Lead, Mercury, and Cadmium Concentrations in Blood Products Transfused to Neonates: Elimination Not Just Mitigation
Abstract
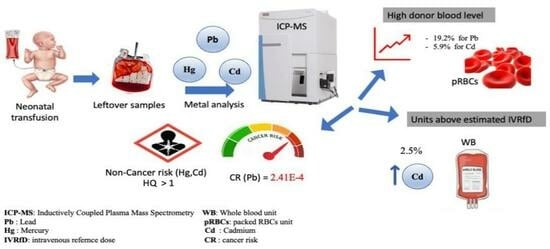
1. Impact Statement
2. Introduction
3. Materials and Methods
3.1. Sample Collection
3.2. ICP-MS Analysis
3.3. Statistical Analysis
4. Results
4.1. Quality Control
4.2. Metal Quantification in Donor Blood Units
4.3. Concurrent Metal Exposure and Representative Ratios of Metals in Blood Products
4.4. Dose per Transfusion Versus IVRfD
4.5. Risk Assessment
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, A.; Dhir, S.K.; Kaur, G.; Gupta, M.; Batta, M. Blood component therapy in neonates in a neonatal intensive care unit of northern India. Clin. Epidemiol. Glob. Health 2015, 3, S38–S42. [Google Scholar] [CrossRef]
- Vassallo, R.; Bachowski, G.; Benjamin, R.; Borge, D.; Dodd, R.; Eder, A.; Eastvold, P.; Goldberg, C.; Hopkins, C.; Lima, J. A Compendium of Transfusion Practice Guidelines; American Red Cross: Washington, DC, USA, 2013; Available online: https://iu.instructure.com/courses/1427995/files/54988388 (accessed on 15 February 2023).
- Sundararajan, S.; Blatz, A.; Dearborn, D.; Varnes, A.; Bearer, C.; El Metwally, D. Toxic metal contamination of banked blood designated for neonatal transfusion. J. Clin. Toxicol. 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Falck, A.J.; Mooney, S.; Kapoor, S.S.; White, K.M.; Bearer, C.; El Metwally, D. Developmental exposure to environmental toxicants. Pediatr. Clin. 2015, 62, 1173–1197. [Google Scholar] [CrossRef] [PubMed]
- García-Niño, W.R.; Pedraza-Chaverrí, J. Protective effect of curcumin against heavy metals-induced liver damage. Food Chem. Toxicol. 2014, 69, 182–201. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Barone, G.; Dambrosio, A.; Storelli, A.; Garofalo, R.; Busco, V.P.; Storelli, M.M. Estimated dietary intake of trace metals from swordfish consumption: A human health problem. Toxics 2018, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Tobwala, S.; Wang, H.-J.; Carey, J.W.; Banks, W.A.; Ercal, N. Effects of lead and cadmium on brain endothelial cell survival, monolayer permeability, and crucial oxidative stress markers in an in vitro model of the blood-brain barrier. Toxics 2014, 2, 258–275. [Google Scholar] [CrossRef]
- Bressler, J.P.; Goldstein, G.W. Mechanisms of lead neurotoxicity. Biochem. Pharmacol. 1991, 41, 479–484. [Google Scholar] [CrossRef]
- Goulart, E.; Pereira, C.; Garcia, R.; Giacomelli, M.; Rodrigues, A. Effects of lead and/or zinc exposure during the second stage of rapid postnatal brain growth on delta-aminolevulinate dehydratase and negative geotaxis of suckling rats. Braz. J. Med. Biol. Res. 2001, 34, 785–790. [Google Scholar] [CrossRef]
- Kumar, A.; MMS, C.-P.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Dack, K.; Fell, M.; Taylor, C.M.; Havdahl, A.; Lewis, S.J. Mercury and prenatal growth: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 7140. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, F.; Majorani, C.; Domanico, F.; Alimonti, A. Mercury in children: Current state on exposure through human biomonitoring studies. Int. J. Environ. Res. Public Health 2017, 14, 519. [Google Scholar] [CrossRef] [PubMed]
- Averina, M.; Hervig, T.; Huber, S.; Kjær, M.; Kristoffersen, E.K.; Bolann, B. Environmental pollutants in blood donors: The multicentre Norwegian donor study. Transfus. Med. 2020, 30, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Yassa, H.A. Autism: A form of lead and mercury toxicity. Environ. Toxicol. Pharmacol. 2014, 38, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, M.; Nakagawa, H.; Suwazono, Y.; Nogawa, K.; Sakurai, M.; Ishizaki, M.; Kido, T. Cancer mortality in residents of the cadmium-polluted Jinzu River Basin in Toyama, Japan. Toxics 2018, 6, 23. [Google Scholar] [CrossRef]
