Conventional Anthelmintic Concentration of Deltamethrin Immersion Disorder in the Gill Immune Responses of Crucian Carp
Abstract
:1. Introduction
2. Material and Methods
2.1. Ethical Statement
2.2. Chemicals
2.3. Experimental Design and Sampling
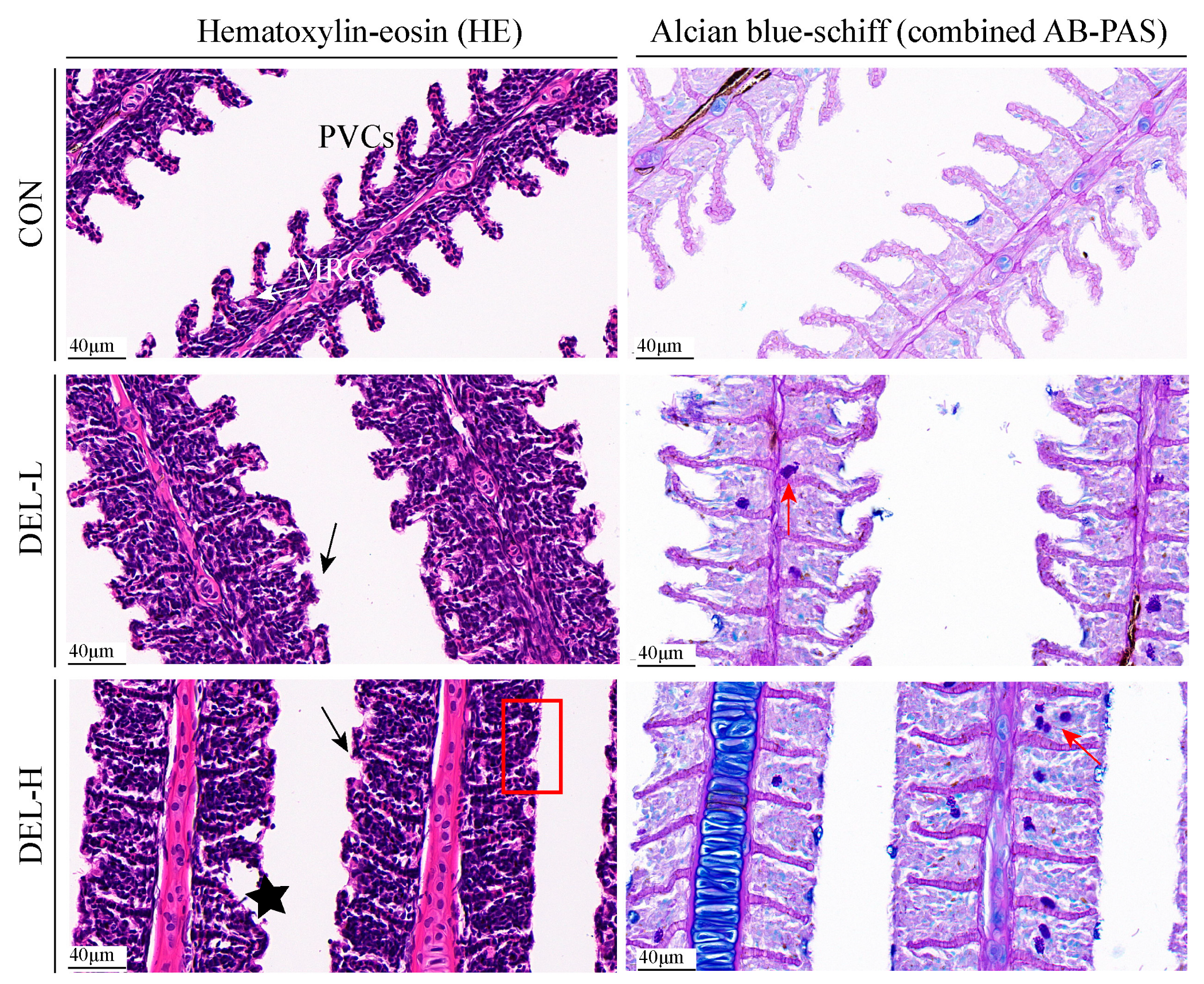
2.4. Histopathological Analyses
2.5. Determination of Gills Filaments Apoptosis
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. TMT-Based Proteomic Analysis
2.8. 16S rRNA Sequencing
2.9. Statistical Analyses
3. Results
3.1. Histoarchitectural Changes and TUNEL Assay
3.2. Gene Expression Analysis
3.2.1. Expression of Tight Junction-Related Genes
3.2.2. Expression of Apoptosis- and Autophagy-Related Genes
3.2.3. Expression of Immune-Related Genes
3.3. TMT-Based Quantitative Proteomic Analysis
3.4. Gill Microbiota Analysis
4. Discussion
4.1. DEL Immersion Caused Destruction of Barrier Function in Gills
4.2. DEL Immersion Caused Disturbance of Mucosal Immune Response in Gills
4.3. DEL Immersion Caused Disorder of Commensal Flora in Gills
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Botwright, N.A.; Mohamed, A.R.; Slinger, J.; Lima, P.C.; Wynne, J.W. Host-parasite interaction of Atlantic salmon (Salmo salar) and the ectoparasite Neoparamoeba perurans in amoebic gill disease. Front. Immunol. 2021, 12, 672700. [Google Scholar] [PubMed]
- Wang, L.; Zhang, D.F.; Xie, J.; Chang, O.; Wang, Q.; Shi, C.; Zhao, F.; Gong, H.; Ren, Y.; Musa, N.; et al. Do ectoparasites on fish gills “talk” with gut microbiota far away? Aquaculture 2023, 562, 738880. [Google Scholar]
- Li, J.; Jiang, H.J.; Wu, P.F.; Li, S.; Han, B.; Yang, Q.; Wang, X.; Han, B.; Deng, N.; Qu, B.; et al. Toxicological effects of deltamethrin on quail cerebrum: Weakened antioxidant defense and enhanced apoptosis. Environ. Pollut. 2021, 286, 117319. [Google Scholar] [PubMed]
- Kong, Y.; Li, M.; Shan, X.F.; Wang, G.; Han, G.H. Effects of deltamethrin subacute exposure in snakehead fish, Channa argus: Biochemicals, antioxidants and immune responses. Ecotoxicol. Environ. Saf. 2021, 209, 111821. [Google Scholar] [PubMed]
- Lu, Q.; Sun, Y.Q.; Ares, I.; Anadón, A.; Martínez, M.; Martínez-Larrañaga, M.; Yuan, Z.; Wang, X.; Martínez, W. Deltamethrin toxicity: A review of oxidative stress and metabolism. Environ. Res. 2019, 170, 260–281. [Google Scholar]
- Yang, C.W.; Lim, W.; Song, G. Immunotoxicological effects of insecticides in exposed fishes. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 247, 109064. [Google Scholar]
- Zhang, L.; Hong, X.S.; Zhao, X.; Yan, S.; Ma, X.; Zha, J. Exposure to environmentally relevant concentrations of deltamethrin renders the Chinese rare minnow (Gobiocypris rarus) vulnerable to Pseudomonas fluorescens infection. Sci. Total Environ. 2020, 715, 136943. [Google Scholar]
- Cengiz, E.I.; Unlu, E. Sublethal effects of commercial deltamethrin on the structure of the gill, liver and gut tissues of mosquitofish, Gambusia affinis: A microscopic study. Environ. Toxicol. Phar. 2006, 21, 246–253. [Google Scholar]
