Aquatic Ecosystem Risk Assessment Generated by Accidental Silver Nanoparticle Spills in Groundwater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Modeling a Spill into a River or Wetland
2.2. Monte Carlo Model
- ➢
- The selection of the analytical models for the calculation of the dependent variables y (i.e., the maximum concentration of AgNPs in a river and/or wetland) as a function of input i and variables zi (e.g., porosity, hydraulic conductivity, and river flow) (see Section 2.1) is as follows:
- ➢
- The determination of the probability distributions functions (PDFs) of the most relevant independent variables zi (e.g., uniform, normal, and lognormal) is based on the knowledge of the variables and the case studies (see Section 2.4).
- ➢
- The generation of N random values for each independent input variable zi1, zi2, zi3, …..ziN is from each PDF. These results are organized in the vector Zi, which are formed by zij in which j is the random simulation order from 1 to N.
- ➢
- The application of the analytical models (see Section 2.1) for the simulation of the outputs (the maximum concentrations in the river and wetland) of the dependent variables for each random value j.
- ➢
- The recalculation of the output results were conducted repeatedly using a different set of random numbers to produce an output vector Y (e.g., concentration).
- ➢
- The obtention of a probability distribution function of the dependent variables (i.e., the maximum concentration of AgNPs in the river and wetland) are given by Y. An analysis of the results (e.g., distribution shape and sensitivity analyses) (see Section 2.5) was also conducted.
