Evaluating Silymarin Extract as a Potent Antioxidant Supplement in Diazinon-Exposed Rainbow Trout: Oxidative Stress and Biochemical Parameter Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish
2.2. Chemicals
2.3. Diet Preparation
2.4. Experimental Design
2.5. Blood Biochemical Parameters
2.6. Oxidative Biomarkers
2.7. Statistical Analysis
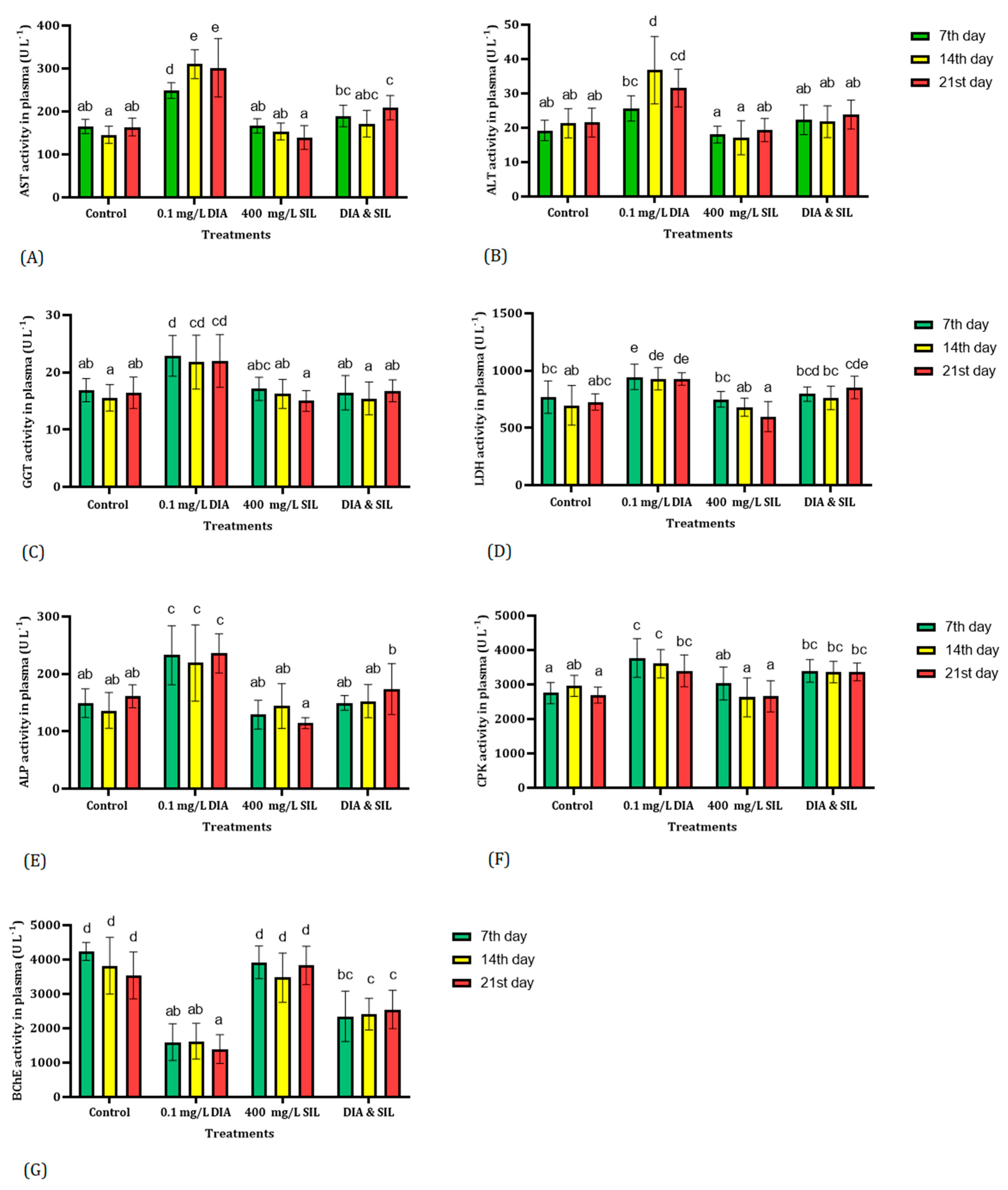
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vali, S.; Majidiyan, N.; Azadikhah, D.; Varcheh, M.; Tresnakova, N.; Faggio, C. Effects of Diazinon on the survival, blood parameters, gills, and liver of grass carp (Ctenopharyngodon idella Valenciennes, 1844; Teleostei: Cyprinidae). Water 2022, 14, 1357. [Google Scholar] [CrossRef]
- Banaee, M.; Sureda, A.; Mirvaghefi, A.R.; Ahmadi, K. Biochemical and histological changes in the liver tissue of rainbow trout (Oncorhynchus mykiss) exposed to sub-lethal concentrations of diazinon. Fish Physiol. Biochem. 2013, 39, 489–501. [Google Scholar] [CrossRef]
- Jamalipour, P.; Choobkar, N.; Abrishamkar, M.; Pournamdari, E. Design of fluorescent method for sensing toxic diazinon in water samples using PbS quantum dots-based gelatin. J. Environ. Sci. Health Part B 2022, 57, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Hassanpoor, S.; Rajabi, M. Application of ecofriendly magnetic nanocomposite synthesized from natural materials for separation and determination of diazinon pesticide in real water samples. Int. J. Environ. Anal. Chem. 2022, 14, 1–20. [Google Scholar] [CrossRef]
- Kakaei, H.; Shahtaheri, S.J.; Abdi, K.; Rahimi Kakavandi, N. Separation and quantification of diazinon in water samples using liquid-phase microextraction-based effervescent tablet-assisted switchable solvent method coupled to gas chromatography-flame ionization detection. Biomed. Chromatogr. 2023, 37, 5624. [Google Scholar] [CrossRef] [PubMed]
- Mighani, S.; Safari, R.; Hoseinifar, S.H.; Shabani, A.; Dadar, M. The effect of diazinon as agricultural pesticides on reproductive indices and related genes expression in zebrafish (Danio rerio). Aquac. Res. 2022, 53, 1019–1025. [Google Scholar] [CrossRef]
- Saha, S.; Chandra Saha, N.; Chatterjee, A.; Banerjee, P.; Garai, P.; Sharma, P.; Patnaik, L.; Nayak, S.; Dhara, K.; Chukwuka, A.V.; et al. Integrated multi-biomarker responses in Mozambique tilapia, Oreochromis mossambicus under acute and chronic Diazinon® exposures. Chem Ecol. 2023, 39, 235–255. [Google Scholar] [CrossRef]
- Dzul-Caamal, R.; Domínguez-Lòpez, M.L.; Olivares-Rubio, H.F.; García-Latorre, E.; Vega-López, A. The relationship between the bioactivation and detoxification of diazinon and chlorpyrifos, and the inhibition of acetylcholinesterase activity in Chirostoma jordani from three lakes with low to high organophosphate pesticides contamination. Ecotoxicology 2014, 23, 779–790. [Google Scholar] [CrossRef]
