Photocatalytic Removal of Metronidazole Antibiotics from Water Using Novel Ag-N-SnO2 Nanohybrid Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Photocatalyst
2.3. Characterization
2.4. Studying Photocatalytic Activity
3. Results and Discussion
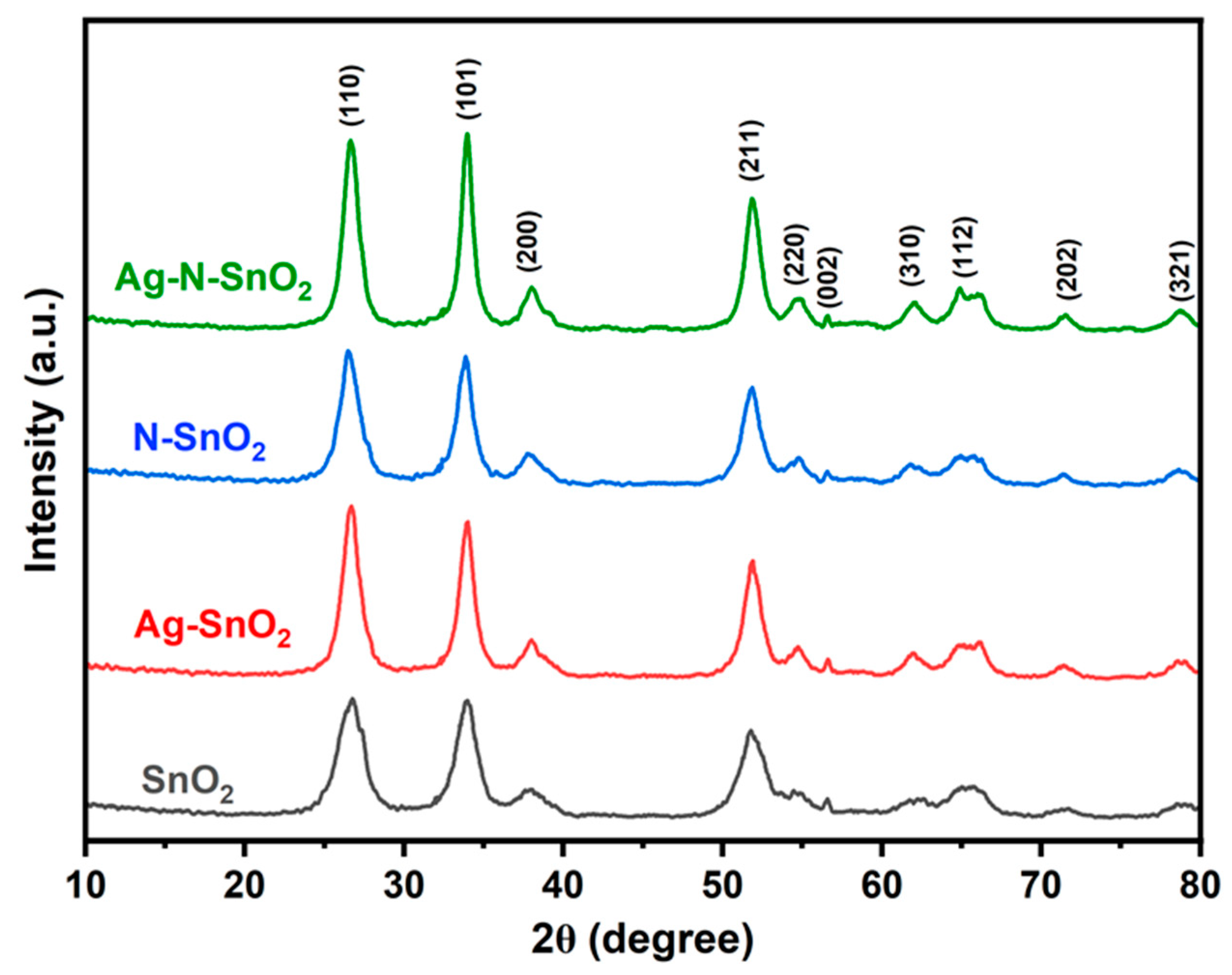
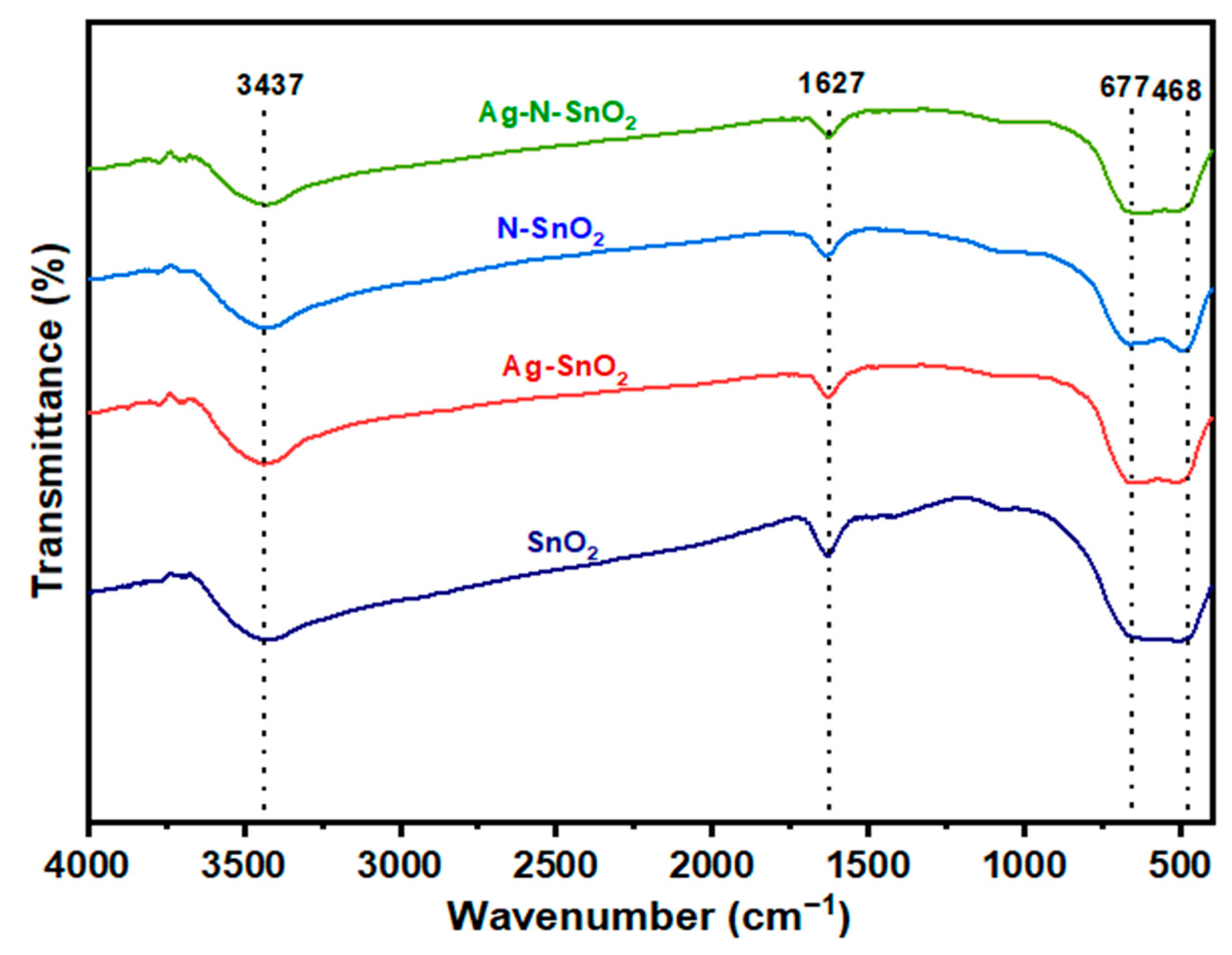
3.1. Morphology and Structure of Photocatalysts
3.2. Photocatalytic Activity
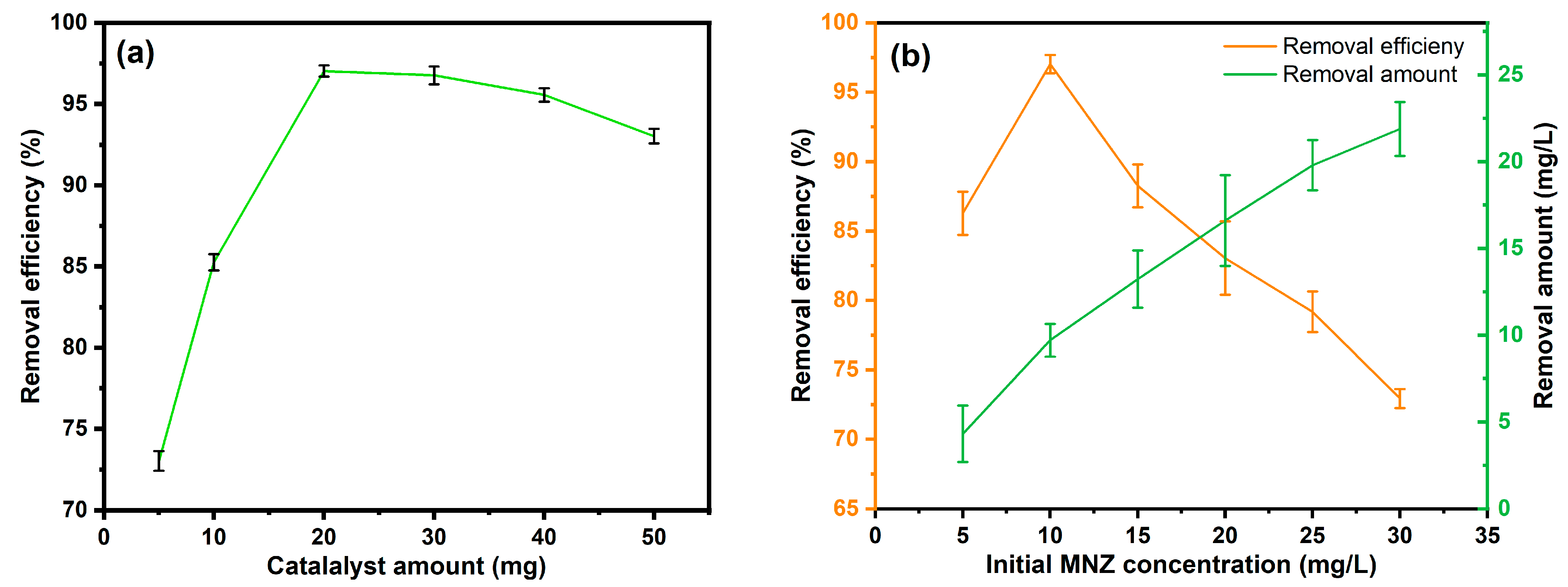
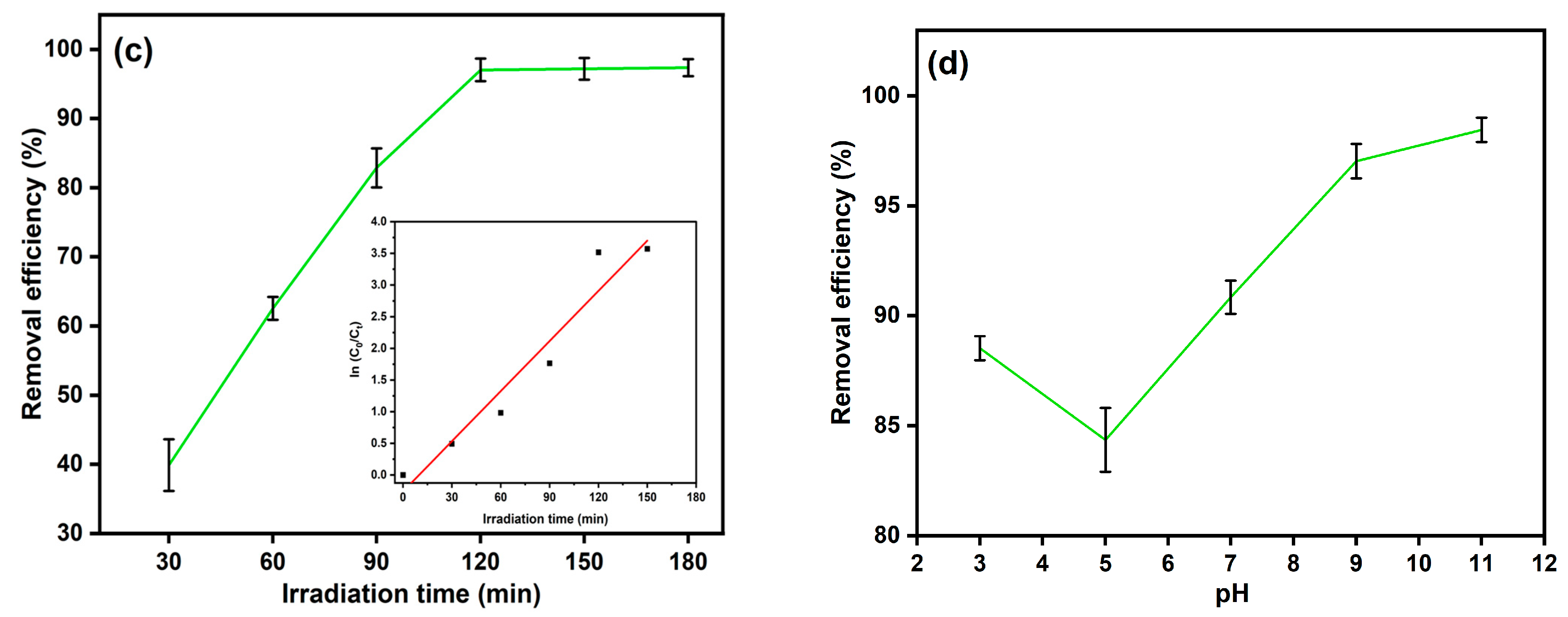
3.3. Operational Parameters Optimization
3.4. MNZ Mineralization
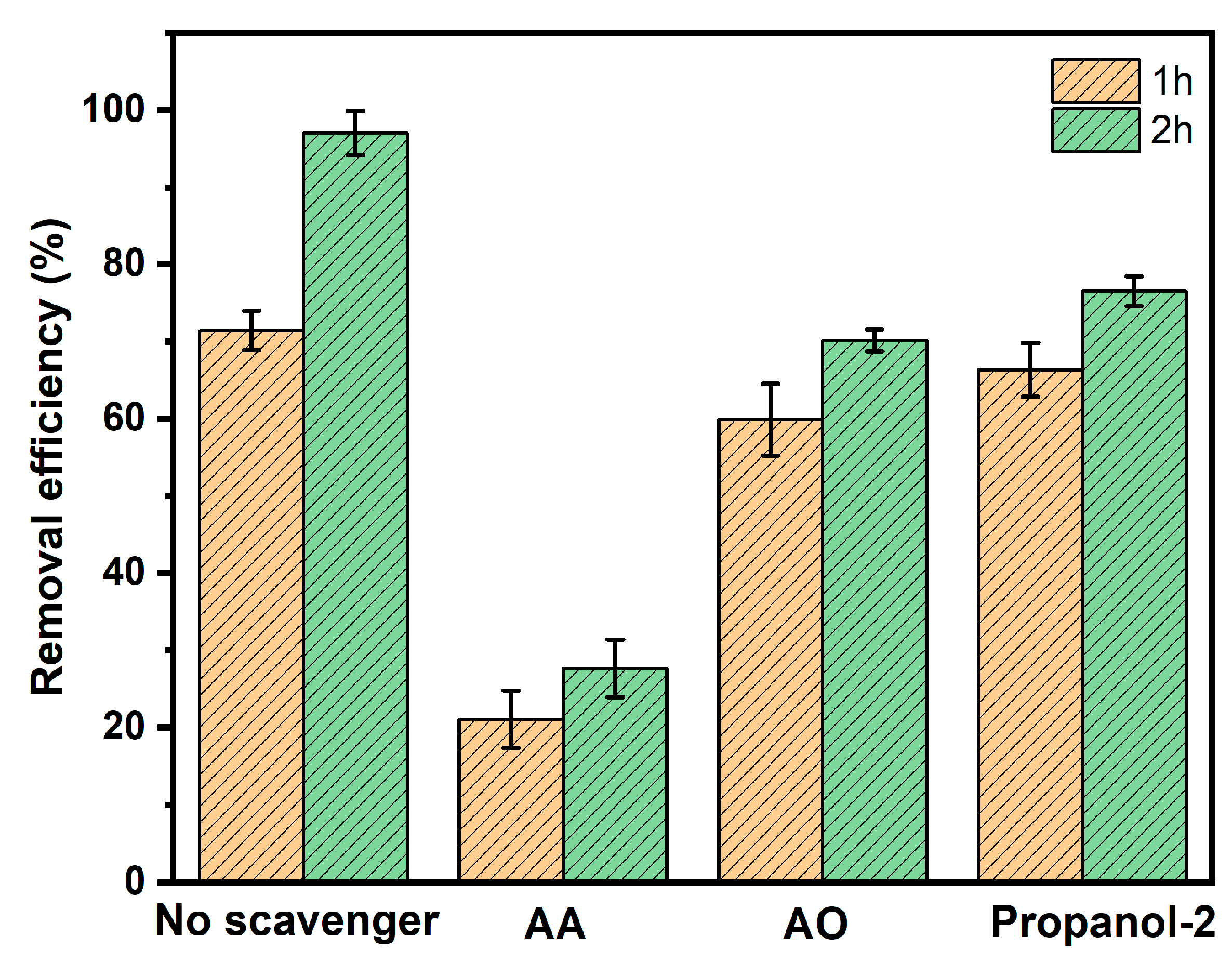
3.5. Radical Scavenges’ Function
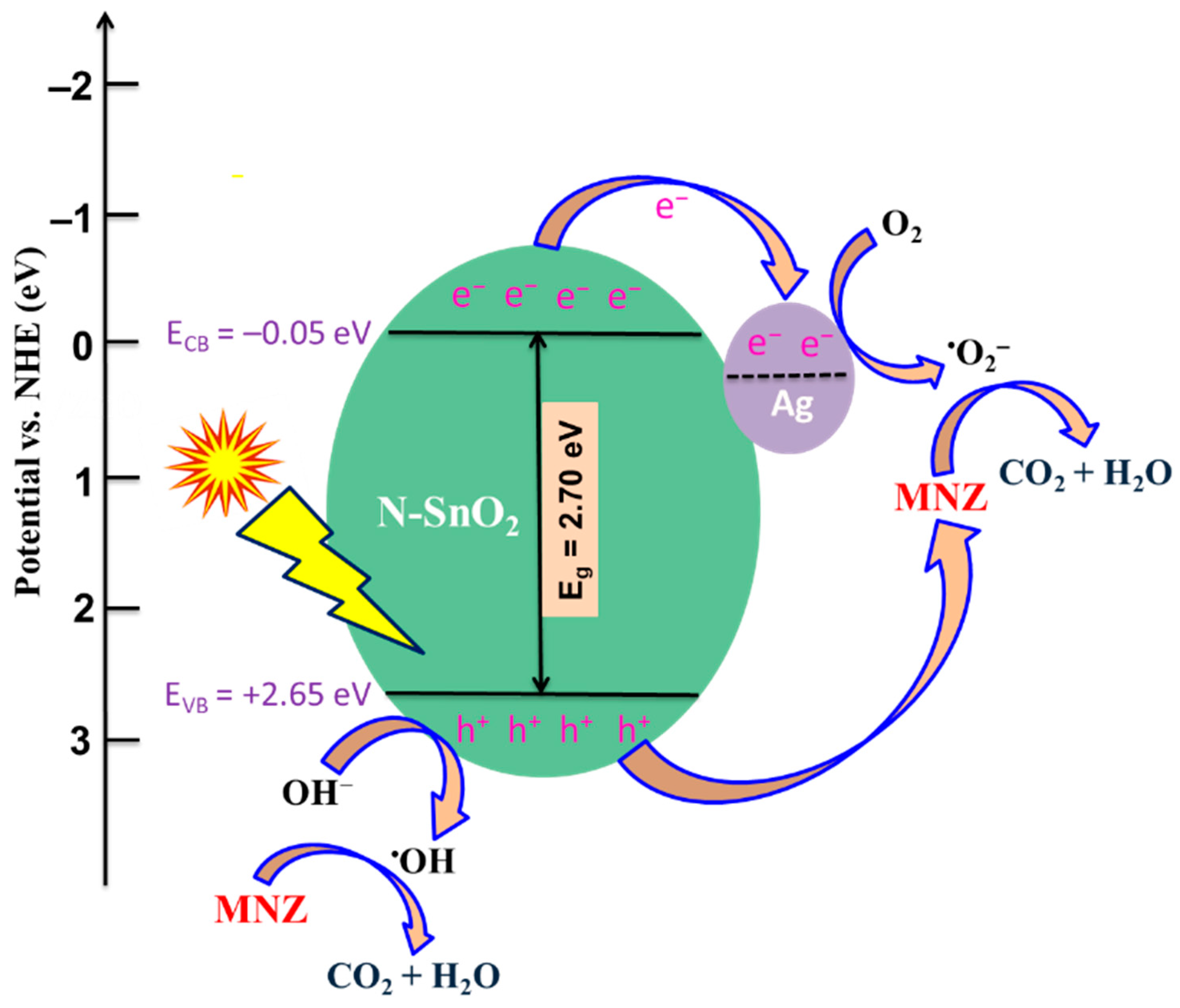
3.6. Plausible Degradation Mechanism for MNZ
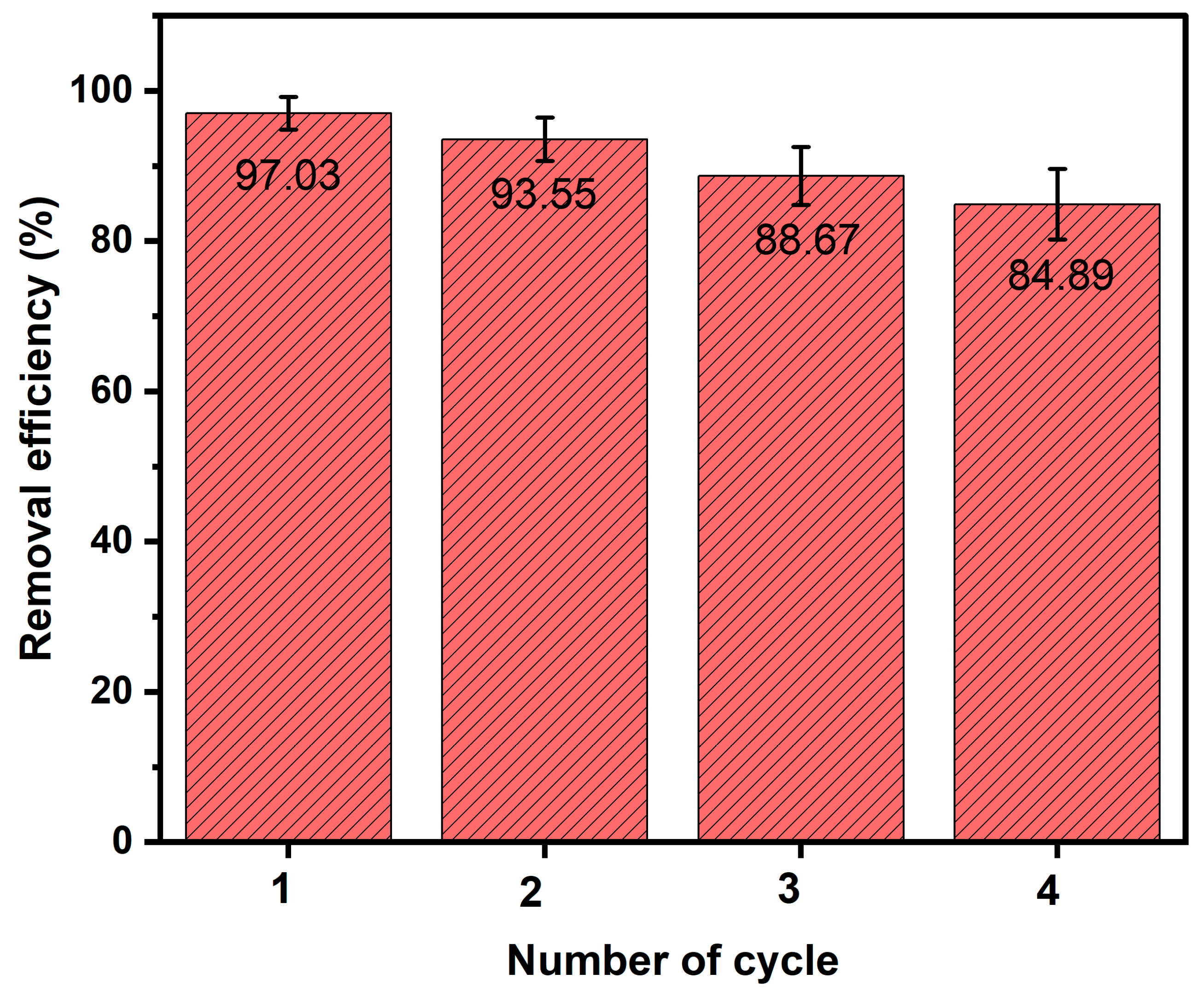
3.7. Ag-N-SnO2 Reusability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hossain, A.; Nakamichi, S.; Mamun, M.H.A.; Tani, K.; Masunaga, S.; Matsuda, H. Occurrence and ecological risk of pharmaceuticals in river surface water of Bangladesh. Environ. Res. 2019, 165, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Fahim, S.A.; Jahan, N.; Sathy, R.A.; Quddus, M.S.; Moniruzzaman, M.; Masum, S.M.; Molla, M.A.I. B–Sn/TiO2 nanoparticles for photodegradation of metronidazole antibiotics under different lights. Mater. Chem. Phys. 2023, 305, 127937. [Google Scholar] [CrossRef]
