Degradation of Sodium Acetate by Catalytic Ozonation Coupled with a Mn-Functionalized Fly Ash: Reaction Parameters and Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Pretreatment Process
2.2. Preparation of Catalyst
2.3. Characterization of Catalyst
2.4. Catalytic Ozonation Reaction
3. Results and Discussion
3.1. Characteristics of Pre-Treated Fly Ash Sample
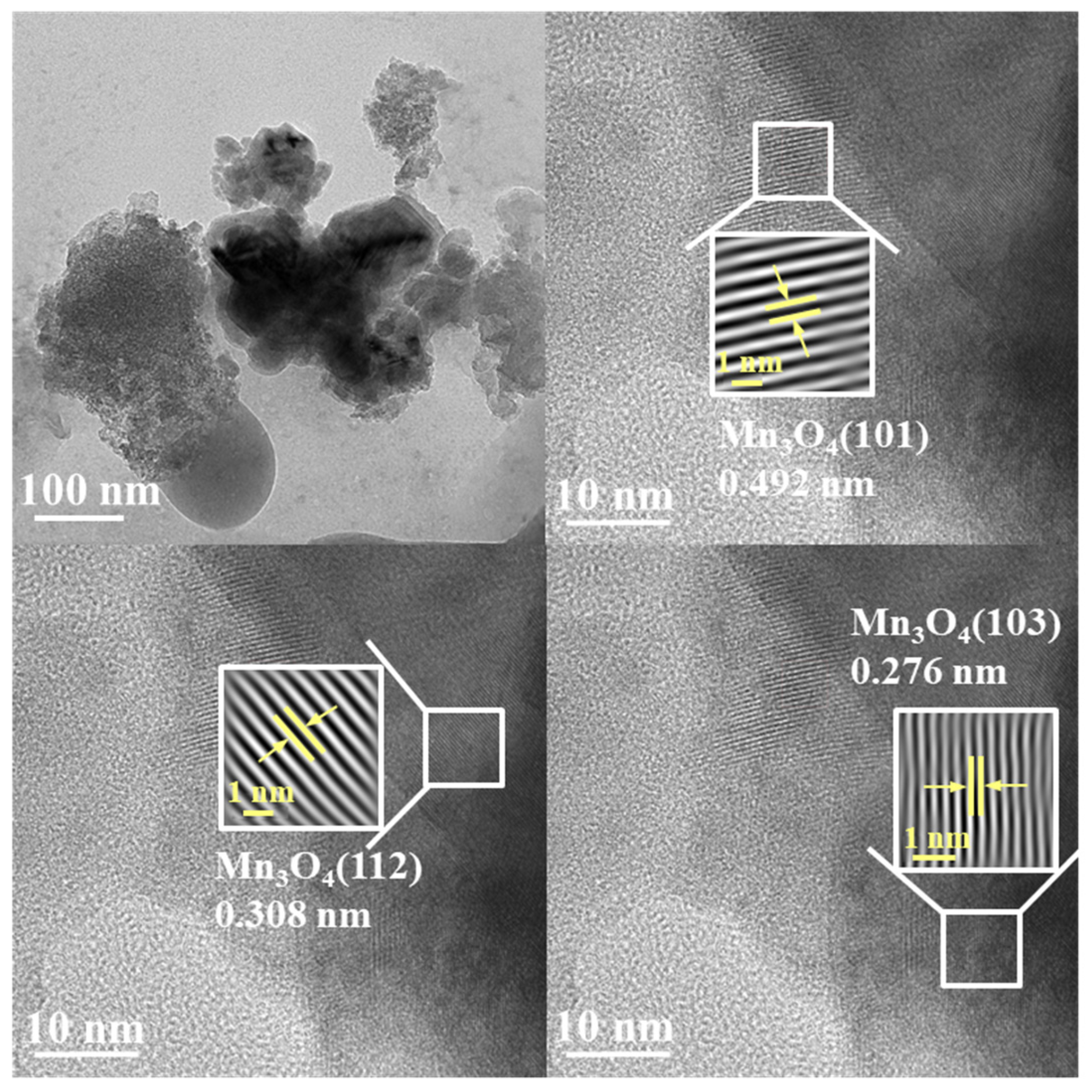
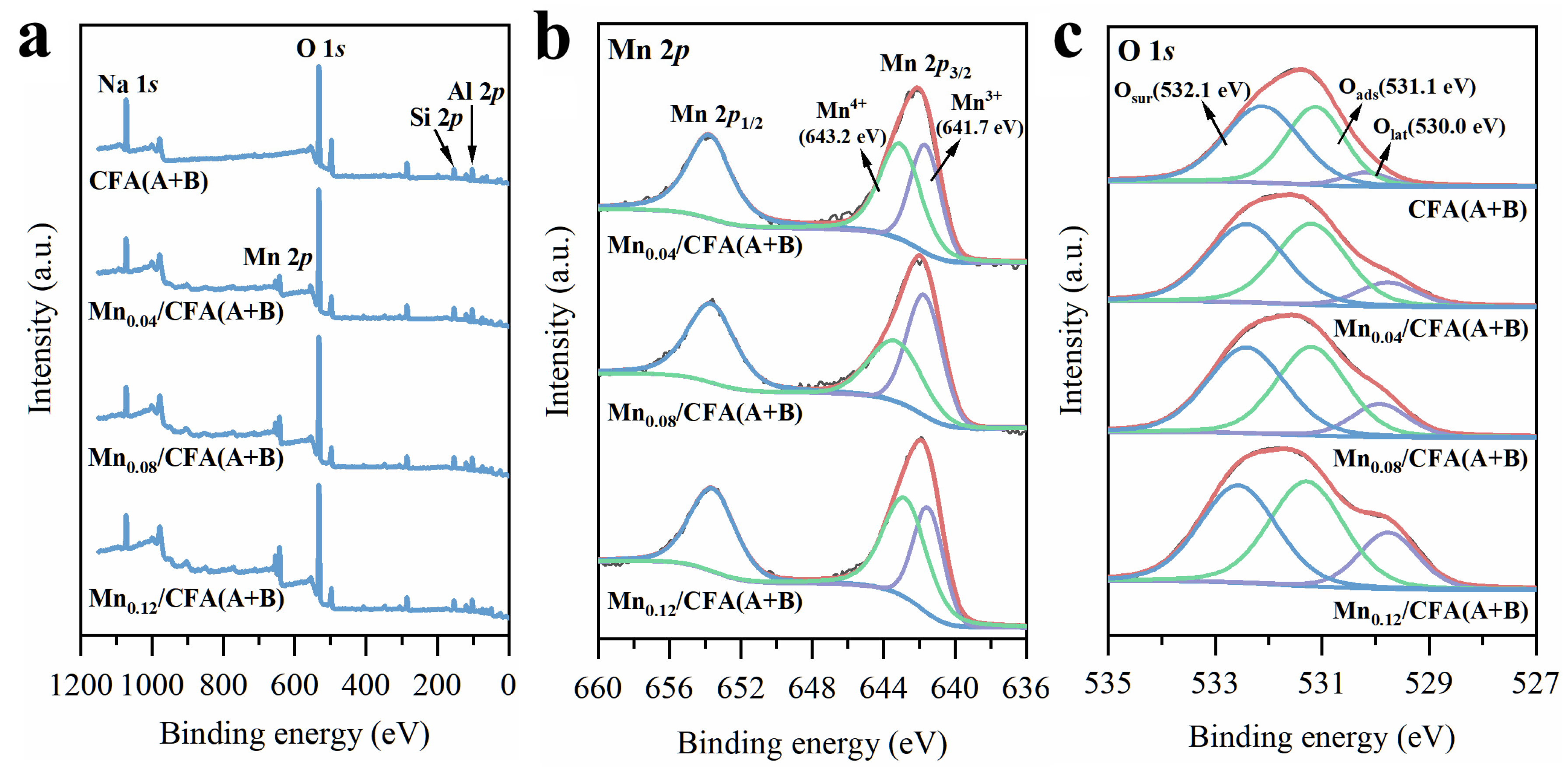
3.2. Characteristics of Mn-Modified Samples
3.3. Catalytic Ozonation Performance
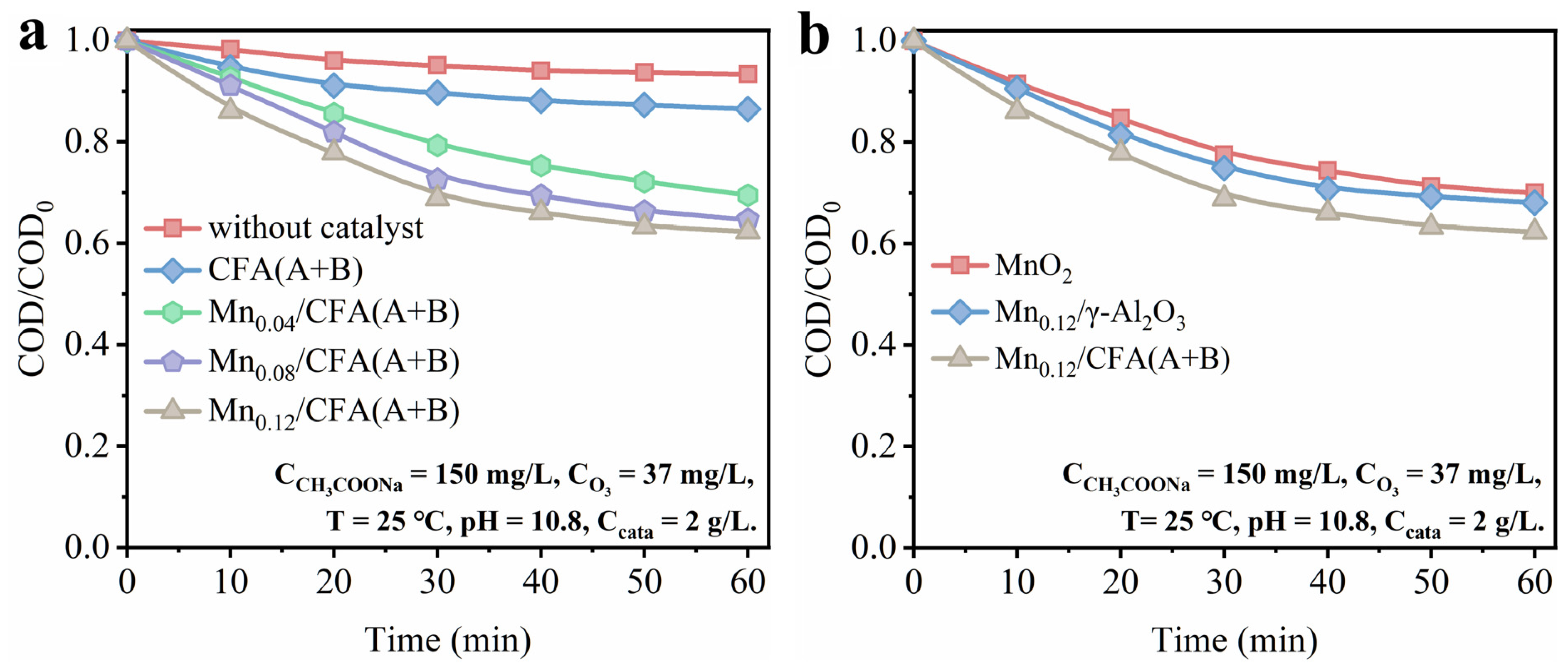
3.3.1. Effect of Loading Amount and Catalyst Carrier
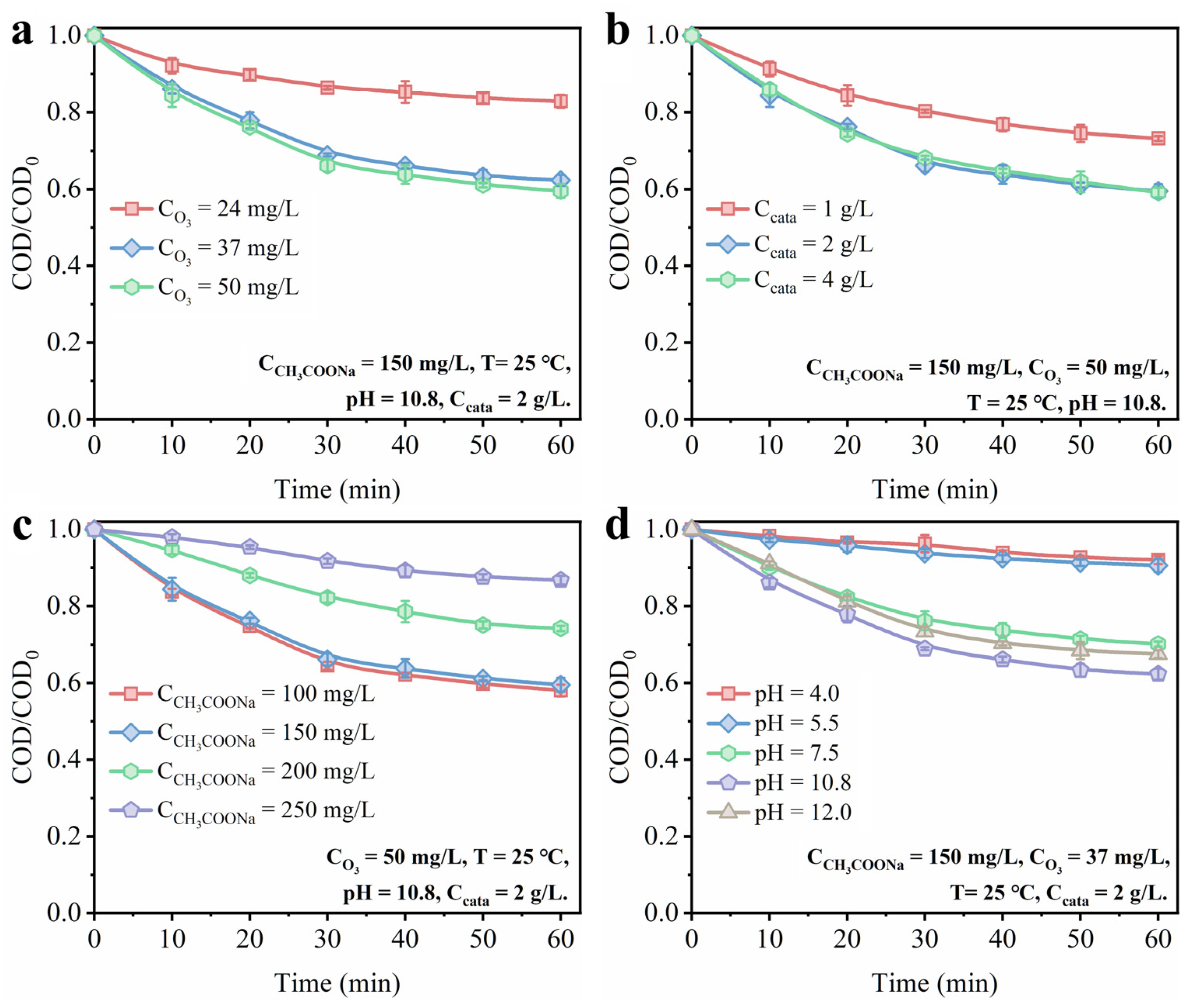
3.3.2. Effect of Different Reaction Parameter
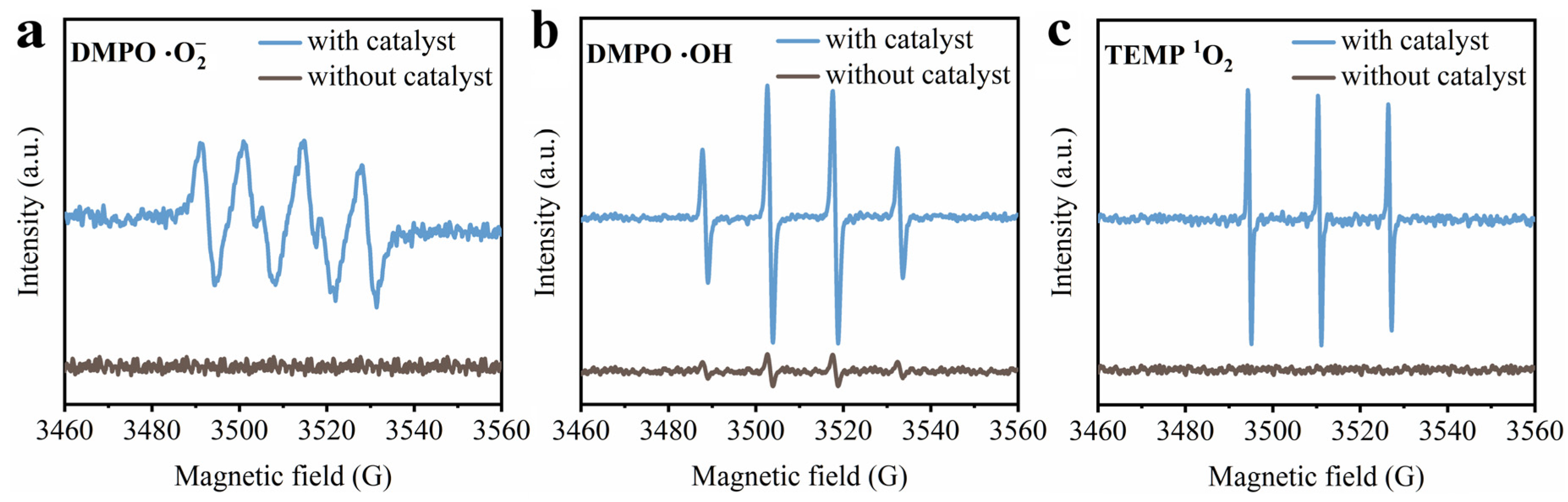
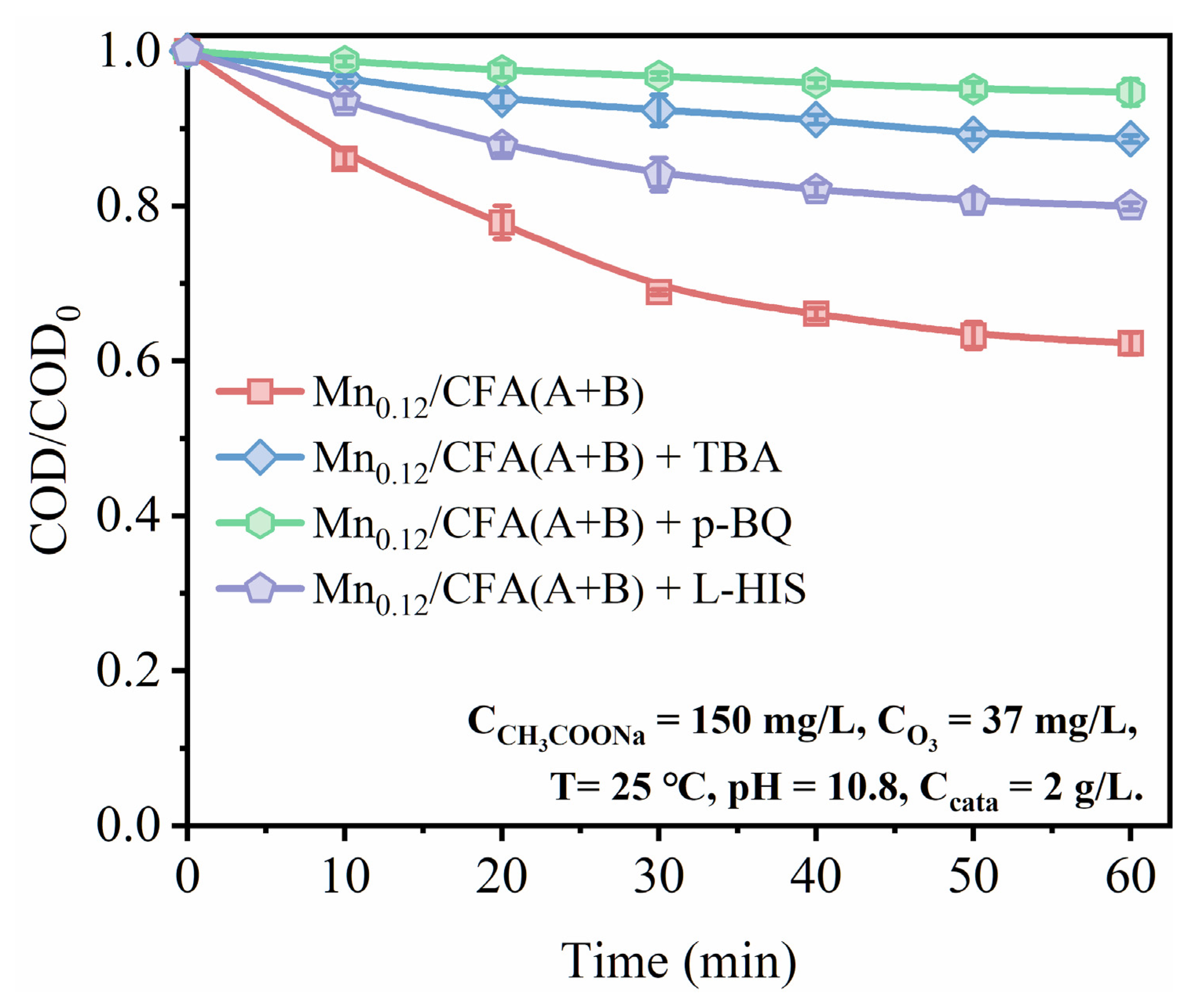
3.4. ROS Generation over Mn-Modified Fly Ash
3.5. Reusability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, J.; Wang, L.; Yu, J.; Guo, B.; Chen, L.; Zhang, Y.; Wang, D.; Shen, Z.; Tsang, D.-C.-W. Cytotoxicity of stabilized/solidified municipal solid waste incineration fly ash. J. Hazard. Mater. 2022, 424, 127369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Chen, L.; Ma, B.; Zhang, Y.; Ni, W.; Tsang, D.-C.-W. Treatment of municipal solid waste incineration fly ash: State-of-the-art technologies and future perspectives. J. Hazard. Mater. 2021, 411, 125132. [Google Scholar]
- Chen, W.; Song, G.; Lin, Y.; Qiao, J.; Wu, T.; Yi, X.; Kawi, S. Synthesis and catalytic performance of Linde-type A zeolite (LTA) from coal fly ash utilizing microwave and ultrasound collaborative activation method. Catal. Today 2022, 397, 407–418. [Google Scholar] [CrossRef]
- Gadore, V.; Ahmaruzzaman, M. Tailored fly ash materials: A recent progress of their properties and applications for remediation of organic and inorganic contaminants from water. J. Water Process. Eng. 2021, 41, 101910. [Google Scholar]
- Wang, N.; Jin, L.; Li, C.; Liang, Y.; Wang, P. Preparation of coal fly ash-based Fenton-like catalyst and its application for the treatment of organic wastewater under microwave assistance. J. Clean. Prod. 2022, 342, 130926. [Google Scholar] [CrossRef]
