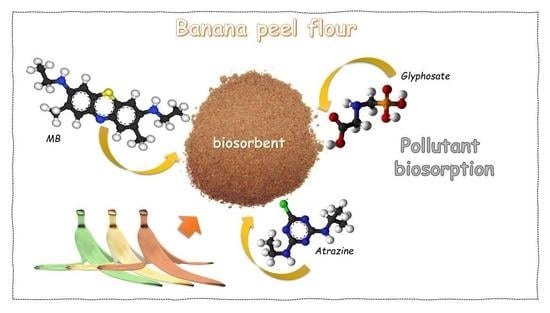
Banana Peel Powder Biosorbent for Removal of Hazardous Organic Pollutants from Wastewater
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Banana Peel Powder Preparation
2.3. Characterization of the Biosorbent
2.4. Adsorption Experiments
2.4.1. Biosorption of the Methylene Blue Azo Dye
2.4.2. Biosorption of the Glyphosate and Atrazine Pesticides
2.4.3. Kinetic Models of Adsorption
3. Results and Discussion
3.1. Characterization of the Biosorbents
3.2. Adsorption Efficiency Evaluation
3.2.1. BPP as Biosorbent for Methylene Blue Removal
3.2.2. Biosorption of Atrazine and Glyphosate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Penagos-Tabares, F.; Sulyok, M.; Faas, J.; Krska, R.; Khiaosa-ard, R.; Zebeli, Q. Residues of Pesticides and Veterinary Drugs in Diets of Dairy Cattle from Conventional and Organic Farms in Austria. Environ. Pollut. 2023, 316, 120626. [Google Scholar] [CrossRef]
- de Aguiar, T.R.; Guimarães Neto, J.O.A.; Şen, U.; Pereira, H. Study of Two Cork Species as Natural Biosorbents for Five Selected Pesticides in Water. Heliyon 2019, 5, e01189. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Kumar, V.; Datta, S.; Dhanjal, D.S.; Sharma, K.; Samuel, J.; Singh, J. Current Advancement and Future Prospect of Biosorbents for Bioremediation. Sci. Total Environ. 2020, 709, 135895. [Google Scholar] [CrossRef] [PubMed]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J. Pesticides in Drinking Water—A Review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef]
- Pandiarajan, A.; Kamaraj, R.; Vasudevan, S.; Vasudevan, S. OPAC (Orange Peel Activated Carbon) Derived from Waste Orange Peel for the Adsorption of Chlorophenoxyacetic Acid Herbicides from Water: Adsorption Isotherm, Kinetic Modelling and Thermodynamic Studies. Bioresour. Technol. 2018, 261, 329–341. [Google Scholar] [CrossRef]
- Ramrakhiani, L.; Ghosh, S.; Mandal, A.K.; Majumdar, S. Utilization of Multi-Metal Laden Spent Biosorbent for Removal of Glyphosate Herbicide from Aqueous Solution and Its Mechanism Elucidation. Chem. Eng. J. 2019, 361, 1063–1077. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and Its Removal from Aqueous Solution by Adsorption: A Review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Amutova, F.; Jurjanz, S.; Akhmetsadykov, N.; Kazankapova, M.; Razafitianamaharavo, A.; Renard, A.; Nurseitova, M.; Konuspayeva, G.; Delannoy, M. Adsorption of Organochlorinated Pesticides: Adsorption Kinetic and Adsorption Isotherm Study. Results Eng. 2023, 17, 100823. [Google Scholar] [CrossRef]
- Helfferich, F.G. Principles of Adsorption & Adsorption Processes, by D. M. Ruthven, John Wiley & Sons, 1984, Xxiv + 433 pp. AIChE J. 1985, 31, 523–524. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar Derived from Non-Customized Matamba Fruit Shell as an Adsorbent for Wastewater Treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Microbial and Plant Derived Biomass for Removal of Heavy Metals from Wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.K.; Malik, D.S.; Yadav, A.K. Applicability of Plant Based Biosorbents in the Removal of Heavy Metals: A Review. Environ. Process. 2016, 3, 495–523. [Google Scholar] [CrossRef]