- Bashiri, F.S.; Khezri, S.M.; Kalantary, R.R.; Kakavandi, B. Enhanced photocatalytic degradation of metronidazole by TiO2 decorated on magnetic reduced graphene oxide: Characterization, optimization and reaction mechanism studies. J. Mol. Liq. 2020, 314, 113608. [Google Scholar] [CrossRef]
- Velasco-Garduño, O.; González-Blanco, G.; Fajardo-Ortiz, M.D.C.; Beristain-Cardoso, R. Influence of metronidazole on activated sludge activity. Environ. Technol. 2021, 42, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Prados-Joya, G.; Sánchez-Polo, M.; Ferro-García, M.A.; Bautista-Toledo, I. Removal of nitroimidazole antibiotics from aqueous solution by adsorption/bioadsorption on activated carbon. J. Hazard. Mater. 2009, 170, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Polo, M.; Rivera-Utrilla, J.; Prados-Joya, G.; Ferro-García, M.A.; Bautista-Toledo, I. Removal of pharmaceutical compounds, nitroimidazoles, from waters by using the ozone/carbon system. Water Res. 2008, 42, 4163–4171. [Google Scholar] [CrossRef] [PubMed]
- Azarpira, H.; Balarak, D. Rice husk as a biosorbent for antibiotic metronidazole removal: Isotherm studies and model validation. Int. J. ChemTech Res. 2016, 9, 566–573. [Google Scholar]
- Pérez, T.; Garcia-Segura, S.; El-Ghenymy, A.; Nava, J.L.; Brillas, E. Solar photoelectro-Fenton degradation of the antibiotic metronidazole using a flow plant with a Pt/air-diffusion cell and a CPC photoreactor. Electrochim. Acta 2015, 165, 173–181. [Google Scholar] [CrossRef]
- Molla, M.A.I.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Fabrication of Ag-doped ZnO by mechanochemical combustion method and their application into photocatalytic Famotidine degradation. J. Environ. Sci. Health—Toxic/Hazard. Subst. Environ. Eng. 2019, 54, 914–923. [Google Scholar] [CrossRef]
- Shathy, R.A.; Fahim, S.A.; Sarker, M.; Quddus, M.S.; Moniruzzaman, M.; Masum, S.M.; Molla, M.A.I. Natural Sunlight Driven Photocatalytic Removal of Toxic Textile Dyes in Water Using B-Doped ZnO/TiO2 Nanocomposites. Catalysts 2022, 12, 3. [Google Scholar] [CrossRef]
- Ahmed, A.Z.; Islam, M.M.; Islam, M.M.U.; Masum, S.M.; Islam, R.; Molla, M.A.I. Fabrication and characterization of B/Sn-doped ZnO nanoparticles via mechanochemical method for photocatalytic degradation of rhodamine B. Inorg. Nano-Metal Chem. 2020, 51, 1369–1378. [Google Scholar] [CrossRef]