- Tapper, M.A.; Serrano, J.A.; Schmieder, P.K.; Hammermeister, D.E.; Kolanczyk, R.C. Metabolism of diazinon in rainbow trout liver slices. Appl. Vitro Toxicol. 2018, 4, 13–23. [Google Scholar] [CrossRef]
- Derikvandy, A.; Pourkhabbaz, H.R.; Banaee, M.; Sureda, A.; Haghi, N.; Pourkhabbaz, A.R. Genotoxicity and oxidative damage in zebrafish (Danio rerio) after exposure to effluent from ethyl alcohol industry. Chemosphere 2020, 251, 126609. [Google Scholar] [CrossRef]
- Tang, J.; Wang, W.; Jiang, Y.; Chu, W. Diazinon exposure produces histological damage, oxidative stress, immune disorders and gut microbiota dysbiosis in crucian carp (Carassius auratus gibelio). Environ. Pollut. 2021, 269, 116129. [Google Scholar] [CrossRef]
- Bayır, M.; Özdemir, E. Genomic organization and transcription of superoxide dismutase genes (sod1, sod2, and sod3b) and response to diazinon toxicity in platyfish (Xiphophorus maculatus) by using SOD enzyme activity. Anim. Biotechnol. 2023, 1–11. [Google Scholar] [CrossRef]
- Ivanović, S.R.; Borozan, N.; Miladinović, D.Ć.; Živković, I.; Borozan, S. The relationship between the cholinergic mechanism of toxicity and oxidative stress in rats during subacute diazinon poisoning. Toxicol. Appl. Pharmacol. 2023, 473, 116598. [Google Scholar] [CrossRef]
- Sharifinasab, Z.; Banaee, M.; Mohiseni, M.; Noori, A. The Protective role of vitamin C and chitosan against paraquat-induced oxidative stress in muscles of common carp (Cyprinus carpio). Croat. J. Fish. 2016, 74, 149–158. [Google Scholar] [CrossRef]
- Ibrahim, A.T.A.; Banaee, M.; Sureda, A. Selenium protection against mercury toxicity on the male reproductive system of Clarias gariepinus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 225, 108583. [Google Scholar] [CrossRef]
- Banaee, M.; Impellitteri, F.; Evaz-Zadeh Samani, H.; Piccione, G.; Faggio, C. Dietary Arthrospira platensis in rainbow trout (Oncorhynchus mykiss): A means to reduce threats caused by CdCl2 exposure? Toxics 2022, 10, 731. [Google Scholar] [CrossRef]
- Banaee, M.; Sureda, A.; Faggio, C. Protective effect of protexin concentrate in reducing the toxicity of chlorpyrifos in common carp (Cyprinus carpio). Environ. Toxicol. Pharmacol. 2022, 94, 103918. [Google Scholar] [CrossRef]
- Nasirin, C.; Najm, M.A.; Chen, T.C.; Dhamija, A.; Lionardo, A.; Bokov, D.O.; Shahbazi Naserabad, S. The protective effects of quercetin on the physiological responses in malathion-exposed common carp, Cyprinus carpio. Trop. Anim. Health Prod. 2023, 55, 22. [Google Scholar] [CrossRef]
- Banaee, M.; Sureda, A.; Shahaf, S.; Fazilat, N. Protective Effects of Silymarin Extract on Malthion-Induced Zebra Cichlid (Cichlasoma Nigrofasciatum) Hepatotoxicity. Iran. J. Toxicol. 2015, 9, 1239–1246. [Google Scholar]
