The Protective Effect of Exogenous 17β-Estradiol against Experimentally Induced Oxidative Damage to Membrane Lipids Is Stronger in Male vs. Female Porcine Thyroids: Preliminary Results
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Assay of Lipid Peroxidation
2.4. Measurement of Lipid Peroxidation Products
2.5. Statistical Analyses
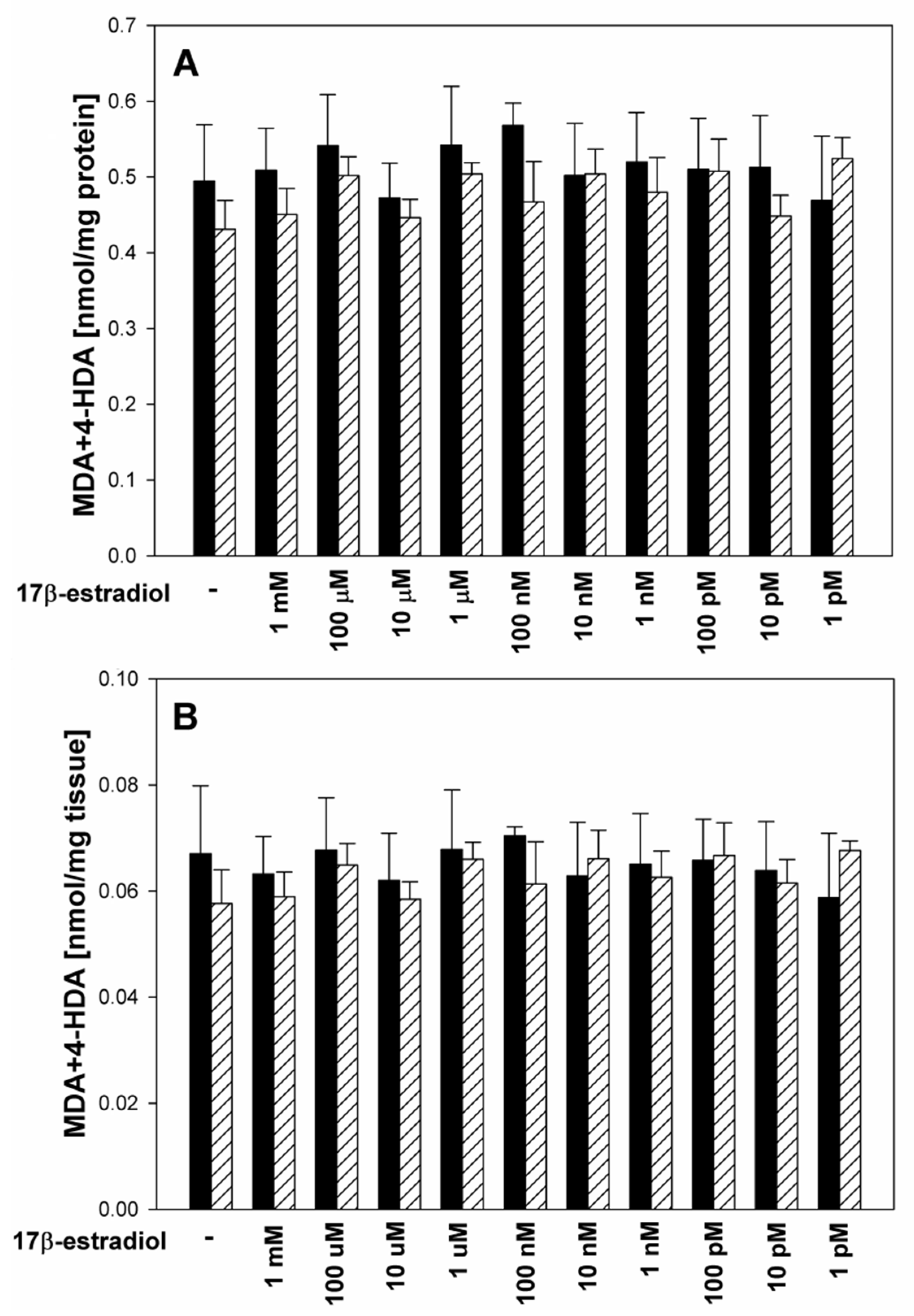
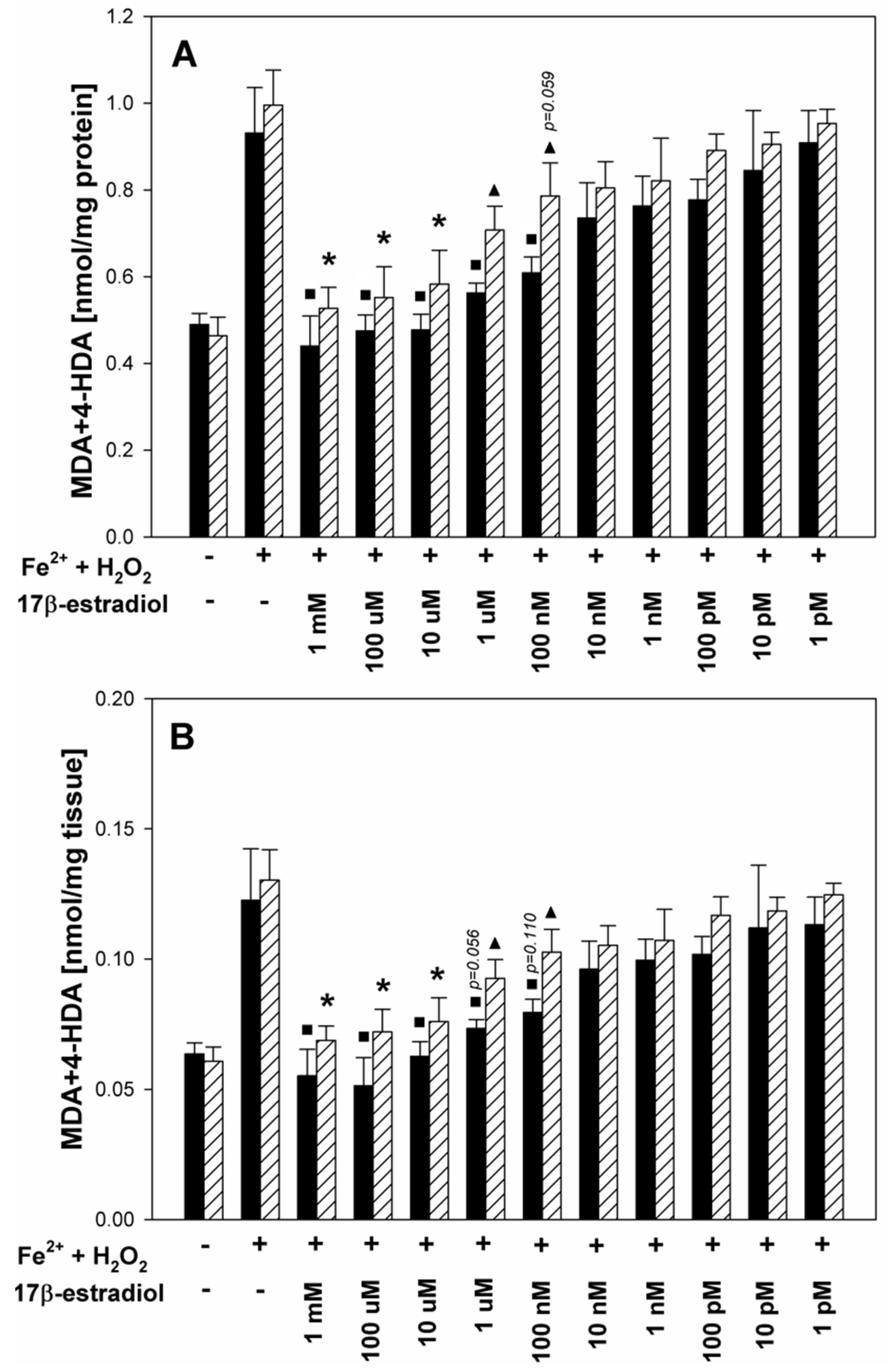
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrow, E.H. The evolution of sex differences in disease. Biol. Sex. Differ. 2015, 6, 5. [Google Scholar] [CrossRef]
- Clocchiatti, A.; Cora, E.; Zhang, Y.; Dotto, G.P. Sexual dimorphism in cancer. Nat. Rev. Cancer 2016, 16, 330–339. [Google Scholar] [PubMed]
- Ober, C.; Loisel, D.A.; Gilad, Y. Sex-specific genetic architecture of human disease. Nat. Rev. Genet. 2008, 9, 911–922. [Google Scholar]
- Zheng, D.; Trynda, J.; Williams, C.; Vold, J.A.; Nguyen, J.H.; Harnois, D.M.; Bagaria, S.P.; McLaughlin, S.A.; Li, Z. Sexual dimorphism in the incidence of human cancers. BMC Cancer 2019, 19, 684. [Google Scholar]
- James, B.C.; Mitchell, J.M.; Jeon, H.D.; Vasilottos, N.; Grogan, R.H.; Aschebrook-Kilfoy, B. An update in international trends in incidence rates of thyroid cancer, 1973–2007. Cancer Causes Control 2018, 29, 465–473. [Google Scholar]
- Suteau, V.; Munier, M.; Briet, C.; Rodien, P. Sex bias in differentiated thyroid cancer. Int. J. Mol. Sci. 2021, 22, 12992. [Google Scholar] [PubMed]
- Garmendia Madariaga, A.; Santos Palacios, S.; Guillén-Grima, F.; Galofré, J.C. The incidence and prevalence of thyroid dysfunction in Europe: A meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 923–931. [Google Scholar]
- Hollowell, J.G.; Staehling, N.W.; Flanders, W.D.; Hannon, W.H.; Gunter, E.W.; Spencer, C.A.; Braverman, L.E. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 2002, 87, 489–499. [Google Scholar] [CrossRef]