- Parlak, V. Evaluation of apoptosis, oxidative stress responses, AChE activity and body malformations in zebrafish (Danio rerio) embryos exposed to deltamethrin. Chemosphere 2018, 207, 397–403. [Google Scholar]
- Arslan, H.; Altun, S.; Ozdemir, S. Acute toxication of deltamethrin results in activation of iNOS, 8-OHdG and up-regulation of caspase 3, iNOS gene expression in common carp (Cyprinus carpio L.). Aquat. Toxicol. 2017, 187, 90–99. [Google Scholar]
- Wu, H.; Gao, J.W.; Xie, M.; Xiang, J.; Zuo, Z.; Tian, X.; Song, R.; Yuan, X.; Wu, Y.; Ou, D. Histopathology and transcriptome analysis reveals the gills injury and immunotoxicity in gibel carp following acute deltamethrin exposure. Ecotoxicol. Environ. Saf. 2022, 234, 113421. [Google Scholar] [PubMed]
- Wu, H.; Gao, J.W.; Xie, M.; Wu, J.; Song, R.; Yuan, X.; Wu, Y. Chronic exposure to deltamethrin disrupts intestinal health and intestinal microbiota in juvenile crucian carp. Ecotoxicol. Environ. Saf. 2022, 241, 113732. [Google Scholar] [PubMed]
- Yuan, X.P.; Wu, H.; Gao, J.W.; Geng, X.; Xie, M.; Song, R.; Zheng, J.; Wu, Y.; Ou, D. Acute deltamethrin exposure induces oxidative stress, triggers endoplasmic reticulum stress, and impairs hypoxic resistance of crucian carp. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 263, 109508. [Google Scholar] [PubMed]
- Eni, G.; Ibor, O.R.; Andem, A.B.; Oku, E.E.; Chukwuka, A.V.; Adeogun, A.O.; Arukwe, A. Biochemical and endocrine-disrupting effects in Clarias gariepinus exposed to the synthetic pyrethroids, cypermethrin and deltamethrin. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 225, 108584. [Google Scholar] [PubMed]
- Kong, Y.; Li, M.; Guo, G.L.; Yu, L.; Sun, L.; Yin, Z.; Li, R.; Chen, X.; Wang, G. Effects of dietary curcumin inhibit deltamethrin-induced oxidative stress, inflammation and cell apoptosis in Channa argus via Nrf2 and NF-κB signaling pathways. Aquaculture 2021, 540, 736744. [Google Scholar]
- Atamanalp, M.; Erdogan, O. Alterations of HSP70 gene expression in rainbow trout (Oncorhyncus mykiss) exposed to deltamethrin. Turk. J. Vet. Anim. Sci. 2010, 34, 359–363. [Google Scholar]
- Parsons, A.E.; Escobar-Lux, R.H.; Sævik, P.N.; Samuelsen, O.B.; Agnalt, A.L. The impact of anti-sea lice pesticides, azamethiphos and deltamethrin, on European lobster (Homarus gammarus) larvae in the Norwegian marine environment. Environ. Pollut. 2020, 264, 114725. [Google Scholar]
- Arnberg, M.; Refseth, G.H.; Allan, I.J.; Benedetti, M.; Regoli, F.; Tassara, L.; Sagerup, K.; Drivdal, M.; Nøst, O.A.; Evenset, A.; et al. Acute and sublethal effects of deltamethrin discharges from the aquaculture industry on Northern Shrimp (Pandalus borealis Krøyer, 1838): Dispersal modeling and field investigations. Environ. Sci. Technol. 2023, 57, 3602–3611. [Google Scholar]
- Kitiyodom, S.; Trullàs, C.; Rodkhum, C.; Thompson, K.D.; Katagiri, T.; Temisak, S.; Namdee, K.; Yata, T.; Pirarat, N. Modulation of the mucosal immune response of red tilapia (Oreochromis sp.) against columnaris disease using a biomimetic-mucoadhesive nanovaccine. Fish Shellfish Immunol. 2021, 112, 81–91. [Google Scholar]