- Hassaan, M.S.; Mohammady, E.Y.; Soaudy, M.R.; El-Garhy, H.A.; Moustafa, M.M.; Mohamed, S.A.; El-Haroun, E.R. Effect of Silybum marianum seeds as a feed additive on growth performance, serum biochemical indices, antioxidant status, and gene expression of Nile tilapia, Oreochromis niloticus (L.) fingerlings. Aquaculture 2019, 509, 178–187. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Wang, X.; Mai, K.; He, G. Effects of silymarin on growth performance, antioxidant capacity and immune response in turbot (Scophthalmus maximus L.). J. World Aqua. Soc. 2019, 50, 1168–1181. [Google Scholar] [CrossRef]
- Wei, L.; Wu, P.; Zhou, X.Q.; Jiang, W.D.; Liu, Y.; Kuang, S.Y.; Tang, L.; Feng, L. Dietary silymarin supplementation enhanced growth performance and improved intestinal apical junctional complex on juvenile grass carp (Ctenopharyngodon idella). Aquaculture 2020, 525, 735311. [Google Scholar] [CrossRef]
- Xiao, P.; Ji, H.; Ye, Y.; Zhang, B.; Chen, Y.; Tian, J.; Liu, P.; Chen, L.; Du, Z. Dietary silymarin supplementation promotes growth performance and improves lipid metabolism and health status in grass carp (Ctenopharyngodon idellus) fed diets with elevated lipid levels. Fish Physiol. Biochem. 2017, 43, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Yang, Z.; Sun, J.; Tian, J.; Chang, Z.; Li, X.; Zhang, B.; Ye, Y.; Ji, H.; Yu, E.; et al. Silymarin inhibits adipogenesis in the adipocytes in grass carp Ctenopharyngodon idellus in vitro and in vivo. Fish Physiol. Biochem. 2017, 43, 1487–1500. [Google Scholar] [CrossRef]
- Veisi, S.; Johari, S.A.; Tyler, C.R.; Mansouri, B.; Esmaeilbeigi, M. Antioxidant properties of dietary supplements of free and nanoencapsulated silymarin and their ameliorative effects on silver nanoparticles induced oxidative stress in Nile tilapia (Oreochromis niloticus). Environ. Sci. Pollut. Res. 2021, 28, 26055–26063. [Google Scholar] [CrossRef] [PubMed]
- Banaee, M.; Sureda, A.; Mirvaghefi, A.R.; Rafei, G.R. Effects of long-term silymarin oral supplementation on the blood biochemical profile of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2011, 37, 885–896. [Google Scholar] [CrossRef]
- Taleb, A.; Ahmad, K.A.; Ihsan, A.U.; Qu, J.; Lin, N.A.; Hezam, K.; Koju, N.; Hui, L.; Qilong, D. Antioxidant effects and mechanism of silymarin in oxidative stress induced cardiovascular diseases. Biomed. Pharmacother. 2018, 102, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Gholamhosseini, A.; Banaee, M.; Sureda, A.; Timar, N.; Zeidi, A.; Faggio, C. Physiological response of freshwater crayfish, Astacus leptodactylus exposed to polyethylene microplastics at different temperature. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 267, 109581. [Google Scholar] [CrossRef]