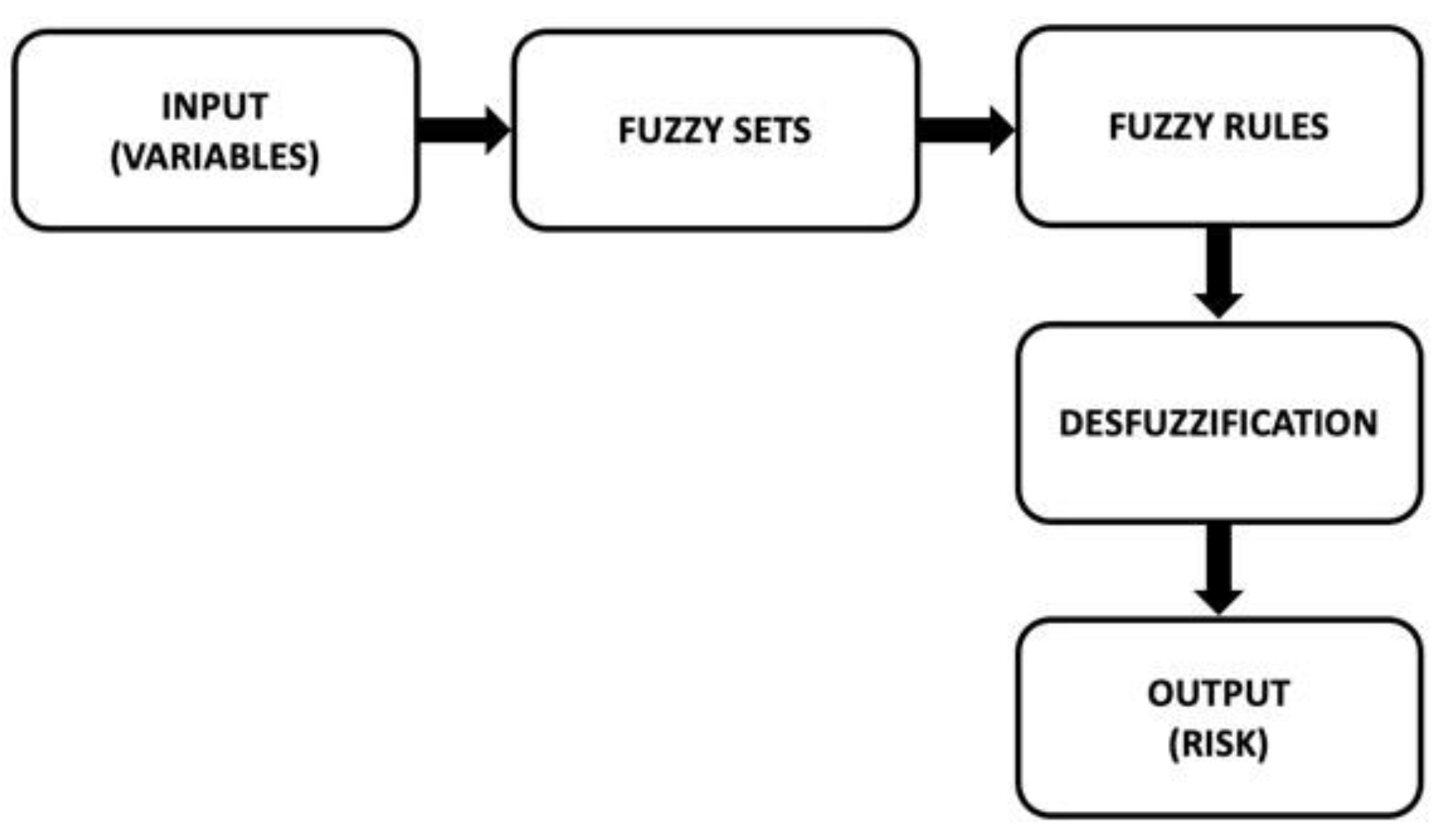
2.3. Fuzzy Logic Model
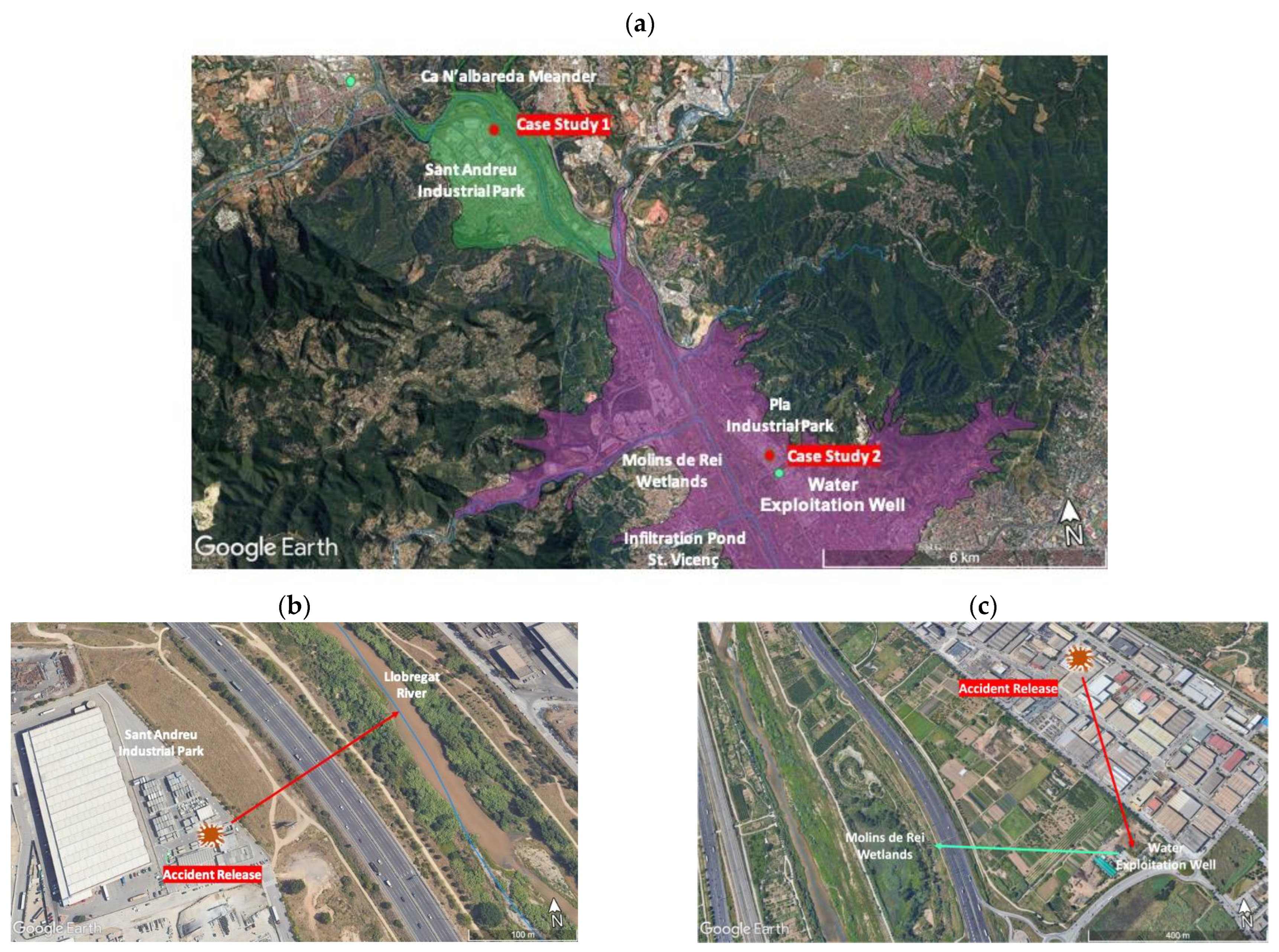
2.4. Case Studies
- 1-
- The presence of silver nanoparticles in the Llobregat River as a consequence of an accidental spill on the soil in the Sant Andreu Industrial Park; this spill reached the groundwater via its discharge and dilution in the Llobregat River (Figure 3b).
- 2-
- An accidental spill in another industrial park (Pla Industrial Park) reached the groundwater, and this was later used to recharge the Molins de Rei Wetlands (Figure 3c).
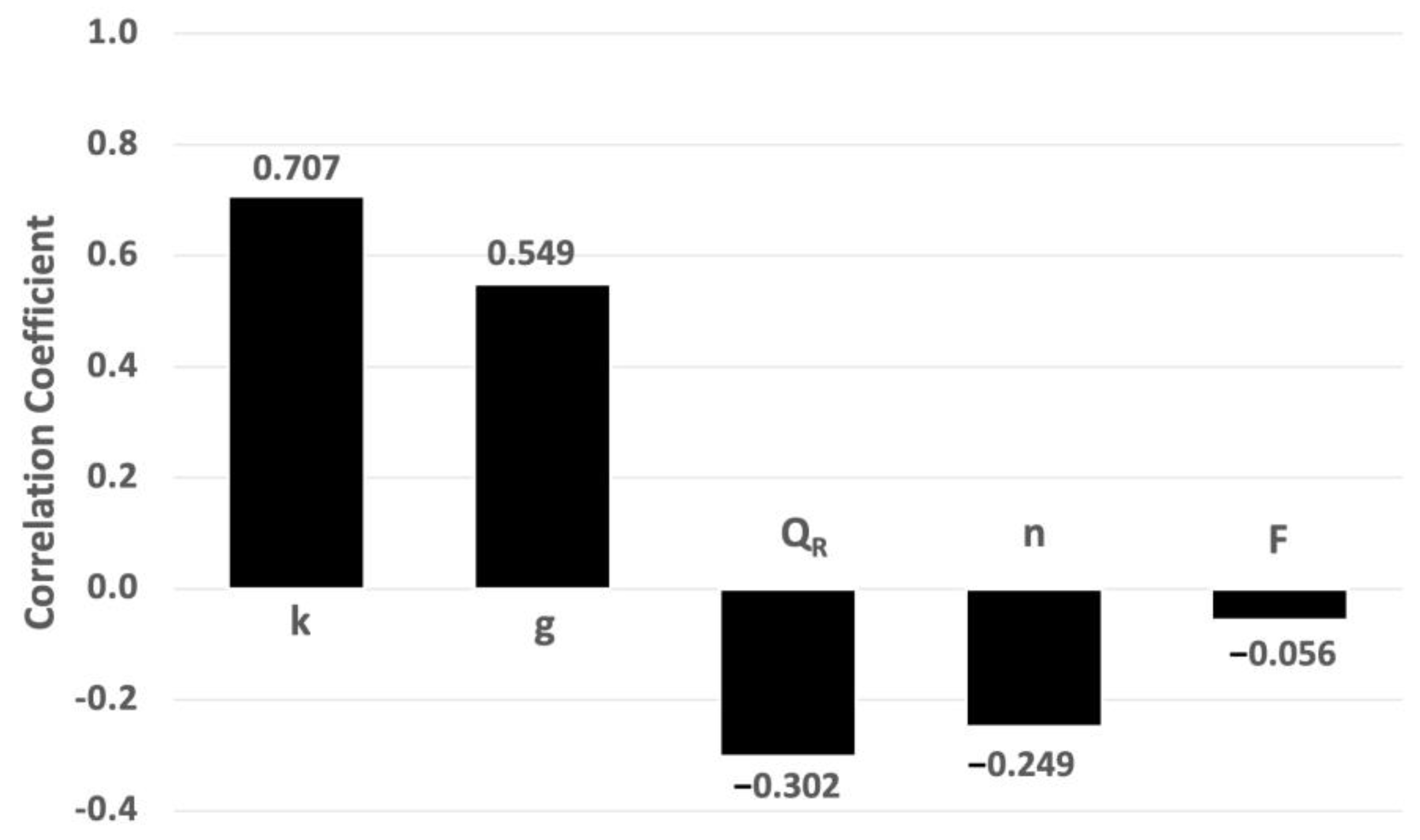
2.5. Sensitivity Analysis
3. Results and Discussion
3.1. Case Study 1
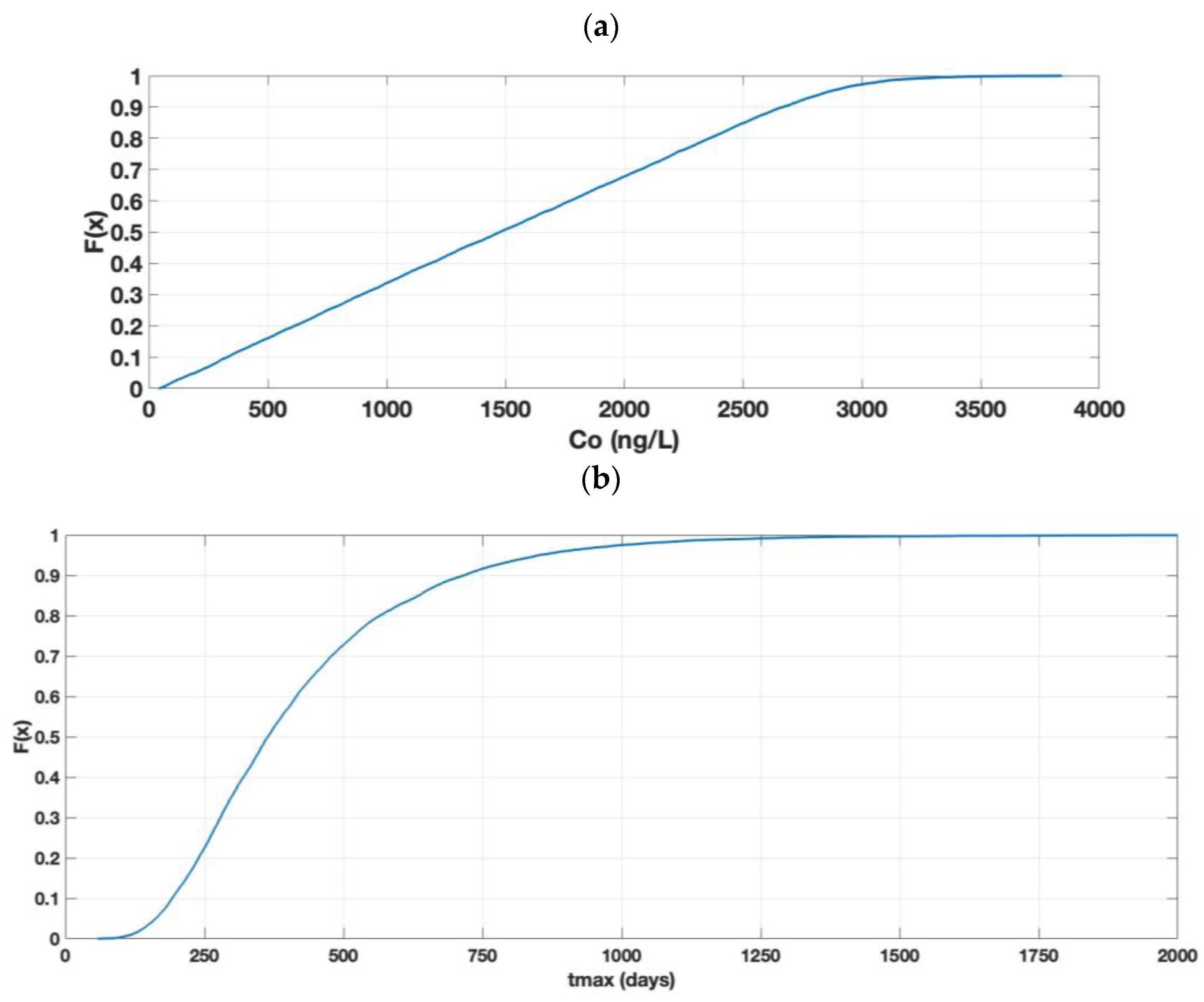
3.1.1. Concentration
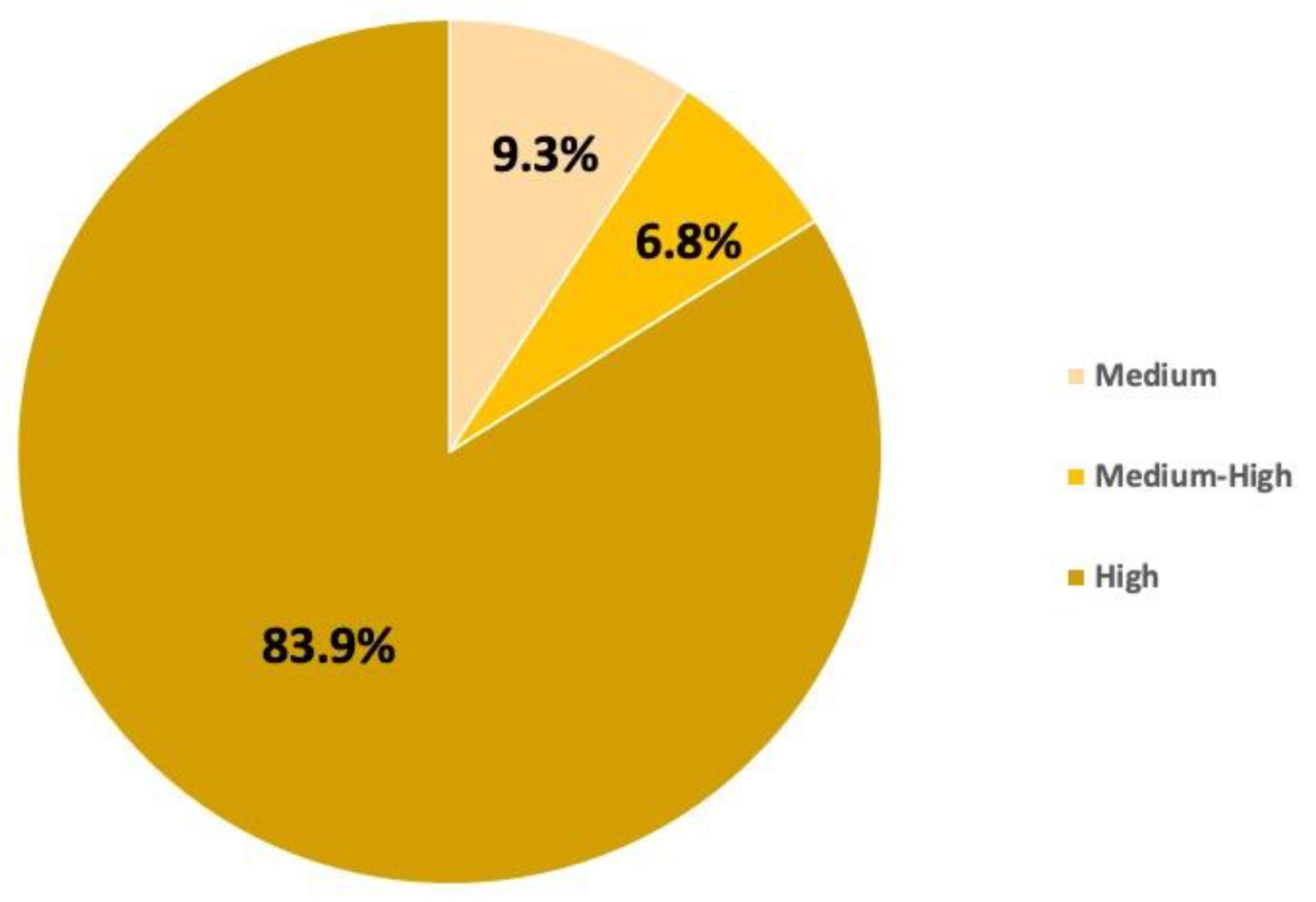
3.1.2. Risk Assessment
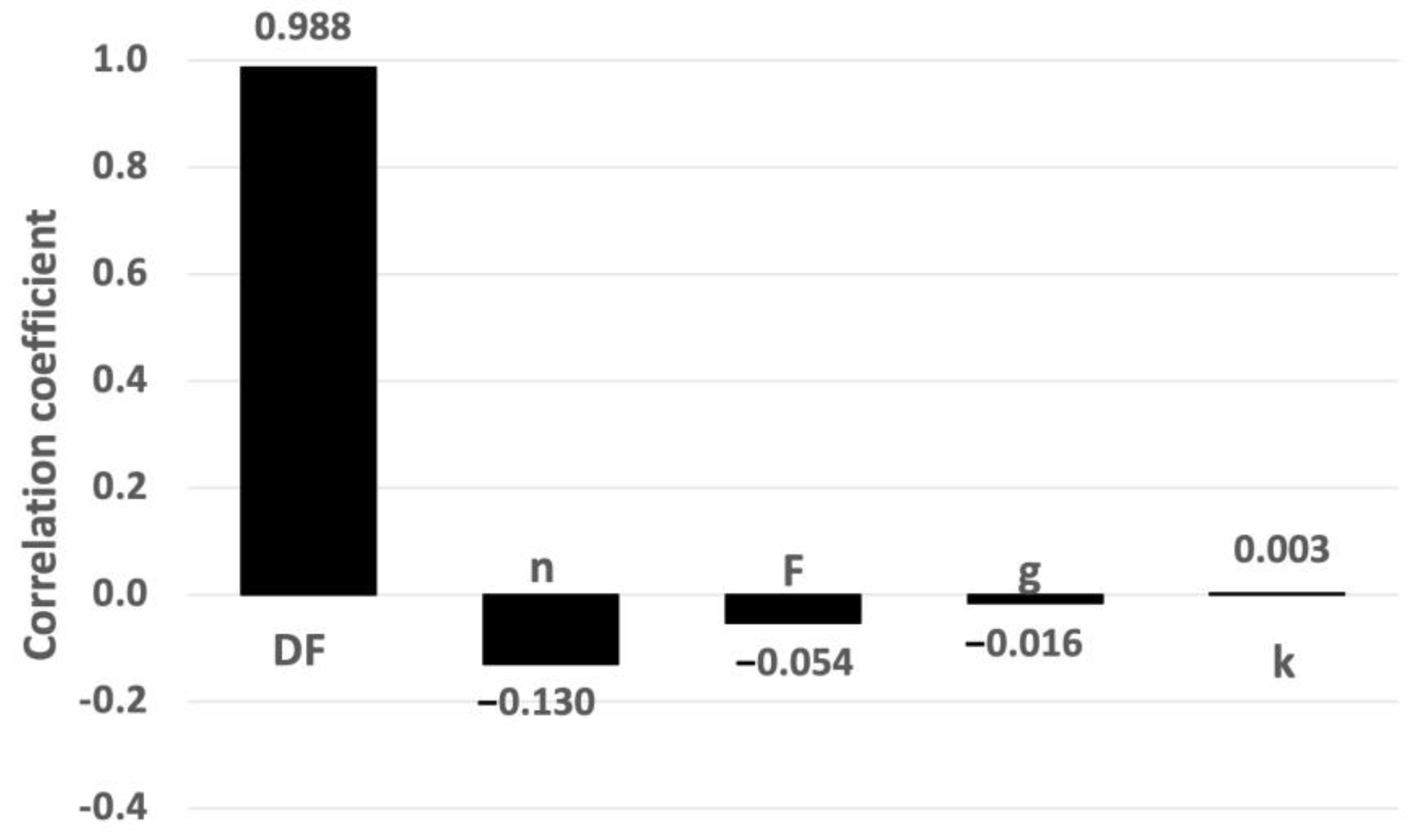
3.1.3. Sensitivity Analysis Applied to AgNP Concentration
3.2. Case Study 2
3.2.1. Concentration
3.2.2. Risk Assessment
3.2.3. Sensitivity Analysis Applied to AgNP Concentration
3.2.4. Sensitivity Analysis Applied to Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lara, H.H.; Ayala-nuñez, N.V.; Ixtepan-turrent, L.; Rodriguez-padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Alsareii, S.A.; Alamri, A.M.; Alasmari, M.Y.; Bawahab, M.A.; Mahnashi, M.H.; Shaikh, I.A.; Shettar, A.K.; Hoskeri, J.H.; Kumbar, V. Synthesis and Characterization of Silver Nanoparticles from Rhizophora apiculata and Studies on Their Wound Healing, Antioxidant, Anti-Inflammatory and ytotoxic Activity. Molecules 2022, 27, 6306. [Google Scholar] [CrossRef]