- Tang, D.; Shi, X.L.; Guo, H.Y.; Bai, Y.; Shen, C.; Zhang, Y.; Wang, Z.F. Comparative transcriptome analysis of the gills of Procambarus clarkii provides novel insights into the immune-related mechanism of copper stress tolerance. Fish Shellfish Immunol. 2020, 96, 32–40. [Google Scholar]
- Liu, Y.; Chen, Z.X.; Li, S.W.; Ding, L.; Wei, X.; Han, S.; Wang, P.; Sun, Y. Multi-omics profiling and biochemical assays reveal the acute toxicity of environmental related concentrations of Di-(2-ethylhexyl) phthalate (DEHP) on the gill of crucian carp (Carassius auratus). Chemosphere 2022, 307 Pt 2, 135814. [Google Scholar] [CrossRef]
- Pan, W.; Godoy, R.S.; Cook, D.P.; Scott, A.L.; Nurse, C.A.; Jonz, M.G. Single-cell transcriptomic analysis of neuroepithelial cells and other cell types of the gills of zebrafish (Danio rerio) exposed to hypoxia. Sci. Rep. 2022, 12, 10144. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Moustafa, E.M.; Gewaily, M.S.; Abdo, S.E.; AbdEl-kader, M.F.; Saadallah, M.S.; Hamouda, A.H. Ameliorative effects of Lactobacillus plantarum L-137 on Nile tilapia (Oreochromis niloticus) exposed to deltamethrin toxicity in rearing water. Aquat. Toxicol. 2020, 219, 105377. [Google Scholar] [CrossRef]
- Macirella, R.; Madeo, G.; Sesti, S.; Tripepi, M.; Bernabo, I.; Godbert, N.; Russa, D.L.; Brunelli, E. Exposure and post-exposure effects of chlorpyrifos on Carassius auratus gills: An ultrastructural and morphofunctional investigation. Chemosphere 2020, 251, 126434. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lim, W.; Song, G. Mediation of oxidative stress toxicity induced by pyrethroid pesticides in fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 234, 108758. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, X.; Li, X.; Yan, X.; Liang, Y.; Huang, Y.; Huang, L.; Zeng, H. Histopathology and transcriptome reveals the tissue-specific hepatotoxicity and gills injury in mosquitofish (Gambusia affinis) induced by sublethal concentration of triclosan. Ecotoxicol. Environ. Saf. 2021, 220, 112325. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.C.; Liu, G.H.; Xu, Y.H.; Luo, Z.; Zhao, T.; Zheng, H.; Tan, X.Y. Physiological and transcriptomic analyses reveal the toxicological mechanism and risk assessment of environmentally-relevant waterborne tetracycline exposure on the gills of tilapia (Oreochromis niloticus). Sci. Total Environ. 2021, 806, 151290. [Google Scholar] [CrossRef]
- Feng, H.; Zhou, P.; Liu, F.; Zhang, W.; Yang, H.; Li, X.; Dong, J. Abamectin causes toxicity to the carp respiratory system by triggering oxidative stress, inflammation, and apoptosis and inhibiting autophagy. Environ. Sci. Pollut. R. 2023, 30, 55200–55213. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, Y.H.; Teng, X.J.; Luan, P.; Teng, X.; Yin, X. Immunosuppression participated in complement activation-mediated inflammatory injury caused by 4-octylphenol via TLR7/IκBα/NF-κB pathway in common carp (Cyprinus carpio) gills. Aquat. Toxicol. 2022, 249, 106211. [Google Scholar] [CrossRef]
- Fuchylo, U.; Alharbi, H.A.; Alcaraz, A.J.; Jones, P.D.; Giesy, J.P.; Hecker, M.; Brinkmann, M. Inflammation of gill epithelia in fish causes increased permeation of petrogenic polar organic chemicals via disruption of tight junctions. Environ. Sci. Technol. 2022, 56, 1820–1829. [Google Scholar] [CrossRef]