- Banaee, M.; Sagvand, S.; Sureda, A.; Amini, M.; Haghi, B.N.; Sopjani, M.; Faggio, C. Evaluation of single and combined effects of mancozeb and metalaxyl on the transcriptional and biochemical response of zebrafish (Danio rerio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 268, 109597. [Google Scholar] [CrossRef]
- Banaee, M.; Beitsayah, A.; Prokić, M.D.; Petrović, T.G.; Zeidi, A.; Faggio, C. Effects of cadmium chloride and biofertilizer (Bacilar) on biochemical parameters of freshwater fish, Alburnus mossulensis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 268, 109614. [Google Scholar] [CrossRef]
- Kocalar, K.; Canli, E.G.; Canli, M. Responses of oxidative stress biomarkers of freshwater fish (Oreochromis niloticus) exposed to Cr6+, Hg2+, Ni2+ and Zn2+ in differing calcium levels. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 267, 109577. [Google Scholar]
- Banaee, M.; Faraji, J.; Amini, M.; Multisanti, C.R.; Faggio, C. Rainbow trout (Oncorhynchus mykiss) physiological response to microplastics and enrofloxacin: Novel pathways to investigate microplastic synergistic effects on pharmaceuticals. Aquat. Toxicol. 2023, 261, 106627. [Google Scholar]
- Rashidian, G.; Bahrami Gorji, S.; Farsani, M.N.; Prokić, M.D.; Faggio, C. The oak (Quercus brantii) acorn as a growth promotor for rainbow trout (Oncorhynchus mykiss): Growth performance, body composition, liver enzymes activity and blood biochemical parameters. Nat. Prod. Res. 2020, 34, 2413–2423. [Google Scholar] [CrossRef]
- Al-Attar, A.M.; Elnaggar, M.H.; Almalki, E.A. Protective effect of some plant oils on diazinon induced hepatorenal toxicity in male rats. Saudi J. Biol. Sci. 2017, 24, 1162–1171. [Google Scholar] [PubMed]
- Hariri, A.T.; Moallem, S.A.; Mahmoudi, M.; Memar, B.; Razavi, B.M.; Hosseinzadeh, H. Effect of Crocus sativus L. stigma (saffron) against subacute effect of diazinon: Histopathological, hematological, biochemical and genotoxicity evaluations in rats. J. Pharmacopuncture. 2018, 21, 61. [Google Scholar] [CrossRef]
- Zhang, H.; Forman, H.J.; Choi, J. γ-Glutamyl transpeptidase in glutathione biosynthesis. Meth. Enzymol. 2005, 401, 468–483. [Google Scholar]
- El Mesallamy, H.O.; Metwally, N.S.; Soliman, M.S.; Ahmed, K.A.; Abdel Moaty, M.M. The chemopreventive effect of Ginkgo biloba and Silybum marianum extracts on hepatocarcinogenesis in rats. Cancer Cell Int. 2011, 11, 38. [Google Scholar] [PubMed]
- Banaee, M.; Sureda, A.; Mirvaghefi, A.R.; Ahmadi, K. Effects of diazinon on biochemical parameters of blood in rainbow trout (Oncorhynchus mykiss). Pestic. Biochem. Phys. 2011, 99, 1–6. [Google Scholar]
- Lotfi, S.; Esfahani, M.; Ranjbar, A.; Mehri, F. Protective effect of flaxseed oil the on diazinon-induced hepatotoxicity in male rats. Proc. Indian Natl. Sci. Acad. 2023, 89, 655–663. [Google Scholar]