- Bureau, S.; Montes-hernandez, G.; Di Girolamo, M.; Vernain, E.E.; Fernandez-martinez, A. In Situ Formation of Silver Nanoparticles (Ag-NPs) onto Textile Fibers. ACS Omega 2021, 6, 1316–1327. [Google Scholar] [CrossRef]
- Zhu, Y.; Deng, Y.; Yi, P.; Peng, L.; Lai, X.; Lin, Z. Flexible Transparent Electrodes Based on Silver Nanowires: Material Synthesis, Fabrication, Performance, and Applications. Adv. Mater. Technol. 2019, 4, 1900413. [Google Scholar] [CrossRef]
- Johnson, A.C.; Jürgens, M.D.; Lawlor, A.J.; Cisowska, I.; Williams, R.J. Particulate and colloidal silver in sewage effluent and sludge discharged from British wastewater treatment plants. Chemosphere 2014, 112, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef]
- Gottschalk, F.; Nowack, B. The release of engineered nanomaterials to the environment. J. Environ. Monit. 2011, 13, 1145–1155. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sun, T.; Nowack, B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 2013, 181, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Lassen, C.; Kjoelholt, J.; Christensen, F.; Nowack, B. Modeling flows and concentrations of nine engineered nanomaterials in the Danish environment. Int. J. Environ. Res. Public Health 2015, 12, 5581–5602. [Google Scholar] [CrossRef] [Green Version]
- Mueller, N.C.; Nowack, B. Exposure modeling of engineered nanoparticles in the environment. Environ. Sci. Technol. 2008, 42, 4447–4453. [Google Scholar] [CrossRef]
- Li, L.; Stoiber, M.; Wimmer, A.; Xu, Z.; Lindenblatt, C.; Helmreich, B.; Schuster, M. To What Extent Can Full-Scale Wastewater Treatment Plant Effluent Influence the Occurrence of Silver-Based Nanoparticles in Surface Waters? Environ. Sci. Technol. 2016, 50, 6327–6333. [Google Scholar] [CrossRef]