- Ramírez-Franco, J.-H.; Galeano, L.-A.; Vicente, M.-A. Fly ash as photo-Fenton catalyst for the degradation of amoxicillin. J. Environ. Chem. Eng. 2019, 7, 103274. [Google Scholar] [CrossRef]
- Sun, P.; Cheng, L.; Gao, S.; Weng, X.; Dong, X. Industrial Chlorinated Organic Removal with Elimination of Secondary Pollution: A Perspective. J. Phys. Chem. C 2023, 127, 6610–6618. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Zhang, J.; Wu, F.; Liu, F.; Zhao, H.; Hu, X.; Zhao, X.; Li, J.; Ju, X.; et al. Stabilization of lead in waste water and farmland soil using modified coal fly ash. J. Clean. Prod. 2021, 314, 127957. [Google Scholar] [CrossRef]
- Singh, N.-B.; Agarwal, A.; De, A.; Singh, P. Coal fly ash: An emerging material for water remediation. Int. J. Coal Sci. Technol. 2022, 9, 44. [Google Scholar]
- Chen, X.; Zhang, G.; Li, J.; Ji, P. Possibility of removing Pb and Cd from polluted water by modified fly ash. Adsorp. Sci. Technol. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Buema, G.; Lupu, N.; Chiriac, H.; Ciobanu, G.; Bucur, R.-D.; Bucur, D.; Favier, L.; Harja, M. Performance assessment of five adsorbents based on fly ash for removal of cadmium ions. J. Mol. Liq. 2021, 333, 115932. [Google Scholar] [CrossRef]
- Wang, N.; Sun, X.; Zhao, Q.; Wang, P. Treatment of polymer-flooding wastewater by a modified coal fly ash-catalysed Fenton-like process with microwave pre-enhancement: System parameters, kinetics, and proposed mechanism. Chem. Eng. J. 2021, 406, 126734. [Google Scholar] [CrossRef]
- Yao, Z.-T.; Ji, X.-S.; Sarker, P.-K.; Tang, J.-H.; Ge, L.-Q.; Xia, M.-S.; Xi, Y.-Q. A comprehensive review on the applications of coal fly ash. Earth Sci. Rev. 2015, 141, 105–121. [Google Scholar]
- Feng, W.; Wan, Z.; Daniels, J.; Li, Z.; Xiao, G.; Yu, J.; Xu, D.; Guo, H.; Zhang, D.; May, E.-F.; et al. Synthesis of high quality zeolites from coal fly ash: Mobility of hazardous elements and environmental applications. J. Clean. Prod. 2018, 202, 390–400. [Google Scholar] [CrossRef]
