of pesticide active ingredients and their product formulations. Environ. Res. 2020, 181, 108926. [Google Scholar]
- Bolognesi, C.; Creus, A.; Ostrosky-Wegman, P.; Marcos, R. Micronuclei and Pesticide exposure. Mutagenesis 2011, 26, 19–26. [Google Scholar]
- Bull, S.; Fletcher, K.; Boobis, A.R.; Battershill, J.M. Evidence for genotoxicity of pesticides in pesticide applicators: A review. Mutagenesis 2006, 21, 93–103. [Google Scholar]
- Dowler, C. Europe Shipping Banned Pesticide Linked to Child Brain Damage to Countries in Global South: Unearthed. 2023. Available online: https://unearthed.greenpeace.org/2023/03/28/eu-banned-pesticide-global-south/ (accessed on 28 March 2023).
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar]
- Sherif, M.; Makame, K.R.; Östlundh, L.; Paulo, S.; Nemmar, A.; Ali, B.R.; Al Rifai, R.; Nagy, K.; Ádám, B. Investigating the genotoxicity of occupational pesticide exposures in Arab countries: Protocol of a systematic review and meta-analysis. medRxiv 2022, 8, 22279322. [Google Scholar]
- Julian, P.T.H.; Sally, G. Cochrane Handbook for Systematic Reviews of Interventions: Chichester, West Sussex; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [PubMed]
- Veritas Health Innovation. Covidence Systematic Review Software; Veritas Health Innovation: Melbourne, Australia, 2021. [Google Scholar]
- Linacre, S. The Predator Effect: Understanding the Past, Present and Future of Deceptive Academic Journals. 2022. Available online: https://blog.cabells.com/tag/cabells-predatory-reports/ (accessed on 9 July 2023).
- Woodruff, T.J.; Sutton, P. The Navigation Guide systematic review methodology: A rigorous and transparent method for translating environmental health science into better health outcomes. Environ. Health Perspect. 2014, 122, 1007–1014. [Google Scholar]
- Pega, F.; Norris, S.L.; Backes, C.; Bero, L.A.; Descatha, A.; Gagliardi, D.; Godderis, L.; Loney, T.; Modenese, A.; Morgan, R.L.; et al. RoB-SPEO: A tool for assessing risk of bias in studies estimating the prevalence of exposure to occupational risk factors from the WHO/ILO Joint Estimates of the Work-related Burden of Disease and Injury. Environ. Int. 2020, 135, 105039. [Google Scholar]
- Amr, M.M. Pesticide monitoring and its health problems in Egypt, a Third World country. Toxicol. Lett. 1999, 107, 1–13. [Google Scholar]
- Mohammad, O.; Walid, A.A.; Ghada, K. Chromosomal aberrations in human lymphocytes from two groups of workers occupationally exposed to pesticides in Syria. Environ. Res. 1995, 70, 24–29. [Google Scholar] [PubMed]
- Omari, Y.I. Cytogenetic Study on Workmen Occupationally Exposed to Pesticides. Balk. J. Med. Genet. 2009, 12, 51–59. [Google Scholar]
- Omari, Y.I. Micronucleus analysis and mitotic index in a Jordanian population exposed to pesticides of organophosphate: Malathion and chlorpyrifos. Caryologia 2011, 64, 173–178. [Google Scholar]
- Qaqish, B.M.; Al-Dalahmah, O.; Al-Motassem, Y.; Battah, A.; Ismail, S.S. Occupational exposure to pesticides and occurrence of the chromosomal translocation t(14;18) among farmers in Jordan. Toxicol. Rep. 2016, 3, 225–229. [Google Scholar]
- Rother, H.A. South African farm workers’ interpretation of risk assessment data expressed as pictograms on pesticide labels. Environ. Res. 2008, 108, 419–427. [Google Scholar] [PubMed]
- Jaga, K.; Dharmani, C. The epidemiology of pesticide exposure and cancer: A review. Rev. Environ. Health 2005, 20, 15–38. [Google Scholar] [PubMed]
- Pesticides and Health Hazards Facts and Figures GLS Gemeinschaftsbank: PAN Germany. 2012. Available online: https://www.pan-germany.org/download/Vergift_EN-201112-web.pdf (accessed on 14 March 2023).