- Markus, A.A.; Krystek, P.; Tromp, P.C.; Parsons, J.R.; Roex, E.W.M.; de Voogt, P.; Laane, R.W.P.M. Determination of metal-based nanoparticles in the river Dommel in the Netherlands via ultrafiltration, HR-ICP-MS and SEM. Sci. Total Environ. 2018, 631–632, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.J.B.; van Bemmel, G.; Milani, N.B.L.; den Hertog, G.C.T.; Undas, A.K.; van der Lee, M.; Bouwmeester, H. Detection of nanoparticles in Dutch surface waters. Sci. Total Environ. 2018, 621, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Sanchís, J.; Jiménez-Lamana, J.; Abad, E.; Szpunar, J.; Farré, M. Occurrence of Cerium-, Titanium-, and Silver-Bearing Nanoparticles in the Besòs and Ebro Rivers. Environ. Sci. Technol. 2020, 54, 3969–3978. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B.; Ranville, J.F.; Diamond, S.; Gallego-Urrea, J.A.; Metcalfe, C.; Rose, J.; Horne, N.; Koelmans, A.A.; Klaine, S.J. Potential scenarios for nanomaterial release and subsequent alteration in the environment. Environ. Toxicol. Chem. 2012, 31, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Debray, B.; Klaessig, F.; Park, B.; Lacome, J.; Vignes, A.; Portillo, V.P.; Vázquez-campos, S.; Hendren, C.O.; Lofts, S.; et al. Predicting accidental release of engineered nanomaterials to the environment. Nat. Nanotechnol. 2023, 18, 412–418. [Google Scholar] [CrossRef]