- Panek, R.; Wdowin, M.; Franus, W.; Czarna, D.; Stevens, L.-A.; Deng, H.; Liu, J.; Sun, C.; Liu, H.; Snape, C.-E. Fly ash-derived MCM-41 as a low-cost silica support for polyethyleneimine in post-combustion CO2 capture. J. CO2 Util. 2017, 22, 81–90. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Sun, Q.; Xu, W.-Q.; Han, Y. Adsorption of lead ion on amino-functionalized fly-ash-based SBA-15 mesoporous molecular sieves prepared via two-step hydrothermal method. Micropor. Mesopor. Mater. 2017, 252, 105–115. [Google Scholar] [CrossRef]
- Cai, C.; Duan, X.; Xie, X.; Kang, S.; Liao, C.; Dong, J.; Liu, Y.; Xiang, S.; Dionysiou, D.-D. Efficient degradation of clofibric acid by heterogeneous catalytic ozonation using CoFe2O4 catalyst in water. J. Hazard. Mater. 2021, 410, 124604. [Google Scholar]
- Ye, R.; Zhu, J.; Tong, Y.; Feng, D.; Chen, P. Metal oxides heterojunction derived Bi-In hybrid electrocatalyst for robust electroreduction of CO2 to formate. J. Energy Chem. 2023, 83, 180–188. [Google Scholar] [CrossRef]
- Kruanak, K.; Jarusutthirak, C. Degradation of 2,4,6-trichlorophenol in synthetic wastewater by catalytic ozonation using alumina supported nickel oxides. J. Environ. Chem. Eng. 2019, 7, 102825. [Google Scholar] [CrossRef]
- Saleh, T.; Badawi, A.; Salama, R.; Mostafa, M. Design and development of novel composites containing nickel ferrites supported on activated carbon derived from agricultural wastes and its application in water remediation. Materials 2023, 16, 2170. [Google Scholar] [CrossRef]
- Gouda, M.; Shehab, M.; Soliman, M.; Helmy, S.; Salama, R. Preparation and characterization of supercapacitor electrodes utilizing catkin plant as an activated carbon source, Delta Univ. Sci. J. 2023, 6, 255–265. [Google Scholar]
- Liu, H.; Gao, Y.; Wang, J.; Pan, J.; Gao, B.; Yue, Q. Catalytic ozonation performance and mechanism of Mn-CeOx@γ-Al2O3/O3 in the treatment of sulfate-containing hypersaline antibiotic wastewater. Sci. Total Environ. 2022, 807, 150867. [Google Scholar] [CrossRef] [PubMed]