- Xu, Y.H.; Wei, X.L.; Xu, Y.C.; Zhang, D.; Zhao, T.; Zheng, H.; Luo, Z. Waterborne enrofloxacin exposure activated oxidative stress and MAPK pathway, induced apoptosis and resulted in immune dysfunction in the gills of yellow catfish Pelteobagrus fulvidraco. Aquaculture 2022, 547, 735541. [Google Scholar] [CrossRef]
- Xu, T.; Liu, Q.H.; Chen, D.; Liu, Y. Atrazine exposure induces necroptosis through the P450/ROS pathway and causes inflammation in the gill of common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2022, 131, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Legrand, T.P.R.A.; Catalano, S.R.; Wos-oxley, M.L.; Stephens, F.; Landos, M.; Bansemer, M.S.; Stone, D.A.; Qin, J.G.; Oxley, A.P.A. The inner workings of the outer surface: Skin and gill microbiota as indicators of changing gut health in Yellowtail Kingfish. Front. Microbiol. 2018, 8, 2664. [Google Scholar] [CrossRef]
- Wang, M.; Yi, M.; Lu, M.X.; Gao, F.; Liu, Z.; Huang, Q.; Li, Q.; Zhu, D. Effects of probiotics Bacillus cereus NY5 and Alcaligenes faecalis Y311 used as water additives on the microbiota and immune enzyme activities in three mucosal tissues in Nile tilapia Oreochromis niloticus reared in outdoor tanks. Aquac. Rep. 2020, 17, 100309. [Google Scholar] [CrossRef]
- Lai, K.P.; Zhu, P.; Boncan, D.A.B.; Yang, L.; Leung, C.C.T.; Ho, J.C.H.; Lin, X.; Chan, T.F.; Kong, R.Y.C.; Tse, W.K.F. Integrated omics approaches revealed the osmotic stress-responsive genes and microbiota in gill of marine medaka. Msystems 2022, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; He, J.; Wang, H.; Zheng, L.; Wang, X.; Zhang, H.; Wu, H.; Shu, Y. Gill junction injury and microbial disorders induced by microcystin-leucine arginine in Lithobates catesbeianus tadpoles. Toxins 2022, 14, 479. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Bai, J.; Liu, R.R.; Lv, A. Comprehensive transcriptomics and proteomics analysis of Carassius auratus gills in response to Aeromonas hydrophila. Fish Shellfish Immunol. Rep. 2023, 4, 100077. [Google Scholar] [CrossRef]
- Xiang, Q.Q.; Yan, H.; Luo, X.W.; Kang, Y.H.; Hu, J.M.; Chen, L. Integration of transcriptomics and metabolomics reveals damage and recovery mechanisms of fish gills in response to nanosilver exposure. Aquat. Toxicol. 2021, 237, 105895. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, Q.H.; Song, Y.; Cheng, B.; Ai, X.H. Effect of copper sulphate exposure on the oxidative stress, gill transcriptome and external microbiota of yellow catfish, Pelteobagrus fulvidraco. Antioxidants 2023, 12, 1288. [Google Scholar] [CrossRef]
- Quezada-Rodriguez, P.R.; Taylor, R.S.; Samsing, F.; Rigby, M.; Wood, A.T.; Nowak, B.F.; Wynne, J.W. Effect of a prophylactic treatment with chloramine-T on gill histology and microbiome of Atlantic salmon (Salmo salar) under commercial conditions. Aquaculture 2022, 546, 737319. [Google Scholar] [CrossRef]