- Morand, G.B.; Tessler, I.; Krasner, J.; Pusztaszeri, M.P.; Yamin, T.; Gecel, N.A.; Avior, G.; Payne, R.J. Investigation of genetic sex-specific molecular profile in well-differentiated thyroid cancer: Is there a difference between females and males? Clin. Otolaryngol. 2023, 48, 748–755. [Google Scholar] [CrossRef]
- Allegra, A.; Caserta, S.; Genovese, S.; Pioggia, G.; Gangemi, S. Gender differences in oxidative stress in relation to cancer susceptibility and survival. Antioxidants 2023, 12, 1255. [Google Scholar]
- Zhang, Y.; Wei, F.; Zhang, J.; Hao, L.; Jiang, J.; Dang, L.; Mei, D.; Fan, S.; Yu, Y.; Jiang, L. Bisphenol A and estrogen induce proliferation of human thyroid tumor cells via an estrogen-receptor-dependent pathway. Arch. Biochem. Biophys. 2017, 633, 29–39. [Google Scholar] [PubMed]
- Stepniak, J.; Lewinski, A.; Karbownik-Lewinska, M. Sexual dimorphism of NADPH oxidase/H₂O₂ system in rat thyroid cells; effect of exogenous 17β-estradiol. Int. J. Mol. Sci. 2018, 19, 4063. [Google Scholar] [CrossRef] [PubMed]
- Spencer, W.A.; Vadhanam, M.V.; Jeyabalan, J.; Gupta, R.C. Oxidative DNA damage following microsome/Cu(II)-mediated activation of the estrogens, 17β-estradiol, equilenin, and equilin: Role of reactive oxygen species. Chem. Res. Toxicol. 2012, 25, 305–314. [Google Scholar]
- Prokai-Tatrai, K.; Perjesi, P.; Rivera-Portalatin, N.M.; Simpkins, J.W.; Prokai, L. Mechanistic investigations on the antioxidant action of a neuroprotective estrogen derivative. Steroids 2008, 73, 280–288. [Google Scholar] [PubMed]
- Stepniak, J.; Karbownik-Lewinska, M. 17β-Estradiol prevents experimentally-induced oxidative damage to membrane lipids and nuclear DNA in porcine ovary. Syst. Biol. Reprod. Med. 2016, 62, 17–21. [Google Scholar]
- Rynkowska, A.; Stępniak, J.; Karbownik-Lewińska, M. Fenton reaction-induced oxidative damage to membrane lipids and protective effects of 17β-estradiol in porcine ovary and thyroid homogenates. Int. J. Environ. Res. Public. Health 2020, 17, E6841. [Google Scholar]
- Rynkowska, A.; Stępniak, J.; Karbownik-Lewińska, M. Melatonin and indole-3-propionic acid reduce oxidative damage to membrane lipids induced by high iron concentrations in porcine skin. Membranes 2021, 11, 571. [Google Scholar] [CrossRef]