- Alshorifi, F.; Alswat, A.; Salama, R. Gold-selenide quantum dots supported onto cesium ferrite nanocomposites for the efficient degradation of rhodamine B. Heliyon 2022, 8, e09652. [Google Scholar] [CrossRef]
- Shen, T.; Su, W.; Yang, Q.; Ni, J.; Tong, S. Synergetic mechanism for basic and acid sites of MgMxOy (M = Fe, Mn) double oxides in catalytic ozonation of p-hydroxybenzoic acid and acetic acid. Appl. Catal. B Environ. 2020, 279, 119346. [Google Scholar] [CrossRef]
- Sun, P.; Zhai, S.; Chen, J.; Yuan, J.; Wu, Z.; Weng, X. Development of a multi-active center catalyst in mediating the catalytic destruction of chloroaromatic pollutants: A combined experimental and theoretical study. Appl. Catal. B Environ. 2020, 272, 119015. [Google Scholar] [CrossRef]
- Xu, K.; Deng, T.; Liu, J.; Peng, W. Study on the phosphate removal from aqueous solution using modified fly ash. Fuel 2010, 89, 3668–3674. [Google Scholar] [CrossRef]
- Slaný, M.; Kuzielová, E.; Žemlička, M.; Matejdes, M.; Struhárová, A.; Palou, M. Metabentonite and metakaolin-based geopolymers/zeolites: Relation between kind of clay, calcination temperature and concentration of alkaline activator. J. Therm. Anal. Calorim. 2023. [Google Scholar] [CrossRef]
- Wang, W.; Qi, L.; Zhang, P.; Luo, J.; Li, J. Removal of COD in wastewater by magnetic coagulant prepared from modified fly ash. Environ. Sci. Pollut. Res. 2022, 29, 52175–52188. [Google Scholar] [CrossRef] [PubMed]
- Niveditha, S.-V.; Gandhimathi, R. Flyash augmented Fe3O4 as a heterogeneous catalyst for degradation of stabilized landfill leachate in Fenton process. Chemosphere 2020, 242, 125189. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Guo, Y.; Guo, Y.; Wang, Y.; Wang, L.; Zhan, W.; Lu, G. In situ assembly of ultrafine Mn3O4 nanoparticles into MIL-101 for selective aerobic oxidation. Catal. Sci. Technol. 2017, 7, 4136–4144. [Google Scholar] [CrossRef]