- Karatas, T.; Yildirim, S.; Arslan, H.; Aggul, A.G. The effects on brown trout (Salmo trutta fario) of different concentrations of deltamethrin. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 226, 108606. [Google Scholar] [CrossRef] [PubMed]
- Ale, A.; Bacchetta, C.; Rossi, A.S.; Galdopórpora, J.; Desimone, M.F.; Torre, F.R.D.; Gervasio, S.; Cazenave, J. Nanosilver toxicity in gills of a neotropical fish: Metal accumulation, oxidative stress, histopathology and other physiological effects. Ecotoxicol. Environ. Saf. 2018, 148, 976–984. [Google Scholar] [CrossRef]
- Gjessing, M.C.; Krasnov, A.; Timmerhaus, G.; Brun, S.; Afanasyev, S.; Dale, O.B.; Dahle, M.K. The Atlantic salmon gill transcriptome response in a natural outbreak of salmon gill pox virus infection reveals new biomarkers of gill pathology and suppression of mucosal defense. Front. Immunol. 2020, 11, 2154. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.M.R.; Shukry, M.; Abd-Elaziz, R.A. Clinico-pathological findings and expression of inflammatory cytokines, apoptosis, and oxidative stress-related genes draw mechanistic insights in Nile tilapia reared under ammonia-N exposure and Aeromonas hydrophila challenge. Fish Shellfish Immunol. 2022, 127, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, Q.C.; Huang, Z.Y.; Ding, L.; Xu, Z. Immunoglobulins, mucosal immunity and vaccination in teleost fish. Front. Immunol. 2020, 11, 567941. [Google Scholar] [CrossRef] [PubMed]
- Sveen, L.R.; Grammes, F.T.; Ytteborg, E.; Takle, H.; Jørgensen, S.M. Genome-wide analysis of Atlantic salmon (Salmo salar) mucin genes and their role as biomarkers. PLoS ONE 2017, 12, e0189103. [Google Scholar] [CrossRef]
- Naama, M.; Telpaz, S.; Awad, A.; Simon, S.B.; Shabso, S.H.; Modilevsky, S.; Rubin, E.; Sawaed, J.; Zelik, L.; Zigdon, M.; et al. Autophagy controls mucus secretion from intestinal goblet cells by alleviating ER stress. Cell Host Microbe 2023, 31, 433–446.e4. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, W.X. Disturbing ion regulation and excretion in medaka (Oryzias melastigma) gills by microplastics: Insights from the gut-gill axis. Sci. Total Environ. 2023, 857 Pt 2, 159353. [Google Scholar] [CrossRef]
- Samanta, P.; Bandyopadhyay, N.; Pal, S.; Mukherjee, A.K.; Ghosh, A.R. Histopathological and ultramicroscopical changes in gill, liver and kidney of Anabas testudineus (Bloch) after chronic intoxication of almix (metsulfuron methyl 10.1%+chlorimuron ethyl 10.1%) herbicide. Ecotoxicol. Environ. Saf. 2015, 122, 360–367. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Guo, M.H.; Liu, Y.; Yu, H.; Xing, M. Environmentally relevant concentration of cypermethrin or/and sulfamethoxazole induce neurotoxicity of grass carp: Involvement of blood-brain barrier, oxidative stress and apoptosis. Sci. Total Environ. 2021, 762, 143054. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.J.; Shang, X.C.; Lu, Y.; Li, Y. Exposure of lead on intestinal structural integrity and the diversity of gut microbiota of common carp. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 239, 108877. [Google Scholar] [CrossRef]