- Chandravanshi, L.; Shiv, K.; Kumar, S. Developmental toxicity of cadmium in infants and children: A review. Environ. Anal. Health Toxicol. 2021, 36, e2021003. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Ierodiakonou, D.; Margetaki, K.; Vafeiadi, M.; Chalkiadaki, G.; Roumeliotaki, T.; Fthenou, E.; Pentheroudaki, E.; McConnell, R.; Kogevinas, M. Associations of prenatal exposure to cadmium with child growth, obesity, and cardiometabolic traits. Am. J. Epidemiol. 2019, 188, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Kippler, M.; Bottai, M.; Georgiou, V.; Koutra, K.; Chalkiadaki, G.; Kampouri, M.; Kyriklaki, A.; Vafeiadi, M.; Fthenou, E.; Vassilaki, M. Impact of prenatal exposure to cadmium on cognitive development at preschool age and the importance of selenium and iodine. Eur. J. Epidemiol. 2016, 31, 1123–1134. [Google Scholar] [CrossRef]
- Echeverría, R.; Vrhovnik, P.; Salcedo-Bellido, I.; Iribarne-Durán, L.M.; Fiket, Ž.; Dolenec, M.; Martin-Olmedo, P.; Olea, N.; Arrebola, J.P. Levels and determinants of adipose tissue cadmium concentrations in an adult cohort from Southern Spain. Sci. Total Environ. 2019, 670, 1028–1036. [Google Scholar] [CrossRef]
- Satarug, S. Dietary cadmium intake and its effects on kidneys. Toxics 2018, 6, 15. [Google Scholar] [CrossRef]
- Horton, L.M.; Mortensen, M.E.; Iossifova, Y.; Wald, M.M.; Burgess, P. What do we know of childhood exposures to metals (arsenic, cadmium, lead, and mercury) in emerging market countries? Int. J. Pediatr. 2013, 2013, 872596. [Google Scholar] [CrossRef] [PubMed]
- Crinnion, W.J. The CDC Fourth National Report on Human Exposure to Environmental Chemicals: What it Tells Us About our Toxic Burden and How it Assists Environmental Medicine Physicians. Altern. Med. Rev. 2010, 15, 101–109. [Google Scholar] [PubMed]
- Aly, S.M.; Omran, A.; Abdalla, M.O.; Gaulier, J.-M.; El-Metwally, D. Lead: A hidden “untested” risk in neonatal blood transfusion. Pediatr. Res. 2019, 85, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Elabiad, M.T.; Hook, R.E. Lead content of blood transfusions for extremely low-birth-weight infants. Am. J. Perinatol. 2013, 30, 765–770. [Google Scholar] [PubMed]
- Torrente, M.; Gascon, M.; Vrijheid, M.; Sunyer, J.; Forns, J.; Domingo, J.L.; Nadal, M. Levels of metals in hair in childhood: Preliminary associations with neuropsychological behaviors. Toxics 2014, 2, 1–16. [Google Scholar] [CrossRef]
- Zubairi, H.; Visintainer, P.; Fleming, J.; Richardson, M.; Singh, R. Lead exposure in preterm infants receiving red blood cell transfusions. Pediatr. Res. 2015, 77, 814–818. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Falck, A.J.; Medina, A.E.; Cummins-Oman, J.; El-Metwally, D.; Bearer, C.F. Mercury, lead, and cadmium exposure via red blood cell transfusions in preterm infants. Pediatr. Res. 2020, 87, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Falck, A.J.; Sundararajan, S.; Al-Mudares, F.; Contag, S.A.; Bearer, C.F. Fetal exposure to mercury and lead from intrauterine blood transfusions. Pediatr. Res. 2019, 86, 510–514. [Google Scholar] [CrossRef]
- EMA. Committee for Medicinal Products for Human Use. Guideline on Bioanalytical Method Validation. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 5 February 2023).
- World Health Organization. Lead in Drinking Water. 2011. Available online: http://www.who.int/water_sanitation_health/dwq/chemicals/lead.pdf (accessed on 15 April 2023).
- U.S. EPA National Center for Environmental Assessment. Chemical Assessment Summary Integrated Risk Information System (IRIS). 2001. Available online: https://cfpub.epa.gov/ncea/iris/search/index.cfm (accessed on 15 April 2023).