- Huang, J.; Zhong, S.; Dai, Y.; Liu, C.-C.; Zhang, H. Effect of MnO2 phase structure on the oxidative reactivity toward bisphenol A degradation. Environ. Sci. Technol. 2018, 52, 11309–11318. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, G.; Chen, C.; Sun, K.; Zeng, L.; Yang, L.; Chen, Y.; Wang, W.; Liu, B.; Lu, Y.; et al. Fe-doped Mn3O4 spinel nanoparticles with highly exposed Feoct-O-Mntet sites for efficient selective catalytic reduction (SCR) of NO with ammonia at low temperatures. ACS Catal. 2020, 10, 6803–6809. [Google Scholar] [CrossRef]
- Pan, H.; Jian, Y.; Chen, C.; He, C.; Hao, Z.; Shen, Z.; Liu, H. Sphere-shaped Mn3O4 catalyst with remarkable low-temperature activity for methyl-ethyl-ketone combustion. Environ. Sci. Technol. 2017, 51, 6288–6297. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xie, M.; Kong, L.; Lu, W.; Feng, Z.; Zhan, J. Mn3O4 nanodots loaded g-C3N4 nanosheets for catalytic membrane degradation of organic contaminants. J. Hazard. Mater. 2020, 390, 122146. [Google Scholar] [CrossRef]
- Ji, D.; Zhou, H.; Zhang, J.; Dan, Y.; Yang, H.; Yuan, A. Facile synthesis of a metal-organic framework-derived Mn2O3 nanowire coated three-dimensional graphene network for high-performance free-standing supercapacitor electrodes. J. Mater. Chem. A 2016, 4, 8283–8290. [Google Scholar] [CrossRef]
- Liu, M.-F.; Du, Z.-Z.; Xie, Y.-L.; Li, X.; Yan, Z.-B.; Liu, J.-M. Unusual ferromagnetism enhancement in ferromagnetically optimal manganite La0.7−yCa0.3+yMn1−yRuyO3 (0≤y<0.3): The role of Mn-Ru t2g super-exchange. Sci. Rep. 2015, 5, 9922. [Google Scholar]
- Ye, Z.; Wang, G.; Giraudon, J.-M.; Nikiforov, A.; Chen, J.; Zhao, L.; Zhang, X.; Wang, J. Investigation of Cu-Mn catalytic ozonation of toluene: Crystal phase, intermediates and mechanism. J. Hazard. Mater. 2022, 424, 127321. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Zhang, T.; Zhang, Y.; Du, J.; Han, X.; Chen, J. Enhancing electrocatalytic oxygen reduction on MnO2 with vacancies. Angew. Chem. Int. Ed. 2013, 125, 2534–2537. [Google Scholar] [CrossRef]