- Sohn, E.K.; Johari, S.A.; Kim, T.G.; Kim, J.K.; Kim, E.; Lee, J.H.; Chung, Y.S.; Yu, I.J. Aquatic toxicity comparison of silver nanoparticles and silver nanowires. Biomed Res. Int. 2015, 2015, 893049. [Google Scholar] [CrossRef] [Green Version]
- Sakka, Y.; Skjolding, L.M.; Mackevica, A.; Filser, J.; Baun, A. Behavior and chronic toxicity of two differently stabilized silver nanoparticles to Daphnia magna. Aquat. Toxicol. 2016, 177, 526–535. [Google Scholar] [CrossRef] [Green Version]
- Kleiven, M.; Macken, A.; Oughton, D.H. Growth inhibition in Raphidocelis subcapita—Evidence of nanospecific toxicity of silver nanoparticles. Chemosphere 2019, 221, 785–792. [Google Scholar] [CrossRef]
- Lacave, J.M.; Fanjul, Á.; Bilbao, E.; Gutierrez, N.; Barrio, I.; Arostegui, I.; Cajaraville, M.P.; Orbea, A. Acute toxicity, bioaccumulation and effects of dietary transfer of silver from brine shrimp exposed to PVP/PEI-coated silver nanoparticles to zebrafish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 199, 69–80. [Google Scholar] [CrossRef]
- Pakrashi, S.; Tan, C.; Wang, W.X. Bioaccumulation-based silver nanoparticle toxicity in Daphnia magna and maternal impacts. Environ. Toxicol. Chem. 2017, 36, 3359–3366. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, Q.; Shen, L.; Ling, J.; Zhou, C.; Hu, J.; Chen, L. Surface charge-dependent bioaccumulation dynamics of silver nanoparticles in freshwater algae. Chemosphere 2020, 247, 125936. [Google Scholar] [CrossRef] [PubMed]
- EU Annexes 1–6 to the Proposal of Directive (EU) COM(2022) 540 Final. Available online: https://eurlex.europa.eu/resource.html?uri=cellar:d0c11ba6-55f8-11ed-92ed%0D%0A01aa75ed71a1.0001.02/DOC_2&format=PDF (accessed on 14 March 2023).