- Center for Disease Control. National Biomonitoring Program: Monitoring Summary, Mercury. 2013. Available online: http://www.cdc.gov/biomonitoring/Mercury_BiomonitoringSummary.html (accessed on 15 April 2023).
- Health Risk Assessment Guide for Metals Gastrointestinal Uptake and Absorption, and Catalogue of Toxicokinetic Models. 2007. Available online: https://www.icmm.com/document/264 (accessed on 15 April 2023).
- Rauf, A.U.; Mallongi, A.; Lee, K.; Daud, A.; Hatta, M.; Al Madhoun, W.; Astuti, R.D.P. Potentially toxic element levels in atmospheric particulates and health risk estimation around industrial areas of Maros, Indonesia. Toxics 2021, 9, 328. [Google Scholar] [CrossRef]
- Cavalleri, A.; Minoia, C.; Pozzoli, L.; Polatti, F.; Bolis, P.F. Lead in red blood cells and in plasma of pregnant women and their offspring. Environ. Res. 1978, 17, 403–408. [Google Scholar] [CrossRef]
- DeSilva, P. Determination of lead in plasma and studies on its relationship to lead in erythrocytes. Occup. Environ. Med. 1981, 38, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Pérez, C.A.; Aguilar-Madrid, G.; Smith, D.R.; Lacasaña-Navarro, M.; Téllez-Rojo, M.M.; Piacitteli, G.; Hu, H.; Hernández-Avila, M. Predictors of plasma lead among lithographic print shop workers in Mexico City. Am. J. Ind. Med. 2004, 46, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, A.S.; Roy, A.; Amarasiriwardena, C.J.; Smith, D.; Lupoli, N.; Mercado-García, A.; Lamadrid-Figueroa, H.; Tellez-Rojo, M.M.; Hu, H.; Hernández-Avila, M. Maternal blood, plasma, and breast milk lead: Lactational transfer and contribution to infant exposure. Environ. Health Perspect. 2014, 122, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Sommar, J.N.; Hedmer, M.; Lundh, T.; Nilsson, L.; Skerfving, S.; Bergdahl, I.A. Investigation of lead concentrations in whole blood, plasma and urine as biomarkers for biological monitoring of lead exposure. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 51–57. [Google Scholar] [CrossRef]
- Angle, C.R.; McIntire, M.S. Red cell lead, whole blood lead, and red cell enzymes. Environ. Health Perspect. 1974, 7, 133–137. [Google Scholar] [CrossRef]
- Cesbron, A.; Saussereau, E.; Mahieu, L.; Couland, I.; Guerbet, M.; Goulle, J.-P. Metallic profile of whole blood and plasma in a series of 106 healthy volunteers. J. Anal. Toxicol. 2013, 37, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Kortei, N.K.; Heymann, M.E.; Essuman, E.K.; Kpodo, F.M.; Akonor, P.T.; Lokpo, S.Y.; Boadi, N.O.; Ayim-Akonor, M.; Tettey, C. Health risk assessment and levels of toxic metals in fishes (Oreochromis noliticus and Clarias anguillaris) from Ankobrah and Pra basins: Impact of illegal mining activities on food safety. Toxicol. Rep. 2020, 7, 360–369. [Google Scholar] [CrossRef]
- Amrutiya, R.J.; Mungala, B.M.; Patel, V.T.; Ganjiwale, J.D.; Nimbalkar, S.M. Blood component transfusion in tertiary care neonatal intensive care unit and neonatal intermediate care unit: An audit. Cureus 2020, 12. [Google Scholar] [CrossRef]
- Rhainds, M.; Delage, G. Health risk assessment of lead exposure from blood transfusion. Epidemiology 2006, 17, S492. [Google Scholar] [CrossRef]