- Yu, J.; Li, X.; Wu, M.; Lin, K.; Xu, L.; Zeng, T.; Shi, H.; Zhang, M. Synergistic role of inherent calcium and iron minerals in paper mill sludge biochar for phosphate adsorption. Sci. Total. Environ. 2022, 834, 155193. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, P.; Chen, L. Catalytic decomposition of gaseous ozone over manganese dioxides with different crystal structures. Appl. Catal. B Environ. 2016, 189, 210–218. [Google Scholar] [CrossRef]
- Li, P.; Zhan, S.; Yao, L.; Xiong, Y.; Tian, S. Highly porous α-MnO2 nanorods with enhanced defect accessibility for efficient catalytic ozonation of refractory pollutants. J. Hazard. Mater. 2022, 437, 129235. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Cao, P.; Qin, X.; Wu, S.; Bai, H.; Chen, S.; Yu, H.; Su, Y.; Quan, X. Oxygen vacancies-driven nonradical oxidation pathway of catalytic ozonation for efficient water decontamination. Appl. Catal. B Environ. 2023, 325, 122321. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, J.; Xu, S.; Lin, K.-Y.-A.; Tong, S. Oxygen vacancy of CeO2 improved efficiency of H2O2/O3 for the degradation of acetic acid in acidic solutions. Sep. Purif. Technol. 2018, 207, 92–98. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Song, Q.; Xin, Q.; Xu, S.; Xu, J. Heterogeneous ceria catalyst with water-tolerant Lewis acidic sites for one-pot synthesis of 1,3-diols via Prins condensation and hydrolysis reactions. J. Am. Chem. Soc. 2013, 135, 1506–1515. [Google Scholar] [CrossRef]
- Li, M.; Yang, K.; Huang, X.; Liu, S.; Jia, Y.; Gu, P.; Miao, H. Efficient degradation of trimethoprim by catalytic ozonation coupled with Mn/FeOx-functionalized ceramic membrane: Synergic catalytic effect and enhanced anti-fouling performance. J. Colloid Interface Sci. 2022, 616, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.-Q.; Qi, J.-Y.; Wang, Y.-P.; Liu, Y.-L.; Wang, L.; Ma, J. Heterogeneous catalytic ozonation of atrazine with Mn-loaded and Fe-loaded biochar. Water Res. 2021, 193, 116860. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ke, L.; Liu, J.; Sun, L.; Yuan, X.; Li, Y.; Xia, D. Enhanced catalytic ozonation towards oxalic acid degradation over novel copper doped manganese oxide octahedral molecular sieves nanorods. J. Hazard. Mater. 2019, 371, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Saputra, E.; Muhammad, S.; Sun, H.; Ang, H.-M.; Tadé, M.-O.; Wang, S. Manganese oxides at different oxidation states for heterogeneous activation of peroxymonosulfate for phenol degradation in aqueous solutions. Appl. Catal. B Environ. 2013, 142, 729–735. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.; Yin, X.; Liu, Y. The role of Mn-doping for catalytic ozonation of phenol using Mn/γ-Al2O3 nanocatalyst: Performance and mechanism. J. Environ. Chem. Eng. 2016, 4, 3415–3425. [Google Scholar] [CrossRef]
- Ma, N.; Ru, Y.; Weng, M.; Chen, L.; Chen, W.; Dai, Q. Synergistic mechanism of supported Mn-Ce oxide in catalytic ozonation of nitrofurazone wastewater. Chemosphere 2022, 308, 136192. [Google Scholar] [CrossRef]
- Li, Y.; Sun, P.; Liu, T.; Cheng, L.; Chen, R.; Bi, X.; Dong, X. Efficient Photothermal Conversion for Oxidation Removal of Formaldehyde using an rGO-CeO2 Modified Nickel Foam Monolithic Catalyst. Sep. Purif. Technol. 2023, 311, 123236. [Google Scholar] [CrossRef]
- Sun, P.; Liu, Y.; Mo, F.; Wu, M.; Xiao, Y.; Xiao, X.; Wang, W.; Dong, X. Efficient photocatalytic degradation of high-concentration moxifloxacin over dodecyl benzene sulfonate modified graphitic carbon nitride: Enhanced photogenerated charge separation and pollutant enrichment. J. Clean. Prod. 2023, 393, 136320. [Google Scholar] [CrossRef]
- Xu, L.; Fu, B.; Sun, Y.; Jin, P.; Bai, X.; Jin, X.; Shi, X.; Wang, Y.; Nie, S. Degradation of organic pollutants by Fe/N co-doped biochar via peroxymonosulfate activation: Synthesis, performance, mechanism and its potential for practical application. Chem. Eng. J. 2020, 400, 125870. [Google Scholar] [CrossRef]
- Chen, J.; Tu, Y.; Shao, G.; Zhang, F.; Zhou, Z.; Tian, S.; Ren, Z. Catalytic ozonation performance of calcium-loaded catalyst (Ca-C/Al2O3) for effective treatment of high salt organic wastewater. Sep. Purif. Technol. 2022, 301, 121937. [Google Scholar] [CrossRef]