- Mohammad, A.; Karim, M.R.; Khan, M.E.; Khan, M.M.; Cho, M.H. Biofilm-Assisted Fabrication of Ag@SnO2-g-C3N4 Nanostructures for Visible Light-Induced Photocatalysis and Photoelectrochemical Performance. J. Phys. Chem. C 2019, 123, 20936–20948. [Google Scholar] [CrossRef]
- Dhanalakshmi, M.; Saravanakumar, K.; Prabavathi, S.; Abinaya, M.; Velluchamy, M. Fabrication of novel surface plasmon resonance induced visible light driven iridium decorated SnO2 nanorods for degradation of organic contaminants. J. Alloys Compd. 2018, 763, 512–524. [Google Scholar] [CrossRef]
- Purkayastha, D.D.; Krishna, M.G. Dopant controlled photoinduced hydrophilicity and photocatalytic activity of SnO2 thin films. Appl. Surf. Sci. 2018, 447, 724–731. [Google Scholar]
- Kabir, R.; Saifullah, M.A.K.; Ahmed, A.Z.; Masum, S.M.; Molla, M.A.I. Synthesis of N-doped ZnO nanocomposites for sunlight photocatalytic degradation of textile dye pollutants. J. Compos. Sci. 2020, 4, 49. [Google Scholar] [CrossRef]