- Rahman, A.N.A.; AbdelMageed, M.A.; Assayed, M.E.M.; Gharib, H.S.A.R.; Nasr, M.A.; Elshopakey, G.E.; Moniem, H.A.; Shahin, S.E.; Elhusseiny, E.; Ahmed, S.A.A. Imidacloprid induced growth, hematological, neuro-behavior, anti-oxidant, economic, genetic, and histopathological alterations in Clarias gariepinus: Alleviative role of dietary Hyphaene thebaica. Aquaculture 2023, 564, 739058. [Google Scholar] [CrossRef]
- Rahman, A.N.A.; Mohamed, A.A.R.; Dahran, N.; Farag, M.F.M.; Alqahtani, L.S.; Nassan, M.A.; Althobaiti, S.A.; El-Naseery, N.I. Appraisal of sub-chronic exposure to lambada-cyhalothrin and/or methomyl on the behavior and hepato-renal functioning in Oreochromis niloticus: Supportive role of taurine-supplemented feed. Aquat. Toxicol. 2022, 250, 106257. [Google Scholar] [CrossRef]
- Wu, L.; Li, H.Y.; Xu, W.J.; Dong, B.; Geng, H.; Jin, J.; Han, D.; Liu, H.; Zhu, X.; Yang, Y.; et al. Emodin alleviates acute hypoxia-induced apoptosis in gibel carp (Carassius gibelio) by upregulating autophagy through modulation of the AMPK/mTOR pathway. Aquaculture 2022, 548, 737689. [Google Scholar] [CrossRef]
- Kumar, S.; Gu, Y.; Abudu, Y.P.; Bruun, S.A.; Jain, A.; Farzam, F.; Mudd, M.; Anonsen, J.H.; Rusten, T.E.; Kasof, G.; et al. Phosphorylation of syntaxin 17 by TBK1 controls autophagy initiation. Dev. Cell 2019, 49, 130–144.e6. [Google Scholar] [CrossRef]
- Zhao, X.L.; Li, P.; Zhang, S.Q.; He, S.W.; Xing, S.Y.; Cao, Z.H.; Lu, R.; Li, Z.H. Effects of environmental norfloxacin concentrations on the intestinal health and function of juvenile common carp and potential risk to humans. Environ. Pollut. 2021, 287, 117612. [Google Scholar] [CrossRef]
- Xiong, G.; Deng, Y.Y.; Li, L.J.; Cao, Z.; Liao, X.; Liu, Y.; Lu, H. Immunotoxicity and transcriptome analysis of zebrafish embryos in response to glufosinate-ammonium exposure. Chemosphere 2019, 236, 124423. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Y.; Guo, M.H.; Mu, M.; Yu, H.; Xing, M. Grass carps co-exposed to environmentally relevant concentrations of cypermethrin and sulfamethoxazole bear immunodeficiency and are vulnerable to subsequent Aeromonas hydrophila infection. Environ. Pollut. 2020, 266 Pt 3, 115156. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Sun, B. Cathepsin H and cathepsin B of Cynoglossus semilaevis are involved in anti-bacterial immunity against Edwardsiella tarda. Fish Shellfish Immunol. 2023, 134, 108594. [Google Scholar] [CrossRef]
- Camara-Ruiz, M.; Cerezo, I.M.; Guardiola, F.A.; Beltrán, J.M.G.; Balebona, M.C.; Moriñigo, M.A.; Esteban, M.A. Alteration of the Immune Response and the Microbiota of the Skin during a Natural Infection by Vibrio harveyi in European Seabass (Dicentrarchus labrax). Microorganisms 2021, 9, 964. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, M.Z.; Li, S.W.; Wei, X.F.; Ding, L.; Han, S.; Wang, P.; Lv, B.; Chen, Z.; Sun, Y. Integrated application of multi-omics approach and biochemical assays provides insights into physiological responses to saline-alkaline stress in the gills of crucian carp (Carassius auratus). Sci. Total Environ. 2022, 822, 153622. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Dong, J.; Liu, Y.T.; Yang, Q.; Xu, N.; Yang, Y.; Ai, X.H. Effects of acute deltamethrin exposure on kidney transcriptome and intestinal microbiota in goldfish (Carassius auratus). Ecotoxicol. Environ. Saf. 2021, 225, 112716. [Google Scholar] [CrossRef] [PubMed]