- Celep, N.A.; Gedikli, S. Protective Effect of Silymarin on Liver in Experimental in the Sepsis Model of Rats. Acta Histochem. Cytochem. 2023, 56, 9–19. [Google Scholar]
- Souza, J.A.D.C.R.; Souza, T.; Quintans, I.L.A.D.C.R.; Farias, D. Network Toxicology and Molecular Docking to Investigate the Non-AChE Mechanisms of Organophosphate-Induced Neurodevelopmental Toxicity. Toxics 2023, 11, 710. [Google Scholar]
- Isik, I.; Celik, I. Acute effects of methyl parathion and diazinon as inducers for oxidative stress on certain biomarkers in various tissues of rainbow trout (Oncorhynchus mykiss). Pestic. Biochem. Phys. 2008, 92, 38–42. [Google Scholar]
- Saha, S.; Chukwuka, A.V.; Mukherjee, D.; Patnaik, L.; Nayak, S.; Dhara, K.; Saha, N.C.; Faggio, C. Chronic effects of Diazinon® exposures using integrated biomarker responses in freshwater walking catfish, Clarias batrachus. J. Appl. Sci. 2021, 11, 10902. [Google Scholar]
- Mohammadi, H.; Manouchehri, H.; Changizi, R.; Bootorabi, F.; Khorramizadeh, M.R. Concurrent metformin and silibinin therapy in diabetes: Assessments in zebrafish (Danio rerio) animal model. J. Diab. Metab. Dis. 2020, 19, 1233–1244. [Google Scholar]
- Abdelkhalek, N.K.; Eissa, I.A.; Ahmed, E.; Kilany, O.E.; El-Adl, M.; Dawood, M.A.; Hassan, A.M.; Abdel-Daim, M.M. Protective role of dietary Spirulina platensis against diazinon-induced Oxidative damage in Nile tilapia; Oreochromis niloticus. Environ. Toxicol. Pharmacol. 2017, 54, 99–104. [Google Scholar]
- Fraschini, F.; Demartini, G.; Esposti, D. Pharmacology of silymarin. Clin. Drug Investig. 2002, 22, 51–65. [Google Scholar]
- Metwally, M.A.A.; El-Gellal, A.M.; El-Sawaisi, S.M. Effects of silymarin on lipid metabolism in rats. World Appl. Sci. J. 2009, 6, 1634–1637. [Google Scholar]
- Durmaz, H.; Sevgiler, Y.; Üner, N. Tissue-specific antioxidative and neurotoxic responses to diazinon in Oreochromis niloticus. Pestic. Biochem. Phys. 2006, 84, 215–226. [Google Scholar]
- Soto, C.; Pérez, J.; García, V.; Uría, E.; Vadillo, M.; Raya, L. Effect of silymarin on kidneys of rats suffering from alloxan-induced diabetes mellitus. Phytomedicine 2010, 17, 1090–1094. [Google Scholar] [PubMed]
- Soto, C.; Recoba, R.; Barron, H.; Alvarez, C.; Favari, L. Silymarin increases antioxidant enzymes in alloxan-induced diabetes in rat pancreas. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003, 136, 205–212. [Google Scholar]
- Shaarawy, S.M.; Tohamy, A.A.; Elgendy, S.M.; Abd Elmageed, Z.Y.; Bahnasy, A.; Mohamed, M.S.; Kandil, E.; Matrougui, K. Protective effects of garlic and silymarin on NDEA-induced rats’ hepatotoxicity. Int. J. Biol. Sci. 2009, 5, 549. [Google Scholar] [PubMed]