- Ahuja, P.; Ujjain, S.K.; Kanojia, R.; Attri, P. Transition metal oxides and their composites for photocatalytic dye degradation. J. Compos. Sci. 2021, 5, 3. [Google Scholar] [CrossRef]
- Sakib, A.A.M.; Masum, S.M.; Hoinkis, J.; Islam, R.; Molla, M.A.I. Synthesis of CuO/ZnO nanocomposites and their application in photodegradation of toxic textile dye. J. Compos. Sci. 2019, 3, 91. [Google Scholar] [CrossRef]
- Duan, Z.; Deng, L.; Shi, Z.; Zhang, H.; Zeng, H.; Crittenden, J. In situ growth of Ag-SnO2 quantum dots on silver phosphate for photocatalytic degradation of carbamazepine: Performance, mechanism and intermediates toxicity assessment. J. Colloid Interface Sci. 2019, 534, 270–278. [Google Scholar] [CrossRef]
- Sharma, R.B.; Kumar, S.; Kumar, R.; Sahu, P.K.; Kumari, V.; Mishra, A.K.; Kumar, V. Unlocking the Potential of N-Doped SnO2 for Sustainable Photocatalytic Degradation of Carcinogenic Dyes. Separations 2023, 10, 322. [Google Scholar]
- Siddique, M.; Subhan, W.; Naz, F.; Nawaz, A. Biosynthesis of highly porous Ag/Bi/SnO2 nanohybrid material using seeds extract of Caesalpinia bonduc and their photocatalytic activity. Phys. B Condens. Matter 2022, 644, 414209. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Bari, G.A.K.M.R.; Vattikuti, S.V.P.; Kim, H. Synthesis of carbon-doped SnO2 nanostructures for visible-light-driven photocatalytic hydrogen production from water splitting. Int. J. Hydrogen Energy 2020, 45, 32789–32796. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Y.; Chen, B.; Ye, F.; Duan, H.; Cui, H. Facile fabrication of N-doped carbon quantum dots modified SnO2 composites for improved visible light photocatalytic activity. Vacuum 2021, 191, 110371. [Google Scholar] [CrossRef]
- Parthibavarman, M.; Sathishkumar, S.; Jayashree, M.; BoopathiRaja, R. Microwave Assisted Synthesis of Pure and Ag Doped SnO2 Quantum Dots as Novel Platform for High Photocatalytic Activity Performance. J. Cluster Sci. 2019, 30, 351–363. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Muthuraj, V. Fabrication of sphere like plasmonic Ag/SnO2 photocatalyst for the degradation of phenol. Optik 2017, 131, 754–763. [Google Scholar] [CrossRef]
- Zhong, X.; Yang, B.; Zhang, X.; Jia, J.; Yi, G. Effect of calcining temperature and time on the characteristics of Sb-doped SnO2 nanoparticles synthesized by the sol-gel method. Particuology 2012, 10, 365–370. [Google Scholar] [CrossRef]