- Stepniak, J.; Lewinski, A.; Karbownik-Lewinska, M. Oxidative damage to membrane lipids in the thyroid—No differences between sexes. Drug Chem. Toxicol. 2019, 2, 1–6. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Al-Rawas, A.M.; Al-Maqbali, M.; Al-Saleh, M.; Enriquez, M.B.; Al-Siyabi, S.; Al-Hashmi, K.; Al-Lawati, I.; Bulthuis, M.L.C.; et al. Systemic oxidative stress is increased in postmenopausal women and independently associates with homocysteine levels. Int. J. Mol. Sci. 2020, 21, 314. [Google Scholar] [CrossRef]
- Moreau, K.L.; Hildreth, K.L.; Klawitter, J.; Blatchford, P.; Kohrt, W.M. Decline in endothelial function across the menopause transition in healthy women is related to decreased estradiol and increased oxidative stress. Geroscience 2020, 42, 1699–1714. [Google Scholar] [PubMed]
- Pech, L.G.M.; Caballero-Chacón, S.D.C.; Guarner-Lans, V.; Díaz-Díaz, E.; Gómez, A.M.; Pérez-Torres, I. Effect of oophorosalpingo-hysterectomy on serum antioxidant enzymes in female dogs. Sci. Rep. 2019, 9, 9674. [Google Scholar] [PubMed]
- Stubbins, R.E.; Najjar, K.; Holcomb, V.B.; Hong, J.; Núñez, N.P. Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes Obes. Metab. 2012, 14, 58–66. [Google Scholar] [PubMed]
- Grasberger, H.; De Deken, X.; Mayo, O.B.; Raad, H.; Weiss, M.; Liao, X.H.; Refetoff, S. Mice deficient in dual oxidase maturation factors are severely hypothyroid. Mol. Endocrinol. 2012, 26, 481–492. [Google Scholar]
- Fortunato, R.S.; Braga, W.M.; Ortenzi, V.H.; Rodrigues, D.C.; Andrade, B.M.; Miranda-Alves, L.; Rondinelli, E.; Dupuy, C.; Ferreira, A.C.; Carvalho, D.P. Sexual dimorphism of thyroid reactive oxygen species production due to higher NADPH oxidase 4 expression in female thyroid glands. Thyroid 2013, 23, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lata, K.; Mukhopadhyay, S.; Mukherjee, T.K. Role of estrogen receptors in pro-oxidative and anti-oxidative actions of estrogens: A perspective. Biochim. Biophys. Acta 2010, 1800, 1127–1135. [Google Scholar]