- Vidovix, T.B.; Januário, E.F.D.; Bergamasco, R.; Vieira, A.M.S. Efficient Removal of Sertraline Hydrochloride from Wastewater Using Banana Peels Functionalized: Performance Adsorption, Mechanisms and Applicability. Environ. Technol. 2023, 2023, 2164745. [Google Scholar] [CrossRef] [PubMed]
- Mishra, L.; Paul, K.K.; Jena, S. Adsorption Isotherm, Kinetics and Optimization Study by Box Behnken Design on Removal of Phenol from Coke Wastewater Using Banana Peel (Musa sp.) Biosorbent. Theor. Found. Chem. Eng. 2022, 56, 1189–1203. [Google Scholar] [CrossRef]
- Lapo, B.; Bou, J.J.; Hoyo, J.; Carrillo, M.; Peña, K.; Tzanov, T.; Sastre, A.M. A Potential Lignocellulosic Biomass Based on Banana Waste for Critical Rare Earths Recovery from Aqueous Solutions. Environ. Pollut. 2020, 264, 114409. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Banana Peel as a Biosorbent for the Decontamination of Water Pollutants. A Review. Environ. Chem. Lett. 2020, 18, 1085–1112. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.; Lee, D. Use of Cellulose-Based Wastes for Adsorption of Dyes from Aqueous Solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- de Carvalho, H.P.; Huang, J.; Zhao, M.; Liu, G.; Dong, L.; Liu, X. Improvement of Methylene Blue Removal by Electrocoagulation/Banana Peel Adsorption Coupling in a Batch System. Alex. Eng. J. 2015, 54, 777–786. [Google Scholar] [CrossRef] [Green Version]
- Mondal, N.K.; Kar, S. Potentiality of Banana Peel for Removal of Congo Red Dye from Aqueous Solution: Isotherm, Kinetics and Thermodynamics Studies. Appl. Water Sci. 2018, 8, 157. [Google Scholar] [CrossRef] [Green Version]
- Stavrinou, A.; Aggelopoulos, C.A.; Tsakiroglou, C.D. Exploring the Adsorption Mechanisms of Cationic and Anionic Dyes onto Agricultural Waste Peels of Banana, Cucumber and Potato: Adsorption Kinetics and Equilibrium Isotherms as a Tool. J. Environ. Chem. Eng. 2018, 6, 6958–6970. [Google Scholar] [CrossRef]
- Mohd Salim, R.; Khan Chowdhury, A.J.; Rayathulhan, R.; Yunus, K.; Sarkar, M.Z.I. Biosorption of Pb and Cu from Aqueous Solution Using Banana Peel Powder. Desalin. Water Treat. 2015, 57, 303–314. [Google Scholar] [CrossRef]
- Vilardi, G.; Di Palma, L.; Verdone, N. Heavy Metals Adsorption by Banana Peels Micro-Powder: Equilibrium Modeling by Non-Linear Models. Chin. J. Chem. Eng. 2018, 26, 455–464. [Google Scholar] [CrossRef]
- Fabre, E.; Lopes, C.B.; Vale, C.; Pereira, E.; Silva, C.M. Valuation of Banana Peels as an Effective Biosorbent for Mercury Removal under Low Environmental Concentrations. Sci. Total Environ. 2020, 709, 135883. [Google Scholar] [CrossRef]
- Alaa El-Din, G.; Amer, A.A.; Malsh, G.; Hussein, M. Study on the Use of Banana Peels for Oil Spill Removal. Alex. Eng. J. 2018, 57, 2061–2068. [Google Scholar] [CrossRef]
- Ingole, R.S.; Lataye, D.H.; Dhorabe, P.T. Adsorption of Phenol onto Banana Peels Activated Carbon. KSCE J. Civ. Eng. 2017, 21, 100–110. [Google Scholar] [CrossRef]