- Tasduq, S.A.; Peerzada, K.; Koul, S.; Bhat, R.; Johri, R.K. Biochemical manifestations of anti-tuberculosis drugs induced hepatotoxicity and the effect of silymarin. Hepatol. Res. 2005, 31, 132–135. [Google Scholar] [PubMed]
- Upadhyay, G.; Kumar, A.; Singh, M.P. Effect of silymarin on pyrogallol-and rifampicin-induced hepatotoxicity in mouse. Eur. J. Pharmacol. 2007, 565, 190–201. [Google Scholar]
- Das, S.K.; Mukherjee, S. Biochemical and immunological basis of silymarin effect, a milk thistle (Silybum marianum) against ethanol-induced oxidative damage. Toxicol. Mech. Methods. 2012, 22, 409–413. [Google Scholar] [PubMed]
- Shabanzadeh, S.; Vatandoust, S.; Hosseinifard, S.M.; Sheikhzadeh, N.; Shahbazfar, A.A. Dietary astaxanthin (Lucantin® Pink) mitigated oxidative stress induced by diazinon in rainbow trout (Oncorhynchus mykiss). Vet. Res. Forum. 2023, 14, 97. [Google Scholar]
- Kim, H.S.; Jo, S.; Yun, K.S.; Lee, K.J. Effects of dietary micelle silymarin on the growth performance, feed utilization and health of olive flounder (Paralichthys olivaceus). Aquac. Int. 2023, 1–18. [Google Scholar] [CrossRef]
- Melo de Almeida, E.; Tisserand, F.; Faria, M.; Chèvre, N. Efficiency of Several Cytochrome P450 Biomarkers in Highlighting the Exposure of Daphnia magna to an Organophosphate Pesticide. Toxics 2022, 10, 482. [Google Scholar]
- Kachel, M.; Krajewska, M.; Stryjecka, M.; Ślusarczyk, L.; Matwijczuk, A.; Rudy, S.; Domin, M. Comparative Analysis of Phytochemicals and Antioxidant Properties of Borage Oil (Borago officinalis L.) and Milk Thistle (Silybum marianum Gaertn). J. Appl. Sci. 2023, 13, 2560. [Google Scholar]
- İpek, E.; Tunca, R. Silymarin protects against doxorubicin induced cardiotoxicity by down-regulating topoisomerase IIβ expression in mice. Biotech. Histochem. 2023, 98, 412–423. [Google Scholar]
- Singh, G.; Mittra, N.; Singh, C. Tempol and silymarin rescue from zinc-induced degeneration of dopaminergic neurons through modulation of oxidative stress and inflammation. Mol. Cell. Biochem. 2023, 478, 1705–1718. [Google Scholar]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef]
- Yu, J.; Ding, Y.; Wu, D.; Liu, P. Rutin, Puerarin and Silymarin Regulated Aluminum-Induced Imbalance of Neurotransmitters and Metal Elements in Brain of Rats. Biol. Trace Elem. Res. 2023, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, X.; Yang, Y.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martinez-Larranaga, M.R.; Wang, X.; Anadón, A.; Martinez, M.A. Neonicotinoids: Mechanisms of systemic toxicity based on oxidative stress-mitochondrial damage. Biol. Trace Elem. Res. 2022, 96, 1493–1520. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, Q.; Guo, J.; Ares, I.; Martínez, M.; Martínez-Larrañaga, M.R.; Wang, X.; Anadón, A.; Martínez, M.A. Oxidative stress and metabolism: A mechanistic insight for glyphosate toxicology. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 617–639. [Google Scholar] [CrossRef]