- Bhawna, A.; Gupta, P.; Kumar, A.; Tyagi, R.; Kumar, A.; Kumar, P.; Singh, R.P.; Singh, V.K. Facile Synthesis of N-Doped SnO2 Nanoparticles: A Cocatalyst-Free Promising Photocatalyst for Hydrogen Generation. ChemistrySelect 2020, 5, 7775–7782. [Google Scholar] [CrossRef]
- Dhinakar, K.G.; Selvalakshmi, T.S.; Sundar, S.M.; Bose, A.C. Structural, optical and impedance properties of SnO2 nanoparticles. J. Mater. Sci. Mater. Electron. 2016, 27, 5818–5824. [Google Scholar] [CrossRef]
- Aslam, M.; Mahmood, T.; Naeem, A.; Ali, R. Investigation of HDTMA mediated sol gel synthesis of N-doped SnO2 nanoparticles: Studies of their electrical and optical properties. Mater. Technol. 2021, 36, 169–178. [Google Scholar] [CrossRef]
- Subbarao, P.S.; Aparna, Y.; Chitturi, K.L. Synthesis and characterization of Ni doped SnO2 nanoparticles by sol-gel method for novel applications. Mater. Today Proc. 2019, 26, 1676–1680. [Google Scholar] [CrossRef]
- Patel, G.H.; Chaki, S.H.; Kannaujiya, R.M.; Parekh, Z.R.; Hirpara, A.B.; Khimani, A.J.; Deshpande, M.P. Sol-gel synthesis and thermal characterization of SnO2 nanoparticles. Physica B Condens. Matter 2021, 613, 412987. [Google Scholar] [CrossRef]