- Bradford, S.A.; Simunek, J.; Bettahar, M.; Van Genuchten, M.T.; Yates, S.R. Modeling colloid attachment, straining, and exclusion in saturated porous media. Environ. Sci. Technol. 2003, 37, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Tosco, T.; Sethi, R. MNM1D: A numerical code for colloid transport in porous media: Implementation and validation. Am. J. Environ. Sci. 2009, 5, 516–524. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Jaisi, D.P.; Yan, J.; Jin, Y.; Zhou, D. Transport and Retention of Polyvinylpyrrolidone-Coated Silver Nanoparticles in Natural Soils. Vadose Zo. J. 2015, 14, vzj2015-01. [Google Scholar] [CrossRef] [Green Version]
- Park, C.M.; Chu, K.H.; Heo, J.; Her, N.; Jang, M.; Son, A.; Yoon, Y. Environmental behavior of engineered nanomaterials in porous media: A review. J. Hazard. Mater. 2016, 309, 133–150. [Google Scholar] [CrossRef]
- Liang, Y.; Bradford, S.A.; Simunek, J.; Heggen, M.; Vereecken, H.; Klumpp, E. Retention and remobilization of stabilized silver nanoparticles in an undisturbed loamy sand soil. Environ. Sci. Technol. 2013, 47, 12229–12237. [Google Scholar] [CrossRef] [Green Version]
- Baalousha, M.; Cornelis, G.; Kuhlbusch, T.A.J.; Lynch, I.; Nickel, C.; Peijnenburg, W.; Van Den Brink, N.W. Modeling nanomaterial fate and uptake in the environment: Current knowledge and future trends. Environ. Sci. Nano 2016, 3, 323–345. [Google Scholar] [CrossRef]
- Bianco, C.; Tosco, T.; Sethi, R. A 3-dimensional micro- and nanoparticle transport and filtration model (MNM3D) applied to the migration of carbon-based nanomaterials in porous media. J. Contam. Hydrol. 2016, 193, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Tosco, T.; Sethi, R. Human health risk assessment for nanoparticle-contaminated aquifer systems. Environ. Pollut. 2018, 239, 242–252. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, M.; Wang, P.; Wang, C.; Miao, L.; Xu, Y.; You, G.; Lv, B.; Yang, Y.; Liu, Z. Transport and long-term release behavior of polymer-coated silver nanoparticles in saturated quartz sand: The impacts of input concentration, grain size and flow rate. Environ. Pollut. 2017, 229, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Leuther, F.; Köhne, J.M.; Metreveli, G.; Vogel, H.J. Transport and Retention of Sulfidized Silver Nanoparticles in Porous Media: The Role of Air-Water Interfaces, Flow Velocity, and Natural Organic Matter. Water Resour. Res. 2020, 56, e2020WR027074. [Google Scholar] [CrossRef]
- Cornelis, G.; Pang, L.; Doolette, C.; Kirby, J.K.; McLaughlin, M.J. Transport of silver nanoparticles in saturated columns of natural soils. Sci. Total Environ. 2013, 463–464, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ge, L.; He, J.; Zhang, W.; Jaisi, D.P.; Zhou, D. Hyperexponential and nonmonotonic retention of polyvinylpyrrolidone-coated silver nanoparticles in an Ultisol. J. Contam. Hydrol. 2014, 164, 35–48. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Aly Hassan, A.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Key factors controlling the transport of silver nanoparticles in porous media. Environ. Sci. Technol. 2013, 47, 4039–4045. [Google Scholar] [CrossRef]
- Braun, A.; Klumpp, E.; Azzam, R.; Neukum, C. Transport and deposition of stabilized engineered silver nanoparticles in water saturated loamy sand and silty loam. Sci. Total Environ. 2015, 535, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Yecheskel, Y.; Dror, I.; Berkowitz, B. Silver nanoparticle (Ag-NP) retention and release in partially saturated soil: Column experiments and modelling. Environ. Sci. Nano 2018, 5, 422–435. [Google Scholar] [CrossRef]
- Taghavy, A.; Mittelman, A.; Wang, Y.; Pennell, K.D.; Abriola, L.M. Mathematical Modeling of the Transport and Dissolution of Citrate-Stabilized Silver Nanoparticles in Porous Media. Environ. Sci. Technol. 2013, 47, 8499–8507. [Google Scholar] [CrossRef]
- Barton, L.E.; Auffan, M.; Durenkamp, M.; McGrath, S.; Bottero, J.Y.; Wiesner, M.R. Monte Carlo simulations of the transformation and removal of Ag, TiO2, and ZnO nanoparticles in wastewater treatment and land application of biosolids. Sci. Total Environ. 2015, 511, 535–543. [Google Scholar] [CrossRef]
- Ramirez, R.; Martí, V.; Darbra, R.M. Environmental Risk Assessment of Silver Nanoparticles in Aquatic Ecosystems Using Fuzzy Logic. Water 2022, 14, 1885. [Google Scholar] [CrossRef]
- Li, W.; Zhou, J.; Xie, K.; Xiong, X. Power System Risk Assessment Using a Hybrid Method of Fuzzy Set and Monte Carlo Simulation. IEEE Trans. Power Syst. 2008, 23, 336–343. [Google Scholar]
- Yang, A.L.; Huang, G.H.; Qin, X.S. An Integrated Simulation-Assessment Approach for Evaluating Health Risks of Groundwater Contamination Under Multiple Uncertainties. Water Resour. Manag. 2010, 24, 3349–3369. [Google Scholar] [CrossRef]
- Kentel, E.; Aral, M. 2D Monte Carlo versus 2D Fuzzy Monte Carlo health risk assessment. Stoch. Environ. Res. Risk Assess. 2005, 19, 86–96. [Google Scholar] [CrossRef]
- Li, H.L.; Huang, G.H.; Zou, Y. An integrated fuzzy-stochastic modeling approach for assessing health-impact risk from air pollution. Stoch. Environ. Res. Risk Assess. 2008, 22, 789–803. [Google Scholar] [CrossRef]