- Lu, Q.; Sun, Y.; Ares, I.; Anadón, A.; Martínez, M.; Martínez-Larrañaga, M.R.; Yuan, Z.; Wang, X.; Martínez, M.A. Deltamethrin toxicity: A review of oxidative stress and metabolism. Environ. Res. 2019, 170, 260–281. [Google Scholar] [CrossRef]
- Wang, X.; Anadón, A.; Wu, Q.; Qiao, F.; Ares, I.; Martínez-Larrañaga, M.R.; Yuan, Z.; Martínez, M.A. Mechanism of neonicotinoid toxicity: Impact on oxidative stress and metabolism. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 471–507. [Google Scholar] [CrossRef] [PubMed]
- Křen, V. Chirality Matters: Biological activity of optically pure silybin and its congeners. Int. J. Mol. Sci. 2021, 22, 7885. [Google Scholar] [CrossRef]
- Martínez, M.A.; Rodríguez, J.L.; Lopez-Torres, B.; Martínez, M.; Martínez-Larrañaga, M.R.; Anadón, A.; Ares, I. Oxidative stress and related gene expression effects of cyfluthrin in human neuroblastoma SH-SY5Y cells: Protective effect of melatonin. Environ. Res. 2019, 177, 108579. [Google Scholar] [CrossRef]
- Alturki, H.A.; Elsawy, H.A.; Famurewa, A.C. Silymarin abrogates acrylamide-induced oxidative stress-mediated testicular toxicity via modulation of antioxidant mechanism, DNA damage, endocrine deficit and sperm quality in rats. Andrologia 2022, 54, e14491. [Google Scholar] [CrossRef]

| Day | Treatments | Biochemical Parameters | |||||
|---|---|---|---|---|---|---|---|
| Total Protein (g dL−1) | Albumin (g dL−1) | Globulins (g dL−1) | Glucose (mg dL−1) | Triglycerides (mg dL−1) | Cholesterol (mg dL−1) | ||
| 7th day | Control | 4.9 ± 0.6 c | 2.4 ± 0.4 b | 2.5 ± 0.4 c | 61.9 ± 3.4 bc | 284.8 ± 31.3 ab | 238.0 ± 26.3 abc |
| 0.1 mg L−1 DIZ | 3.6 ± 0.3 a | 1.8 ± 0.2 a | 1.8 ± 0.2 a | 73.1 ± 5.7 d | 336.7 ± 28.0 b | 324.2 ± 59.1 b | |
| 400 mg kg−1 SIL | 4.8 ± 0.6 c | 2.3 ± 0.3 ab | 2.4 ± 0.3 c | 61.3 ± 4.8 bc | 246.1 ± 33.4 a | 224.6 ± 45.4 ab | |
| SIL + DIZ | 4.5 ± 0.3 bc | 2.2 ± 0.4 ab | 2.3 ± 0.4 bc | 64.9 ± 2.3 c | 288.8 ± 33.2 ab | 223.3 ± 22.5 ab | |
| 14th day | Control | 4.5 ± 0.6 bc | 2.2 ± 0.4 ab | 2.3 ± 0.4 c | 61.7 ± 4.9 bc | 318.1 ± 44.2 b | 254.7 ± 32.7 bc |
| 0.1 mg L−1 DIZ | 3.7 ± 0.3 a | 1.9 ± 0.2 ab | 1.9 ± 0.2 ab | 76.8 ± 2.8 d | 293.6 ± 32.8 ab | 281.8 ± 21.6 cde | |
| 400 mg kg−1 SIL | 4.9 ± 0.9 c | 2.3 ± 0.4 b | 2.5 ± 0.5 c | 57.9 ± 8.2 ab | 243.9 ± 37.4 a | 215.7 ± 41.8 ab | |
| SIL + DIZ | 4.1 ± 0.3 ab | 1.9 ± 0.1 a | 2.2 ± 0.3 abc | 67.0 ± 3.4 c | 259.3 ± 22.7 a | 247.4 ± 36.5 abc | |
| 21st day | Control | 4.7 ± 0.7 bc | 2.3 ± 0.3 b | 2.4 ± 0.4 c | 61.6 ± 4.3 bc | 290.0 ± 52.6 ab | 267.3 ± 38.0 bcd |
| 0.1 mg L−1 DIZ | 3.7 ± 0.5 a | 2.0 ± 0.3 ab | 1.8 ± 0.2 a | 73.6 ± 2.9 d | 327.1 ± 36.3 b | 318.7 ± 39.7 de | |