- de Sousa, P.A.R.; Tavares Furtado, L.; Lima Neto, J.L.; de Oliveira, F.M.; Martins Siqueira, J.G.; Silva, L.F.; Melo Coelho, L. Evaluation of the Adsorption Capacity of Banana Peel in the Removal of Emerging Contaminants Present in Aqueous Media—Study Based on Factorial Design. Braz. J. Anal. Chem. 2019, 6, 14–28. [Google Scholar] [CrossRef]
- Silva, C.R.; Gomes, T.F.; Andrade, G.C.R.M.; Monteiro, S.H.; Dias, A.C.R.; Zagatto, E.A.G.; Tornisielo, V.L. Banana Peel as an Adsorbent for Removing Atrazine and Ametryne from Waters. J. Agric. Food Chem. 2013, 61, 2358–2363. [Google Scholar] [CrossRef] [PubMed]
- ul Haq, A.; Shah, J.; Jan, M.R.; Din, S.U. Kinetic, Equilibrium and Thermodynamic Studies for the Sorption of Metribuzin from Aqueous Solution Using Banana Peels, an Agro-Based Biomass. Toxicol. Environ. Chem. 2015, 97, 124–134. [Google Scholar] [CrossRef]
- Chaparadza, A.; Hossenlopp, J.M. Adsorption Kinetics, Isotherms and Thermodynamics of Atrazine Removal Using a Banana Peel Based Sorbent. Water Sci. Technol. 2012, 65, 940–947. [Google Scholar] [CrossRef]
- Ahmad, T.; Danish, M. Prospects of Banana Waste Utilization in Wastewater Treatment: A Review. J. Environ. Manag. 2018, 206, 330–348. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Munyengabe, A.; Mavhungu, M.L.; Mbaya, R.; Baloyi, J. Breakthrough Studies for the Sorption of Methylene Blue Dye from Wastewater Samples Using Activated Carbon Derived from Waste Banana Peels. Biomass Convers. Refin. 2023, 1–13. [Google Scholar] [CrossRef]
- Gad, H.M.H.; El-Sayed, A.A. Activated Carbon from Agricultural By-Products for the Removal of Rhodamine-B from Aqueous Solution. J. Hazard. Mater. 2009, 168, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Mitrogiannis, D.; Markou, G.; Çelekli, A.; Bozkurt, H. Biosorption of Methylene Blue onto Arthrospira Platensis Biomass: Kinetic, Equilibrium and Thermodynamic Studies. J. Environ. Chem. Eng. 2015, 3, 670–680. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Alshirifi, A.N.; Alkaim, A.F. Kinetics and Equilibrium Study for the Adsorption of Textile Dyes on Coconut Shell Activated Carbon. Arab. J. Chem. 2017, 10, S3381–S3393. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, N.F.; Lima, E.C.; Pinto, I.S.; Amavisca, C.V.; Royer, B.; Pinto, R.B.; Alencar, W.S.; Pereira, S.F.P. Application of Cupuassu Shell as Biosorbent for the Removal of Textile Dyes from Aqueous Solution. J. Environ. Manag. 2011, 92, 1237–1247. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.L.; Hameed, B.H. Insight into the Adsorption Kinetics Models for the Removal of Contaminants from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Thompson, A.K.; Supapvanich, S.; Sirison, J. Banana Ripening; Springer Briefs in Food, Health, and Nutrition; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-27738-3. [Google Scholar]
- Memon, J.R.; Memon, S.Q.; Bhanger, M.I.; Memon, G.Z.; El-Turki, A.; Allen, G.C. Characterization of Banana Peel by Scanning Electron Microscopy and FT-IR Spectroscopy and Its Use for Cadmium Removal. Colloids Surf. B Biointerfaces 2008, 66, 260–265. [Google Scholar] [CrossRef]
- Leandro-Silva, E.; Pipi, A.R.F.; Magdalena, A.G.; Piacenti-Silva, M. Application of Langmuir and Freundlich Models in the Study of Banana Peel as Bioadsorbent of Copper (II) in Aqueous Medium. Rev. Mater. 2020, 25, 1–12. [Google Scholar] [CrossRef]