- Delage, G.; Gingras, S.; Rhainds, M. A population-based study on blood lead levels in blood donors. Transfusion 2015, 55, 2633–2640. [Google Scholar] [CrossRef]
- King, E.; Shih, G.; Ratnapradipa, D.; Quilliam, D.N.; Morton, J.; Magee, S.R. Mercury, lead, and cadmium in umbilical cord blood. J. Environ. Health 2013, 75, 38–43. [Google Scholar] [PubMed]
- Simić, A.; Hansen, A.F.; Syversen, T.; Lierhagen, S.; Ciesielski, T.M.; Romundstad, P.R.; Midthjell, K.; Åsvold, B.O.; Flaten, T.P. Trace elements in whole blood in the general population in Trøndelag County, Norway: The HUNT3 Survey. Sci. Total Environ. 2022, 806, 150875. [Google Scholar] [CrossRef] [PubMed]
- White, K.M.; Anderson Berry, A.L.; White, S.F.; Moran, D.; Lyden, E.; Bearer, C.F. Donor blood remains a source of heavy metal exposure. Pediatr. Res. 2019, 85, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Gehrie, E.; Keiser, A.; Dawling, S.; Travis, J.; Strathmann, F.G.; Booth, G.S. Primary prevention of pediatric lead exposure requires new approaches to transfusion screening. J. Pediatr. 2013, 163, 855–859. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic effects and biomarkers of lead exposure: A review. Rev. Environ. Health 2009, 24, 15–46. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.S.; Nelson, L.S.; Howland, M.A.; Lewin, N.A.; Flomenbaum, N.E.; Goldfrank, L.R. Goldfrank’s Manual of Toxicologic Emergencies, 9th ed.; McGraw Hill Professional: New York, NY, USA, 2011; pp. 1237–1242, 1299–1307. [Google Scholar]
- Al-Sulaiti, M.M.; Soubra, L.; Al-Ghouti, M.A. The causes and effects of mercury and methylmercury contamination in the marine environment: A review. Curr. Pollut. Rep. 2022, 8, 249–272. [Google Scholar] [CrossRef]
- Smith, D.; Hernandez-Avila, M.; Téllez-Rojo, M.M.; Mercado, A.; Hu, H. The relationship between lead in plasma and whole blood in women. Environ. Health Perspect. 2002, 110, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, M.F.; Barbosa, F., Jr.; Tanus-Santos, J.E. Assessment of how pregnancy modifies plasma lead and plasma/whole blood lead ratio in ALAD 1-1 genotype women. Basic Clin. Pharmacol. Toxicol. 2008, 102, 347–351. [Google Scholar] [CrossRef]
- Flora, S.J.; Pachauri, V. Chelation in metal intoxication. Int J Environ. Res Public Health 2010, 7, 2745–2788. [Google Scholar] [CrossRef]
- Rahbar, M.H.; Samms-Vaughan, M.; Dickerson, A.S.; Hessabi, M.; Bressler, J.; Coore Desai, C.; Shakespeare-Pellington, S.; Reece, J.-A.; Morgan, R.; Loveland, K.A. Concentration of lead, mercury, cadmium, aluminum, arsenic and manganese in umbilical cord blood of Jamaican newborns. Int. J. Environ. Res. Public Health 2015, 12, 4481–4501. [Google Scholar] [CrossRef]
| ClinChek Whole Blood—Lot 2192 | ClinChek Serum—Lot 2062 | Seronorm Whole Blood—Lot 2011933 ¤ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd | Pb | Cd | Pb £ | Hg | Hg | |||||||
| Results * | Target $ | Results | Target | Results | Target | Result | Target | Results | Target | Results | Target | |
| Level 1 | 1.42–1.56 | 1.49 (1.12–1.86) | 31.1–34.5 | 35 (28–42) | 1.82–1.87 | 1.91 (1.34–2.48) | 2.21–2.44 | 2.03 (1.63–2.44) | 1.38–1.73 | 1.63 (1.30–1.96) | ||
| Level 2 | 3.37–3.72 | 3.52 (2.82–4.23) | 77.6–87.8 | 90.7 (72.5–109) | 5.51–5.90 | 5.51 (4.41–6.61) | ||||||