| 400 mg kg−1 SIL | 4.9 ± 0.4 c | 2.2 ± 0.3 ab | 2.7 ± 0.6 c | 55.1 ± 3.1 a | 244.0 ± 42.4 a | 198.1 ± 37.9 a | |
| SIL + DIZ | 4.6 ± 0.3 bc | 2.1 ± 0.3 ab | 2.5 ± 0.4 c | 63.3 ± 4.4 bc | 286.0 ± 42.3 ab | 267.0 ± 52.7 bcd | |
| Day | Treatments | Plasma Metabolites | |||
|---|---|---|---|---|---|
| Creatinine (mg dL−1) | Urea (mg dL−1) | Uric Acid (mg dL−1) | Ammonia (mg dL−1) | ||
| 7th day | Control | 0.28 ± 0.11 a | 0.91 ± 0.03 a | 0.23 ± 0.04 ab | 0.63 ± 0.12 a |
| 0.1 mg L−1 DIZ | 0.58 ± 0.12 b | 1.61 ± 0.21 b | 0.30 ± 0.06 c | 1.98 ± 0.37 d | |
| 400 mg kg−1 SIL | 0.37 ± 0.07 a | 0.88 ± 0.13 a | 0.20 ± 0.02 a | 0.67 ± 0.10 a | |
| SIL + DIZ | 0.31 ± 0.08 a | 0.94 ± 0.17 a | 0.27 ± 0.07 abc | 1.74 ± 0.48 cd | |
| 14th day | Control | 0.36 ± 0.12 a | 0.91 ± 0.15 a | 0.21 ± 0.04 a | 0.72 ± 0.26 a |
| 0.1 mg L−1 DIZ | 0.91 ± 0.18 c | 1.57 ± 0.27 b | 0.32 ± 0.05 c | 1.74 ± 0.38 cd | |
| 400 mg kg−1 SIL | 0.32 ± 0.11 a | 0.89 ± 0.12 a | 0.26 ± 0.03 abc | 0.50 ± 0.08 a | |
| SIL + DIZ | 0.26 ± 0.14 a | 0.91 ± 0.12 a | 0.26 ± 0.09 abc | 1.67 ± 0.31 bcd | |
| 21st day | Control | 0.29 ± 0.14 a | 0.89 ± 0.16 a | 0.21 ± 0.03 a | 0.69 ± 0.12 a |
| 0.1 mg L−1 DIZ | 0.86 ± 0.20 c | 1.69 ± 0.32 b | 0.30 ± 0.04 c | 1.49 ± 0.37 bc | |
| 400 mg kg−1 SIL | 0.31 ± 0.12 a | 0.88 ± 0.23 a | 0.21 ± 0.06 a | 0.66 ± 0.20 a | |
| SIL + DIZ | 0.32 ± 0.11 a | 0.99 ± 0.23 a | 0.28 ± 0.05 bc | 1.32 ± 0.44 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banaee, M.; Impellitteri, F.; Multisanti, C.R.; Sureda, A.; Arfuso, F.; Piccione, G.; Faggio, C. Evaluating Silymarin Extract as a Potent Antioxidant Supplement in Diazinon-Exposed Rainbow Trout: Oxidative Stress and Biochemical Parameter Analysis. Toxics 2023, 11, 737. https://doi.org/10.3390/toxics11090737
Banaee M, Impellitteri F, Multisanti CR, Sureda A, Arfuso F, Piccione G, Faggio C. Evaluating Silymarin Extract as a Potent Antioxidant Supplement in Diazinon-Exposed Rainbow Trout: Oxidative Stress and Biochemical Parameter Analysis. Toxics. 2023; 11(9):737. https://doi.org/10.3390/toxics11090737
Chicago/Turabian StyleBanaee, Mahdi, Federica Impellitteri, Cristiana Roberta Multisanti, Antoni Sureda, Francesca Arfuso, Giuseppe Piccione, and Caterina Faggio. 2023. "Evaluating Silymarin Extract as a Potent Antioxidant Supplement in Diazinon-Exposed Rainbow Trout: Oxidative Stress and Biochemical Parameter Analysis" Toxics 11, no. 9: 737. https://doi.org/10.3390/toxics11090737
APA StyleBanaee, M., Impellitteri, F., Multisanti, C. R., Sureda, A., Arfuso, F., Piccione, G., & Faggio, C. (2023). Evaluating Silymarin Extract as a Potent Antioxidant Supplement in Diazinon-Exposed Rainbow Trout: Oxidative Stress and Biochemical Parameter Analysis. Toxics, 11(9), 737. https://doi.org/10.3390/toxics11090737