- Afolabi, F.O.; Musonge, P.; Bakare, B.F. Bio-Sorption of Copper and Lead Ions in Single and Binary Systems onto Banana Peels. Cogent Eng. 2021, 8, 1886730. [Google Scholar] [CrossRef]
- Branca, C.; Blasi, C. Di A Lumped Kinetic Model for Banana Peel Combustion. Thermochim. Acta 2015, 614, 68–75. [Google Scholar] [CrossRef]
- Guimarães, J.L.; Frollini, E.; da Silva, C.G.; Wypych, F.; Satyanarayana, K.G. Characterization of Banana, Sugarcane Bagasse and Sponge Gourd Fibers of Brazil. Ind. Crop. Prod. 2009, 30, 407–415. [Google Scholar] [CrossRef]
- Pravin Kumar, S.A.; Nagarajan, R.; Midhun Prasad, K.; Anand, B.; Murugavelh, S. Thermogravimetric Study and Kinetics of Banana Peel Pyrolysis: A Comparison of ‘Model-Free’ Methods. Biofuels 2019, 13, 129–138. [Google Scholar] [CrossRef]
- Choudhary, M.; Kumar, R.; Neogi, S. Activated Biochar Derived from Opuntia Ficus-Indica for the Efficient Adsorption of Malachite Green Dye, Cu+2 and Ni+2 from Water. J. Hazard. Mater. 2020, 392, 122441. [Google Scholar] [CrossRef] [PubMed]
- Ungarish, M.; Aharoni, C. Kinetics of Chemisorption. Deducing Kinetic Laws from Experimental Data. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1981, 77, 975. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated Lignin-Chitosan Extruded Blends for Efficient Adsorption of Methylene Blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Dotto, G.L.; Costa, J.A.V.; Pinto, L.A.A. Kinetic Studies on the Biosorption of Phenol by Nanoparticles from Spirulina sp. LEB 18. J. Environ. Chem. Eng. 2013, 1, 1137–1143. [Google Scholar] [CrossRef]
- Rudzinski, W.; Plazinski, W. Kinetics of Solute Adsorption at Solid/Solution Interfaces: On the Special Features of the Initial Adsorption Kinetics. Langmuir 2008, 24, 6738–6744. [Google Scholar] [CrossRef]





| Kinetic Model | Nonlinear Equation | Equation Number |
|---|---|---|
| Pseudo-first-order | 4 | |
| Pseudo-second-order | 5 | |
| 6 | ||
| Pseudo-general-order | 7 | |
| Avrami fractional-order | 8 | |
| Elovich | 9 | |
| Intraparticle diffusion | 10 |
| Model Parameter | Ripeness Stage | ||
|---|---|---|---|
| Green | Semi-Ripe | Ripe | |
| Fractional-order | |||
| 0.24 ± 0.01 | 0.25 ± 0.01 | 0.28 ± 1.87 × 10−6 | |
| 27.24 ± 1.67 × 10−8 | 29.15 ± 1.56 | 28.53 ± 1.37 | |
| 1.85 | 1.83 | 1.63 | |
| 0.8793 | 0.8793 | 0.8839 | |
| Residual sum of squares | 10.09 | 10.09 | 11.19 |
| Pseudo-first-order | |||
| 0.46 ± 0.11 | 0.45 ± 1.13 | 0.35 ± 0.08 | |
| 27.24 ± 1.18 | 29.15 ± 1.48 | 28.53 ± 1.30 | |
| 0.8914 | 0.8526 | 0.8955 | |
| Residual sum of squares | 9.08 | 14.41 | 10.07 |
| Pseudo-second-order | |||
| 0.03 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | |
| 28.92 ± 0.19 | 31.17 ± 1.48 | 30.62 ± 1.21 | |
| 24.11 ± 0.04 | 24.09 ± 0.04 | 18.27 ± 0.02 | |
| 0.9343 | 0.9152 | 0.9482 | |
| Residual sum of squares | 5.49 | 8.29 | 5.00 |
| Pseudo general-order | |||
| 0.027 ± 0.001 | 0.025 ± 0.001 | 0.019 ± 0.001 | |
| 28.95 ± 1.19 | 31.17 ± 0.01 | 30.43 ± 1.01 | |
| 2.02 ± 0.01 | 2.03 ± 0.10 | 2.03 ± 0.10 | |
| 0.9347 | 0.9233 | 0.9485 | |
| Residual sum of squares | 5.46 | 7.50 | 5.00 |