- Toe, A.M.; Ouedraogo, M.; Ouedraogo, R.; Ilboudo, S.; Guissou, P.I. Pilot Study on Agricultural Pesticide Poisoning in Burkina Faso. Interdiscip. Toxicol. 2010, 6, 185–195. [Google Scholar]
- Kaur, K.; Kaur, R. Occupational Pesticide Exposure, Impaired DNA Repair, and Diseases. Indian J. Occup. Environ. Med. 2018, 22, 74–81. [Google Scholar] [PubMed]
- Valencia-Quintana, R.; Milić, M.; Bonassi, S.; Ochoa-Ocaña, M.A.; Campos-Peña, V.; Tenorio-Arvide, M.G.; Pérez-Flores, G.A.; Sánchez-Alarcón, J. Effect of Pesticide Exposure over DNA Damage in Farmers from Los Reyes, Michoacan in Mexico. Toxics 2023, 11, 122. [Google Scholar] [PubMed]
- Janoš, T.; Ottenbros, I.; Bláhová, L.; Šenk, P.; Šulc, L.; Pálešová, N.; Sheardová, J.; Vlaanderen, J.; Čupr, P. Effects of pesticide exposure on oxidative stress and DNA methylation urinary biomarkers in Czech adults and children from the CELSPAC-SPECIMEn cohort. Environ. Res. 2023, 222, 115368. [Google Scholar]
- Dara, S.K. The New Integrated Pest Management Paradigm for the Modern Age. J. Integr. Pest Manag. 2019, 10, 12. [Google Scholar]
- Ilyushina, N.A.; Egorova, O.V.; Masaltsev, G.V.; Averianova, N.S.; Revazova, Y.A.; Rakitskii, V.N.; Goumenou, M.; Vardavas, A.; Stivaktakis, P.; Tsatsakis, A. Genotoxicity of mixture of imidacloprid, imazalil and tebuconazole. Toxicol. Rep. 2020, 7, 1090–1094. [Google Scholar]
- DeMarini, D.M. Genotoxicity of tobacco smoke and tobacco smoke condensate: A review. Mutat. Res. 2004, 567, 447–474. [Google Scholar]
- Hartmann, A.; Speit, G. The contribution of cytotoxicity to DNA-effects in the single cell gel test (comet assay). Toxicol. Lett. 1997, 90, 183–188. [Google Scholar] [PubMed]
- de-Assis, M.P.; Barcella, R.C.; Padilha, J.C.; Pohl, H.H.; Krug, S.B.F. Health problems in agricultural workers occupationally exposed to pesticides. Rev. Bras. Med. Trab. 2021, 18, 352–363. [Google Scholar] [PubMed]
- FAO. Women in Agriculture: Closing the Gender Gap for Development. Rome: Food and Agriculture Organization of the United Nations. 2011. Available online: https://www.fao.org/3/am307e/am307e00.pdf (accessed on 12 May 2023).
- Dereumeaux, C.; Fillol, C.; Quenel, P.; Denys, S. Pesticide exposures for residents living close to agricultural lands: A review. Environ. Int. 2020, 134, 105210. [Google Scholar] [PubMed]
- Jallow, M.F.; Awadh, D.G.; Albaho, M.S.; Devi, V.Y.; Thomas, B.M. Pesticide Knowledge and Safety Practices among Farm Workers in Kuwait: Results of a Survey. Int. J. Environ. Res. Public Health 2017, 14, 340. [Google Scholar]
- Osaili, T.M.; Al Sallagi, M.S.; Dhanasekaran, D.K.; Odeh, W.A.B.; Al Ali, H.J.; Al Ali, A.A.; Ismail, L.C.; Mehri, K.O.A.; Pisharath, V.A.; Holley, R.; et al. Pesticide residues in fresh fruits imported into the United Arab Emirates. Heliyon 2022, 8, e11946. [Google Scholar]


| PECO Element | Description |
|---|---|