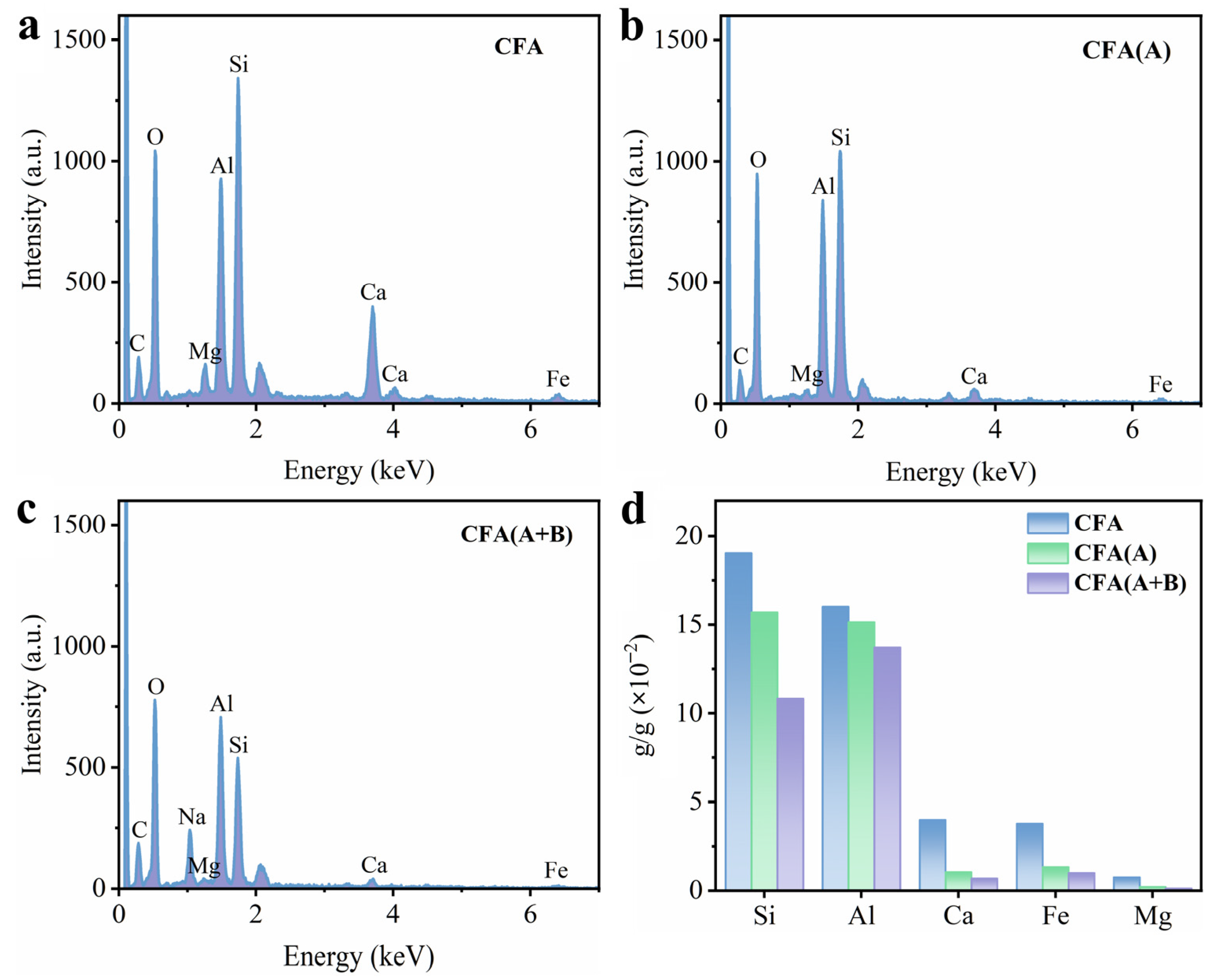



| Samples | SBET (m2/g) | Vtotal (cm3/g) | Pore Size (nm) | ICP Analysis (10−2 g/g) | ||||
|---|---|---|---|---|---|---|---|---|
| Si | Al | Ca | Fe | Mg | ||||
| CFA | 1.47 ± 0.02 | 0.0047 | 8.14 | 19.04 | 16.03 | 3.99 | 3.77 | 0.74 |
| CFA(A) | 11.37 ± 0.26 | 0.0083 | 9.72 | 15.71 | 15.15 | 1.03 | 1.32 | 0.21 |
| CFA(A+B) | 59.64 ± 0.81 | 0.13 | 12.761 | 10.83 | 13.73 | 0.67 | 0.98 | 0.13 |
| Samples | ICP Analysis | XPS Analysis | |||
|---|---|---|---|---|---|
| Mn wt.% | Mn3+/Mn4+ | Oads | Osur | Olat | |
| CFA(A+B) | / | / | 0.56 | 0.39 | 0.05 |
| Mn0.04/CFA(A+B) | 3.61% | 0.75 | 0.46 | 0.43 | 0.11 |
| Mn0.08/CFA(A+B) | 6.97% | 1.23 | 0.40 | 0.48 | 0.12 |
| Mn0.12/CFA(A+B) | 11.10% | 0.93 | 0.32 | 0.50 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Chen, R.; Chang, X.; Yan, J.; Gu, Y.; Xi, S.; Sun, P.; Dong, X. Degradation of Sodium Acetate by Catalytic Ozonation Coupled with a Mn-Functionalized Fly Ash: Reaction Parameters and Mechanism. Toxics 2023, 11, 700. https://doi.org/10.3390/toxics11080700
Chen Y, Chen R, Chang X, Yan J, Gu Y, Xi S, Sun P, Dong X. Degradation of Sodium Acetate by Catalytic Ozonation Coupled with a Mn-Functionalized Fly Ash: Reaction Parameters and Mechanism. Toxics. 2023; 11(8):700. https://doi.org/10.3390/toxics11080700
Chicago/Turabian StyleChen, Yaoji, Ruifu Chen, Xinglan Chang, Jingying Yan, Yajie Gu, Shuang Xi, Pengfei Sun, and Xiaoping Dong. 2023. "Degradation of Sodium Acetate by Catalytic Ozonation Coupled with a Mn-Functionalized Fly Ash: Reaction Parameters and Mechanism" Toxics 11, no. 8: 700. https://doi.org/10.3390/toxics11080700
APA StyleChen, Y., Chen, R., Chang, X., Yan, J., Gu, Y., Xi, S., Sun, P., & Dong, X. (2023). Degradation of Sodium Acetate by Catalytic Ozonation Coupled with a Mn-Functionalized Fly Ash: Reaction Parameters and Mechanism. Toxics, 11(8), 700. https://doi.org/10.3390/toxics11080700