| Elovich | |||
| 798.53 ± 108.62 | 735.74 ± 127.11 | 373.85 ± 39.95 | |
| 0.32 ± 0.01 | 0.29 ± 0.01 | 0.28 ± 0.01 | |
| 0.9982 | 0.9969 | 0.9983 | |
| Residual sum of squares | 0.15 | 0.31 | 0.16 |
| Intraparticle diffusion | |||
| 11.80 ± 3.54 | 13.64 ± 3.62 | 12.37 ± 2.44 | |
| C1 () | 2.43 ± 2.92 | 2.49 ± 2.99 | 1.68 ± 2.02 |
| 0.8349 | 0.8687 | 0.9249 | |
| Residual sum of squares | 14.47 | 15.10 | 6.89 |
| 4.09 ± 0.45 | 2.42 ± 0.10 | 2.82 ± 0.17 | |
| C2 () | 13.14 ± 1.00 | 17.86 ± 0.30 | 15.40 ± 0.48 |
| 0.9647 | 0.9910 | 0.9828 | |
| Residual sum of squares | 0.45 | 0.24 | 0.62 |
| 1.20 ± 0.04 | 1.35 ± 0.06 | 1.29 ± 0.07 | |
| C3 () | 20.91 ± 0.20 | 22.48 ± 0.35 | 21.80 ± 0.45 |
| 0.9948 | 0.9935 | 0.9879 | |
| Residual sum of squares | 0.14 | 0.06 | 0.10 |
| Model Parameter | Pesticide | |
|---|---|---|
| Glyphosate | Atrazine | |
| Fractional-order | ||
| 0.02608 ± 0.01 | 0.02270 ± 0.01 | |
| 3.22 ± 0.01 | 3.46 ± 0.15 | |
| 1.41 ± 0.10 | 0.99 ± 0.01 | |
| 0.9609 | 0.9446 | |
| Residual sum of squares | 0.03 | 0.09 |
| Pseudo-first-order | ||
| 0.04 ± 0.01 | 0.02 ± 0.01 | |
| 3.22 ± 0.14 | 3.46 ± 0.40 | |
| 0.9554 | 0.9366 | |
| Residual sum of squares | 0.03738 | 0.10 |
| Pseudo-second-order | ||
| 0.010 ± 0.001 | 0.003 ± 0.002 | |
| 3.99 ± 0.19 | 5.01 ± 1.05 | |
| 0.15 ± 0.01 | 0.08 ± 0.01 | |
| 0.9776 | 0.9197 | |
| Residual sum of squares | 0.02 | 0.12 |
| Pseudo-general-order | ||
| 0.009 ± 0.001 | 0.006 ± 0.003 | |
| 4.03 ± 0.17 | 4.56 ± 0.81 | |
| 2.05 ± 0.01 | 1.72 ± 0.01 | |
| 0.9870 | 0.9394 | |
| Residual sum of squares | 0.02 | 0.10 |
| Elovich | ||
| 0.37 ± 0.04 | 0.18 ± 0.02 | |
| 1.20 ± 0.07 | 0.90 ± 0.07 | |
| 0.9845 | 0.9578 | |
| Residual sum of squares | 0.02 | 0.07 |
| Intraparticle diffusion | ||
| 0.333 ± 0.01 | 0.556 ± 0.04 | |
| C1 () | 0.144 ± 0.07 | −1.357 ± 0.20 |
| 0.9928 | 0.9775 | |
| Residual sum of squares | 0.04 | 0.13 |
| 0.008 ± 0.001 | 0.05 ± 0.01 | |
| C2 () | 3.17 ± 0.01 | 2.47 ± 0.06 |
| ---- | 0.9499 | |
| Residual sum of squares | ---- | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farias, K.C.S.; Guimarães, R.C.A.; Oliveira, K.R.W.; Nazário, C.E.D.; Ferencz, J.A.P.; Wender, H. Banana Peel Powder Biosorbent for Removal of Hazardous Organic Pollutants from Wastewater. Toxics 2023, 11, 664. https://doi.org/10.3390/toxics11080664
Farias KCS, Guimarães RCA, Oliveira KRW, Nazário CED, Ferencz JAP, Wender H. Banana Peel Powder Biosorbent for Removal of Hazardous Organic Pollutants from Wastewater. Toxics. 2023; 11(8):664. https://doi.org/10.3390/toxics11080664
Chicago/Turabian StyleFarias, Kelly C. S., Rita C. A. Guimarães, Karla R. W. Oliveira, Carlos E. D. Nazário, Julio A. P. Ferencz, and Heberton Wender. 2023. "Banana Peel Powder Biosorbent for Removal of Hazardous Organic Pollutants from Wastewater" Toxics 11, no. 8: 664. https://doi.org/10.3390/toxics11080664
APA StyleFarias, K. C. S., Guimarães, R. C. A., Oliveira, K. R. W., Nazário, C. E. D., Ferencz, J. A. P., & Wender, H. (2023). Banana Peel Powder Biosorbent for Removal of Hazardous Organic Pollutants from Wastewater. Toxics, 11(8), 664. https://doi.org/10.3390/toxics11080664