- Sethi, R.; Molfetta, A. Di Groundwater Engineering A Technical Approach to Hydrogeology, Contaminant Transport and Groundwater Remediation, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Xu, M.; Eckstein, Y. Use of Weighted Least-Squares Method in Evaluation of the Relationship Between Dispersivity and Field Scale. Groundwater 1995, 33, 905–908. [Google Scholar] [CrossRef]
- Abramowitz, M.; Stegun, I.A. Handbook of Mathematical Functions with Formulas, Graphs, and Mathematical Tables. Natl. Bur. Stand. Appl. Math. 1964, 55, 1046. [Google Scholar] [CrossRef] [Green Version]
- Metropolis, N.; Ulam, S. The Monte Carlo Method. J. Am. Stat. Assoc. 1949, 44, 335–341. [Google Scholar] [CrossRef]
- Hendren, C.O.; Badireddy, A.R.; Casman, E.; Wiesner, M.R. Modeling nanomaterial fate in wastewater treatment: Monte Carlo simulation of silver nanoparticles (nano-Ag). Sci. Total Environ. 2013, 449, 418–425. [Google Scholar] [CrossRef]
- Gottschalk, F.; Scholz, R.W.; Nowack, B. Probabilistic material flow modeling for assessing the environmental exposure to compounds: Methodology and an application to engineered nano-TiO2 particles. Environ. Model. Softw. 2010, 25, 320–332. [Google Scholar] [CrossRef]
- Christian, J.; Christensen, S.; Sonnenborg, T.O.; Seifert, D.; Lajer, A.; Troldborg, L. Advances in Water Resources Review of strategies for handling geological uncertainty in groundwater flow and transport modeling. Adv. Water Resour. 2012, 36, 36–50. [Google Scholar] [CrossRef]
- Thomopoulos, N.T. Essentials of Monte Carlo Simulation Statistical Methods for Building Simulation Models, 1st ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Zio, E. The Monte Carlo Simulation Method for System Reliability and Risk Analysis, 1st ed.; Springer: London, UK, 2013. [Google Scholar]
- González, J.R.; Arnaldos, J.; Darbra, R.M. Introduction of the human factor in the estimation of accident frequencies through fuzzy logic. Saf. Sci. 2017, 97, 134–143. [Google Scholar] [CrossRef]
- Ocampo-Duque, W.; Ferré-Huguet, N.; Domingo, J.L.; Schuhmacher, M. Assessing water quality in rivers with fuzzy inference systems: A case study. Environ. Int. 2006, 32, 733–742. [Google Scholar] [CrossRef] [PubMed]
- González, R.J.; Darbra, R.M.; Arnaldos, J. Using fuzzy logic to introduce the human factor in the failure frequency estimation of storage vessels in chemical plants. Chem. Eng. Trans. 2013, 32, 193–198. [Google Scholar] [CrossRef]
- González Dan, J.R.; Darbra Roman, R.M. Reduction of the uncertainty in the frequency calculation in port accidents through fuzzy logic. Fresenius Environ. Bull. 2014, 23, 2840–2846. [Google Scholar]
- Cabanillas, J.; Ginebreda, A.; Guillén, D.; Martínez, E.; Barceló, D.; Moragas, L.; Robusté, J.; Darbra, R.M. Fuzzy logic based risk assessment of effluents from waste-water treatment plants. Sci. Total Environ. 2012, 439, 202–210. [Google Scholar] [CrossRef]
- Seguí, X.; Pujolasus, E.; Betrò, S.; Àgueda, A.; Casal, J.; Ocampo-Duque, W.; Rudolph, I.; Barra, R.; Páez, M.; Barón, E.; et al. Fuzzy model for risk assessment of persistent organic pollutants in aquatic ecosystems. Environ. Pollut. 2013, 178, 23–32. [Google Scholar] [CrossRef]
- Topuz, E.; van Gestel, C.A.M. An approach for environmental risk assessment of engineered nanomaterials using Analytical Hierarchy Process (AHP) and fuzzy inference rules. Environ. Int. 2016, 92–93, 334–347. [Google Scholar] [CrossRef]
- Mielgo, Á.D. Study of Llobregat River Morphodynamic Evolution in Its Final Section; Polytechnic University of Catalonia: Barcelona, Spain, 2015. [Google Scholar]
- Agència Catalana de l’Aigua. Area Fluviodeltaíca del Llobregat; Agència Catalana de l’Aigua: Barcelona, Spain, 2005.
- Agència Catalana de l’Aigua. Massa d’Aigua 38. Cubeta de Sant Andreu i Vall Baixa del Llobregat; Agència Catalana de l’Aigua: Barcelona, Spain, 2004.
- Silva, T.; Pokhrel, L.R.; Dubey, B.; Tolaymat, T.M.; Maier, K.J.; Liu, X. Particle size, surface charge and concentration dependent ecotoxicity of three organo-coated silver nanoparticles: Comparison between general linear model-predicted and observed toxicity. Sci. Total Environ. 2014, 468–469, 968–976. [Google Scholar] [CrossRef]
- Xue, Y.; Mishra, P.; Eivazi, F.; Afrasiabi, Z. Effects of silver nanoparticle size, concentration and coating on soil quality as indicated by arylsulfatase and sulfite oxidase activities. Pedosphere 2022, 32, 733–743. [Google Scholar] [CrossRef]
- He, J.; Wang, D.; Zhou, D. Transport and retention of silver nanoparticles in soil: Effects of input concentration, particle size and surface coating. Sci. Total Environ. 2019, 648, 102–108. [Google Scholar] [CrossRef]
- Lim, M.; Hwang, G.; Bae, S.; Jang, M.H.; Choi, S.; Kim, H.; Hwang, Y.S. Transport of citrate-coated silver nanoparticles in saturated porous media. Environ. Geochem. Health 2020, 42, 1753–1766. [Google Scholar] [CrossRef] [PubMed]
- Agencia Catalana de l’Aigua Consulta de les Dades de Control de la Qualitat i la Quantitat de l’aigua al Medi. Available online: http://aca-web.gencat.cat/sdim21/seleccioXarxes.do;jsessionid=4B6442745A6C3695855A89488EBD1B3F (accessed on 12 October 2022).