- Ruan, X.; Mueck, A.O. The WHO claims estrogens are ‘carcinogenic’: Is this true? Climacteric 2023, 26, 263–270. [Google Scholar] [CrossRef]
- Abbassi-Ghanavati, M.; Greer, L.G.; Cunningham, F.G. Pregnancy and laboratory studies: A reference table for clinicians. Obstet. Gynecol. 2009, 114, 1326–1331. [Google Scholar] [CrossRef]
- Yang, L.; Ma, J.; Lei, P.; Yi, J.; Ma, Y.; Huang, Z.; Wang, T.; Ping, H.; Ruan, D.; Sun, D.; et al. Advances in antioxidant applications for combating 131I side effects in thyroid cancer treatment. Toxics 2023, 11, 529. [Google Scholar]
- Iwan, P.; Stepniak, J.; Karbownik-Lewinska, M. Cumulative protective effect of melatonin and indole-3-propionic acid against KIO3-induced lipid peroxidation in porcine thyroid. Toxics 2021, 9, 89. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępniak, J.; Koziróg, E.; Karbownik-Lewińska, M. The Protective Effect of Exogenous 17β-Estradiol against Experimentally Induced Oxidative Damage to Membrane Lipids Is Stronger in Male vs. Female Porcine Thyroids: Preliminary Results. Toxics 2023, 11, 746. https://doi.org/10.3390/toxics11090746
Stępniak J, Koziróg E, Karbownik-Lewińska M. The Protective Effect of Exogenous 17β-Estradiol against Experimentally Induced Oxidative Damage to Membrane Lipids Is Stronger in Male vs. Female Porcine Thyroids: Preliminary Results. Toxics. 2023; 11(9):746. https://doi.org/10.3390/toxics11090746
Chicago/Turabian StyleStępniak, Jan, Edward Koziróg, and Małgorzata Karbownik-Lewińska. 2023. "The Protective Effect of Exogenous 17β-Estradiol against Experimentally Induced Oxidative Damage to Membrane Lipids Is Stronger in Male vs. Female Porcine Thyroids: Preliminary Results" Toxics 11, no. 9: 746. https://doi.org/10.3390/toxics11090746
APA StyleStępniak, J., Koziróg, E., & Karbownik-Lewińska, M. (2023). The Protective Effect of Exogenous 17β-Estradiol against Experimentally Induced Oxidative Damage to Membrane Lipids Is Stronger in Male vs. Female Porcine Thyroids: Preliminary Results. Toxics, 11(9), 746. https://doi.org/10.3390/toxics11090746