| Level 3 | 6.68–6.95 | 6.69 (5.35–8.03) | 226–247 | 250 (200–300) | ||||||||
| Plasma | Platelets | pRBCs | WB | p | |
|---|---|---|---|---|---|
| Pb | |||||
| median | 0.5 | 0.5 | 33.0 | 22.0 | <0.00001 * |
| max | 7.7 | 7.1 | 54.0 | 57.0 | |
| min | 0.2 | 0.2 | 9.0 | 5.0 | |
| Hg | |||||
| median | 0.2 | 0.2 | 0.6 | 0.6 | 0.32 |
| max | 0.3 | 0.6 | 3.0 | 1.2 | |
| min | 0.2 | 0.2 | 0.6 | 0.2 | |
| Cd | |||||
| median | 0.2 | 0.2 | 0.6 | 0.2 | 0.069 |
| max | 0.2 | 0.6 | 5.8 | 3.0 | |
| min | 0.2 | 0.2 | 0.6 | 0.2 |
| Plasma/Plts | Plasma/PRBCs | Plasma/WB | Plts/PRBCs | Plts/WB | PRBCs/WB | |
|---|---|---|---|---|---|---|
| Pb | 0.66:26.4 [36] 0.74:100 [37] | 0.4:12 [38] 0.1:7.7 [39] 0.3:10 [36] 0.1:21 [40] 0.57:227 [40] | 2–3.5:1 [41] | |||
| 0.9:1 | 1:33 | 1:22.6 | 0.9:33.8 | 0.9:22.6 | 33.8:22.6 | |
| Hg | 2.69:5.8 [42] | |||||
| 0.2:0.2 | 0.2:0.9 | 0.2:0.6 | 0.2:0.9 | 0.2:0.6 | 0.9:0.6 | |
| Cd | 2.27: 6 [42] | |||||
| 0.2:0.2 | 0.2:1.2 | 0.2:0.5 | 0.2:1.2 | 0.2:0.5 | 1.2:0.5 |
| Metal | Blood Products | |||
|---|---|---|---|---|
| Plasma | Platelets | pRBCs | WB | |
| Pb n | 30 | 30 | 30 | 30 |
| Median | 0.01 | 0.01 | 0.55 | 1.83 |
| Max | 0.13 | 0.12 | 0.90 | 4.75 |
| Min | 0.003 | 0.003 | 0.15 | 0.003 |
| Hg n | 30 | 25 | 30 | 17 |
| Median | 0.003 | 0.003 | 0.010 | 0.046 |
| Max | 0.005 | 0.004 | 0.050 | 0.100 |
| Min | 0.003 | 0.003 | 0.010 | 0.003 |
| Cd n | 30 | 30 | 30 | 30 |
| Median | 0.003 | 0.003 | 0.01 | 0.017 |
| Max | 0.003 | 0.15 | 0.10 | 0.3 |
| Min | 0.003 | 0.003 | 0.003 | 0.003 |
| Metal | IVRfD (μg/kg/Day) | Number of Transfusions with Doses >IVRfD | Frequency (%) | Plasma | Platelets | pRBCs | WB |
|---|---|---|---|---|---|---|---|
| Hg | 0.095 | 0/102 | 0 | 0 | 0 | 0 | 0 |
| Cd | 0.1 | 3/120 | 2.5 | 0 | 0 | 0 | 3 |
| Measured Metal | MDD | ADI | HQ |
|---|---|---|---|
| Hg | 0.12 | 0.095 | 1.26 |
| Cd | 0.25 | 0.1 | 2.5 |
| - | CSF | LADD | Cancer Risk |
| Pb | 8.5 × 10−3 | 4.8 × 10−3 | 2.41 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aly, S.M.; Elfiky, S.; Mohamed, Y.G.; Soliman, R.A.M.; Shalaby, N.; Beauval, N.; Gaulier, J.-M.; Allorge, D.; Omran, A. Lead, Mercury, and Cadmium Concentrations in Blood Products Transfused to Neonates: Elimination Not Just Mitigation. Toxics 2023, 11, 712. https://doi.org/10.3390/toxics11080712
Aly SM, Elfiky S, Mohamed YG, Soliman RAM, Shalaby N, Beauval N, Gaulier J-M, Allorge D, Omran A. Lead, Mercury, and Cadmium Concentrations in Blood Products Transfused to Neonates: Elimination Not Just Mitigation. Toxics. 2023; 11(8):712. https://doi.org/10.3390/toxics11080712
Chicago/Turabian StyleAly, Sanaa M., Samar Elfiky, Yasmine G. Mohamed, Radwa A. M. Soliman, Nancy Shalaby, Nicolas Beauval, Jean-Michel Gaulier, Delphine Allorge, and Ahmed Omran. 2023. "Lead, Mercury, and Cadmium Concentrations in Blood Products Transfused to Neonates: Elimination Not Just Mitigation" Toxics 11, no. 8: 712. https://doi.org/10.3390/toxics11080712
APA StyleAly, S. M., Elfiky, S., Mohamed, Y. G., Soliman, R. A. M., Shalaby, N., Beauval, N., Gaulier, J.-M., Allorge, D., & Omran, A. (2023). Lead, Mercury, and Cadmium Concentrations in Blood Products Transfused to Neonates: Elimination Not Just Mitigation. Toxics, 11(8), 712. https://doi.org/10.3390/toxics11080712