- Toda, H.; Araki, K.; Moritome, T.; Nakaninshi, T. Perforin-dependent cytotoxic mechanism in killing by CD8 positive T cells in ginbuna crucian carp, Carassius auratus langsdorfii. Dev. Comp. Immunol. 2011, 35, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wu, G.T.; Zhu, J.Y.; Wang, X.W.; Liu, L.L.; Li, H.J.; Zhu, H. Povidone iodine exposure alters the immune response and microbiota of the gill and skin in koi carp, Cyprinus carpio. Aquaculture 2023, 563, 738926. [Google Scholar] [CrossRef]
- Zhao, N.; Guo, J.M.; Zhang, B.; Liu, K.; Liu, Y.; Shen, Y.; Li, J. Heterogeneity of the tissue-specific mucosal microbiome of normal grass carp (Ctenopharyngodon idella). Mar. Biotechnol. 2022, 24, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Ding, L.G.; Huang, Z.Y.; Xu, H.; Xu, Z. Commensal bacteria-immunity crosstalk shapes mucosal homeostasis in teleost fish. Rev. Aquacult. 2021, 13, 2322–2343. [Google Scholar] [CrossRef]
- Salinas, I.; Magadán, S. Omics in fish mucosal immunity. Dev. Comp. Immunol. 2017, 75, 99–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Ni, J.; Ma, Y.; Xiong, H.; Jian, W. Toxicity and modulation of silver nanoparticles synthesized using abalone viscera hydrolysates on bacterial community in aquatic environment. Front. Microbiol. 2022, 13, 968650. [Google Scholar] [CrossRef]
- Jung, A.; Jung-Schroers, V. Detection of Deefgea chitinilytica in freshwater ornamental fish. Lett. Appl. Microbiol. 2011, 52, 497–500. [Google Scholar] [CrossRef]
- Li, S.; Heng, X.; Guo, L.Y.; Lessing, D.J.; Chu, W.H. SCFAs improve disease resistance via modulate gut microbiota, enhance immune response and increase antioxidative capacity in the host. Fish Shellfish Immunol. 2022, 120, 560–568. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Yuan, X.; Gao, J.; Xie, M.; Tian, X.; Xiong, Z.; Song, R.; Xie, Z.; Ou, D. Conventional Anthelmintic Concentration of Deltamethrin Immersion Disorder in the Gill Immune Responses of Crucian Carp. Toxics 2023, 11, 743. https://doi.org/10.3390/toxics11090743
Wu H, Yuan X, Gao J, Xie M, Tian X, Xiong Z, Song R, Xie Z, Ou D. Conventional Anthelmintic Concentration of Deltamethrin Immersion Disorder in the Gill Immune Responses of Crucian Carp. Toxics. 2023; 11(9):743. https://doi.org/10.3390/toxics11090743
Chicago/Turabian StyleWu, Hao, Xiping Yuan, Jinwei Gao, Min Xie, Xing Tian, Zhenzhen Xiong, Rui Song, Zhonggui Xie, and Dongsheng Ou. 2023. "Conventional Anthelmintic Concentration of Deltamethrin Immersion Disorder in the Gill Immune Responses of Crucian Carp" Toxics 11, no. 9: 743. https://doi.org/10.3390/toxics11090743
APA StyleWu, H., Yuan, X., Gao, J., Xie, M., Tian, X., Xiong, Z., Song, R., Xie, Z., & Ou, D. (2023). Conventional Anthelmintic Concentration of Deltamethrin Immersion Disorder in the Gill Immune Responses of Crucian Carp. Toxics, 11(9), 743. https://doi.org/10.3390/toxics11090743