| Population | Adult (>18 years old) professional agricultural workers, defined as farmers and pesticide applicators, in Arabic-speaking countries of the MENA region (19 countries: Algeria, Bahrain, Egypt, Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Mauritania, Oman, Palestine, Qatar, Saudi Arabia, Sudan, Syria, Tunisia, the United Arab Emirates, and Yemen), while those who work in other sectors, who are located outside the region, and who are less than 18 years old were excluded. |
| Exposure | Exposure to a variety of pesticide products used in agricultural settings, while excluding exposure to non-agricultural pesticides, other chemicals, and genotoxic agents. |
| Comparator | No comparators were used for assessing the prevalence and extent of DNA damage. The comparator group for identifying and determining the effect size of genotoxic pesticide exposures and risk factors were populations not directly exposed to pesticides, or the general population. |
| Outcome | Biomarkers of DNA damage detected by established genotoxicity tests, such as DNA strand break measurements, cytogenetic assays, and mutagenicity assays. Additional outcomes included prevalence and risk factors of genotoxicity among agricultural workers exposed to pesticides in Arab countries. |
| Risk of Bias Domain | 1—Bias in Selection of Participants into the Study | 2—Bias Due to a Lack of Blinding of Study Personnel | 3—Bias Due to Exposure Misclassification | 4—Bias Due to Incomplete Exposure Data | 5—Bias Due to Outcome Misclassification | 6—Bias Due to Selective Reporting of Exposures/Outcomes | 7—Bias Due to Differences in Numerator and Denominator | 8—Bias Due to Confounding | 9—Bias Due to Conflicts of Interest | 10—Other Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Amr 1999 [25] | ||||||||||
| Mohammad 1995 [26] | ||||||||||
| Omari 2009 [27] | ||||||||||
| Omari 2011 [28] | ||||||||||
| Qaqish 2016 [29] |
| Country | Exposed Participants | Non-Exposed | Type of Pesticide, Duration, and Pattern of Exposure | Assay and Type of Biomarker | DNA Damage in Exposed Participants | DNA Damage in Non-Exposed Participants | Comparison of Exposed and Non-Exposed Participants | Additional Risk Factors/Confounders | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Egypt | 300 pesticide formulators 300 pesticide applicators Cytogenetics was assessed in only 32 applicators and 39 formulators | 20 to compare with applicators, another 20 to compare with formulators | Chlorinated hydrocarbons, organophosphates (dimethoate, malathion, dichlorvos), carbamates (propoxur), as well as pyrethroids (cypermethrin, deltamethrin, tetramethrin, sumithrin, D-allethrin); Formulators 5–25 yrs exposure, applicators 5–15 yrs exposure; pesticide spraying: 3 x/yr, June–Sept. | Chromosome aberration assay; gaps, breaks, exchanges, dicentrics, fragments, and deletions | Formulators: gaps: 1.58 ± 0.81, breaks: 1.13 ± 0.86, exchanges: 0.7 ± 0.7, dicentrics: 0.79 ± 0.6, fragments: 0.54 ± 0.6, deletions: 0.3 ± 0.5 Applicators: gaps: 4.13, breaks: 1.8, isobreaks: 0.28, deletions: 8.89 (N.B: The paper was not clear regarding the number of cells from which they calculated those averages and standard deviations) | Gaps: 1.05 ± 0.06, breaks: 0.7 ± 0.86, exchanges: 0.1 ± 0.3, dicentrics: 0.2 ± 0.5, fragments: 0.25 ± 0.4, deletions: 0.1 ± 0.3 | Significant differences (p < 0.001) in gap, exchange, and dicentric Significant differences (p < 0.05) in break, fragment, and deletion between formulators and applicators | There were no additional risk factors reported | Amr 1999 [25] |