- Banjac, Z.; Ginebreda, A.; Kuzmanovic, M.; Marcé, R.; Nadal, M.; Riera, J.M.; Barceló, D. Emission factor estimation of ca. 160 emerging organic microcontaminants by inverse modeling in a Mediterranean river basin (Llobregat, NE Spain). Sci. Total Environ. 2015, 520, 241–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institut Geològic de Catalunya. Mapa Hidrogeològic del Tram Baix del Llobregat i el Seu Delta 1:30,000; Institut Cartogràfic de Catalunya—ICC: Barcelona, Spain, 2006. [Google Scholar]
- Domenico, P.A.; Schwartz, F.W. Physical and Chemical Hydrogeology, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Sendrós Brea-Iglesias, A. Using Geophysical Techniques in Planning and Management of Groundwater Resources. Application in Mediterranean Aquifers. Ph.D. Thesis, Universitat de Barcelona, Barcelona, Spain, 2016. [Google Scholar]
- Sendrós, A.; Himi, M.; Lovera, R.; Rivero, L.; Garcia-Artigas, R.; Urruela, A.; Casas, A. Geophysical characterization of hydraulic properties around a managed aquifer recharge system over the llobregat river alluvial aquifer (Barcelona metropolitan area). Water 2020, 12, 3455. [Google Scholar] [CrossRef]
- Valhondo, C.; Carrera, J.; Ayora, C.; Barbieri, M.; Nödler, K.; Licha, T.; Huerta, M. Behavior of nine selected emerging trace organic contaminants in an artificial recharge system supplemented with a reactive barrier. Environ. Sci. Pollut. Res. 2014, 21, 11832–11843. [Google Scholar] [CrossRef]
- Valhondo, C.; Carrera, J.; Ayora, C.; Tubau, I.; Martinez-Landa, L.; Nödler, K.; Licha, T. Characterizing redox conditions and monitoring attenuation of selected pharmaceuticals during artificial recharge through a reactive layer. Sci. Total Environ. 2015, 512–513, 240–250. [Google Scholar] [CrossRef]
- Catalunya, Generalitat de Departament d’Agricultura, Ramaderia Pesca, A. i M.N. Aiguamolls de Mollins de Rei. Available online: https://mediambient.gencat.cat/web/.content/home/ambits_dactuacio/patrimoni_natural/sistemes_dinformacio/inventari_zones_humides/documents_fitxes/llobregat/fitxers_estatics/08001109_aiguamolls_molins_rei.pdf (accessed on 14 March 2023).
- RuralCat Dades D’evapotranspiració i Precipitació de la XAC. Available online: https://ruralcat.gencat.cat/reg.dadesmeteo (accessed on 14 March 2023).
- USEPA. Risk Assessment Guidance for Superfund (RAGS) Volume III—Part A: Process for Conducting Probabilistic Risk Assessment, Appendix B; U.S. Environmental Protection Agency: Washington, DC, USA, 2001; Volume III.
- Vásquez, G.A.; Busschaert, P.; Haberbeck, L.U.; Uyttendaele, M.; Geeraerd, A.H. An educationally inspired illustration of two-dimensional Quantitative Microbiological Risk Assessment (QMRA) and sensitivity analysis. Int. J. Food Microbiol. 2014, 190, 31–43. [Google Scholar] [CrossRef]
- Carlos García-Díaz, J.; Gozalvez-Zafrilla, J.M. Uncertainty and sensitive analysis of environmental model for risk assessments: An industrial case study. Reliab. Eng. Syst. Saf. 2012, 107, 16–22. [Google Scholar] [CrossRef]




| Variables zi (Units) | PDF Type | Distribution Parameters | ||
|---|---|---|---|---|
| Accident from Case Study 1 | Accident from Case Study 2 | |||
| Source | m (µg) | Constant | 2*109 | |
| Aquifer | b (m) | Constant | 3 | |
| L (m) | Constant | 200 | 600 | |
| n (-) | Normal | Mean = 0.15 St. dev. = 0.02 | Mean = 0.25 St. dev. = 0.02 | |
| g (-) | Uniform | Min = 0.0005 Max = 0.0015 | ||
| k (m/day) | LogNormal | Mean = 5.5 St dev. = 0.4 | Mean = 6.1 St. dev. = 0.4 | |
| Interaction NPs—Aquifer | F (-) | Uniform | Min = 1 Max = 1.1 | |
| River | QR (m3/s) | LogNormal | Mean = 1.887 St. dev. = 0.248 | - |
| Wetland | DF (-) | Uniform | - | Min = 4.2*10−4 Max =2.7*10−2 |
| SRXY | |||
|---|---|---|---|
| X-Y | Co (ng/L) | Case Study 1 | Case Study 2 |
| Size *-T, Size *-R | - | 0 | 0 |
| Co-R | 3–8 | 0–0.2 | - |
| 8–12 | 0.2–0.5 | - | |
| 12–19 | 0.5–2 | - | |
| 19.0–21.9 | 2–21.3 | - | |
| 21.90–21.99 | 0.2–2 | - | |
| 22–300 | 0 ** | ||
| 300–370 | 0–0.2 | ||
| 370–400 | 0.2–0.5 | ||
| 400–430 | 0.5–2 | ||
| 430–475 | 2–3.1 | ||
| 475–490 | 0.2–2 | ||
| >490 | 0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez, R.; Martí, V.; Darbra, R.M. Aquatic Ecosystem Risk Assessment Generated by Accidental Silver Nanoparticle Spills in Groundwater. Toxics 2023, 11, 671. https://doi.org/10.3390/toxics11080671
Ramirez R, Martí V, Darbra RM. Aquatic Ecosystem Risk Assessment Generated by Accidental Silver Nanoparticle Spills in Groundwater. Toxics. 2023; 11(8):671. https://doi.org/10.3390/toxics11080671
Chicago/Turabian StyleRamirez, Rosember, Vicenç Martí, and R. M. Darbra. 2023. "Aquatic Ecosystem Risk Assessment Generated by Accidental Silver Nanoparticle Spills in Groundwater" Toxics 11, no. 8: 671. https://doi.org/10.3390/toxics11080671