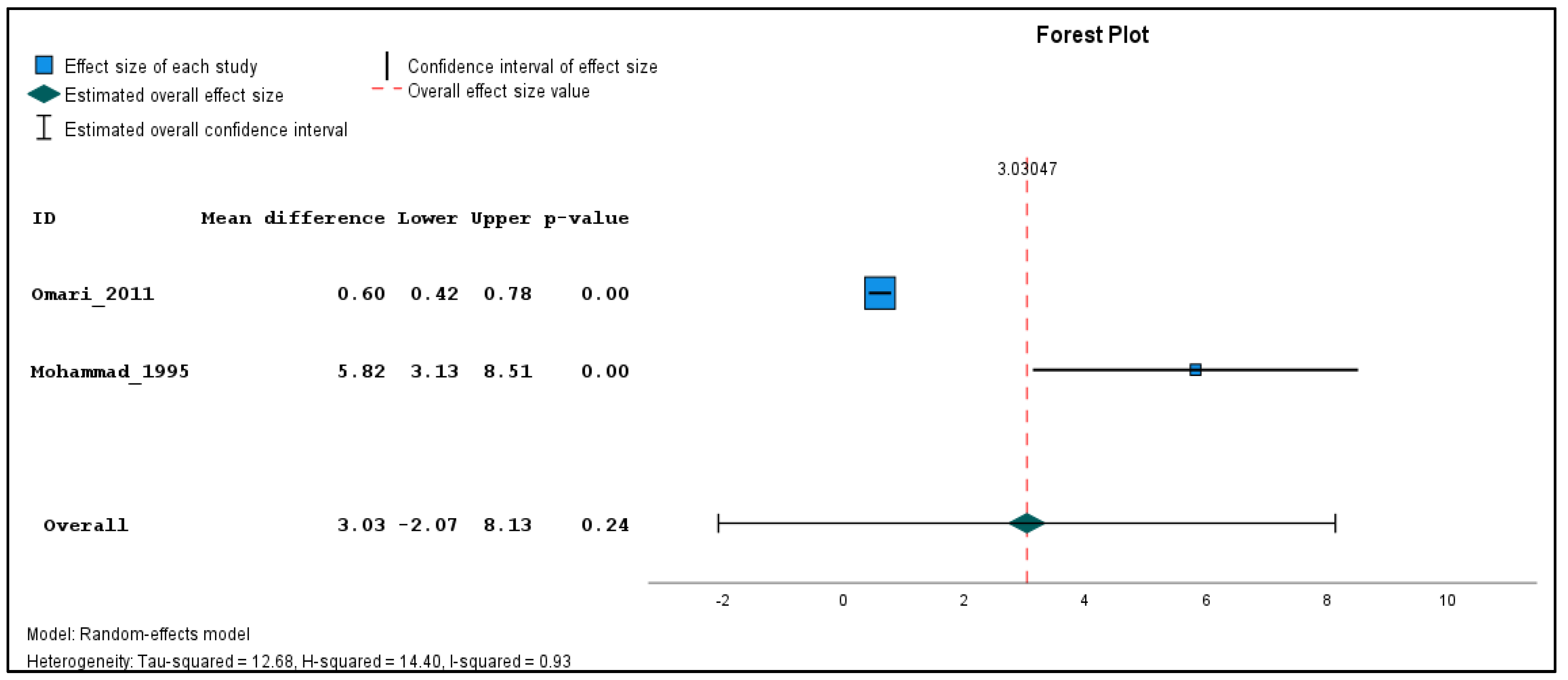
| Syria | 9 sprayers, 7 dealers, and quality controllers | 6 | Sprayers: deltamethrin and cypermethrin, 3 years exposure Dealers and quality controllers: mixture of pesticides including pyrethrins; year-round exposure | Chromosome aberration assay; chromatid breaks, chromatid exchanges, chromosomal breaks, dicentrics, rings, minutes | Average number ± SD of aberrations per 100 cells in sprayers: Beginning of season: aberrations: 7 ± 1.85, breaks: 7.5 ± 2.62, chromatid breaks: 6 ± 2.69 Middle of season: 10 ± 1.32, 12.11 ± 2.37, 8.78 ± 1.72 End of season: 13.78 ± 2.73, 15.33 ± 3.43, 12.44 ± 2.65 Average number ± SD of aberrations per 100 cells in dealers and quality controllers: 13.52 ± 3.40, 15.38 ± 3.18, 11.95 ± 3.85 | Aberrations: 4.34 ± 1.39, breaks: 5.16 ± 1.59, chromatid breaks: 3.64 ± 1.47 | Sprayers: Significant differences in chromatid breaks at the beginning, middle and end of season (p < 0/05) Dealers and quality controllers: Significant difference in chromatid breaks (p < 0.05) and in all genetic damage (p < 0.05) | There were no additional risk factors reported | Moham-mad 1995 [26] |
| Jordan | 40 farmers | 30 | Malathion and chlorpyrifos; Duration of exposure: 2 to 5 years | Chromosome aberration assay; gaps, chromatid breaks, isochromatid breaks, and exchanges such as dicentric, rings, and trivalents | Smokers had 5.75 ± 0.05 abnormal cells, and 6.10 ± 0.23 aberrations/100 cells, while non-smokers had 3.35 ± 0.26 abnormal cells, and 5.13 ± 0.28 aberrations/100 cells. | Smokers had 5.13 ± 0.36 abnormal cells, and 4.59 ± 0.35 aberrations/100 cells, while non-smokers had 4.14 ± 0.32 abnormal cells, and 2.04 ± 0.21 aberrations/100 cells | In both the smokers and non-smokers subsets, the pesticide-exposed group exhibited significantly higher rates (p < 0.05 for individual analysis, p < 0.01 for combined analysis) of abnormal cells, gaps, chromatid breaks, and chromosomal aberrations compared to the pesticide non-exposed control group. | Confounders such as age and duration of exposure were controlled, and the individuals were stratified based on smoking status. Significantly higher incidence of DNA damage was observed in smokers among the exposed group compared to both non-smokers within the same group and the unexposed controls (p < 0.05) Individuals who had been exposed to potentially genotoxic agents were excluded from the analysis | Omari 2009 [27] |
| Jordan | 23 farmers | 22 | Insecticide mixture Malathion and chlorpyrifosDuration of use: 3–30 years | Micronucleus test; frequency of micronuclei (MN) | The examination of 11,500 binucleated lymphocytes revealed after 8 months of exposure: 0 MN: 11,230, 1 MN: 201, 2 MN: 28, 3 MN: 26, 4 MN: 15 cells After 8 months free from exposure: 0 MN: 11,345, 1 MN: 128, 2 MN: 19, 3 MN: 6, 4 MN: 2 cells | The examination of 11,500 binucleated lymphocytes revealed 0 MN: 10,918, 1 MN: 75, 2 MN: 7 cells, with no cells observed with 3 MN or 4 MN | After 8 months of exposure: highly significant increase in MN frequency (p < 0.01) After 8 months free from exposure: significant increase in MN frequency (p < 0.05) | There were no additional factors reported Significant decrease in mitotic index in exposed groups compared to control group; no specific causes mentioned | Omari 2011 [28] |
| Jordan | 96 farmers | 96 community members | Pesticide types not reported Open field pesticide use: 80.2% Herbicide use: 95.8% Insecticide use on animals: 47.9% Duration of exposure: 1–40 years (mean 10.9 ± 7.9 years) | Nested polymerase chain reaction (PCR) assay; BCL2-IGH t(14;18) fusion frequency | 63.5% (61 out of 96) | 11.5% (11 out of 96) | Significant increase for all exposure; OR = 13.5 (95%CI = 6.3–28.6), p < 0.0001 Significant increase for pesticide use on open fields; OR = 3.0 (95%CI = 1.1–8.5), p = 0.03 Significant increase for insecticide use on animals; OR = 2.4 (95% CI = 1.02–5.7), p = 0.043 No significant association for herbicide use; OR = 0.57 (95%CI = 0.06–5.7), p = 0.627 | No significant association for duration of pesticide use; p = 0.51 No significant association for wearing a mask; OR = 0.7 (95%CI = 0.04–0.7), p = 0.99 No significant association for wearing a mask and gloves, OR = 2.3 (95%CI = 0.8–6.6), p = 0.15) | Qaqish 2016 [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherif, M.; Makame, K.R.; Östlundh, L.; Paulo, M.S.; Nemmar, A.; Ali, B.R.; Al-Rifai, R.H.; Nagy, K.; Ádám, B. Genotoxicity of Occupational Pesticide Exposures among Agricultural Workers in Arab Countries: A Systematic Review and Meta-Analysis. Toxics 2023, 11, 663. https://doi.org/10.3390/toxics11080663
Sherif M, Makame KR, Östlundh L, Paulo MS, Nemmar A, Ali BR, Al-Rifai RH, Nagy K, Ádám B. Genotoxicity of Occupational Pesticide Exposures among Agricultural Workers in Arab Countries: A Systematic Review and Meta-Analysis. Toxics. 2023; 11(8):663. https://doi.org/10.3390/toxics11080663
Chicago/Turabian StyleSherif, Moustafa, Khadija Ramadhan Makame, Linda Östlundh, Marilia Silva Paulo, Abderrahim Nemmar, Bassam R. Ali, Rami H. Al-Rifai, Károly Nagy, and Balázs Ádám. 2023. "Genotoxicity of Occupational Pesticide Exposures among Agricultural Workers in Arab Countries: A Systematic Review and Meta-Analysis" Toxics 11, no. 8: 663. https://doi.org/10.3390/toxics11080663
APA StyleSherif, M., Makame, K. R., Östlundh, L., Paulo, M. S., Nemmar, A., Ali, B. R., Al-Rifai, R. H., Nagy, K., & Ádám, B. (2023). Genotoxicity of Occupational Pesticide Exposures among Agricultural Workers in Arab Countries: A Systematic Review and Meta-Analysis. Toxics, 11(8), 663. https://doi.org/10.3390/toxics11080663