- Amouhadi, E.; Aliyan, H.; Aghaei, H.; Fazaeli, R.; Richeson, D. Photodegradation and mineralization of metronidazole by a novel quadripartite SnO2@TiO2/ZrTiO4/ZrO2 photocatalyst: Comprehensive photocatalyst characterization and kinetic study. Mater. Sci. Semicond. Process. 2022, 143, 106560. [Google Scholar] [CrossRef]
- Agarwal, S.; Tyagi, I.; Gupta, V.K.; Sohrabi, M.; Mohammadi, S.; Golikand, A.N.; Fakhri, A. Iron doped SnO2/Co3O4 nanocomposites synthesized by sol-gel and precipitation method for metronidazole antibiotic degradation. J. Mater. Sci. Eng. C 2017, 70, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Sheikhsamany, R.; Faghihian, H.; Fazaeli, R. Synthesis of novel HKUST-1-based SnO2 porous nanocomposite with the photocatalytic capability for degradation of metronidazole. Mater. Sci. Semicond. Process. 2022, 138, 106310. [Google Scholar] [CrossRef]
- Derikvandi, H.; Nezamzadeh-Ejhieh, A. Synergistic effect of p-n heterojunction, supporting and zeolite nanoparticles in enhanced photocatalytic activity of NiO and SnO2. J. Colloid Interface Sci. 2017, 490, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, K.; Hariharan, R.; Rajarajan, M.; Suganthi, A. Photocatalytic performance of Ag doped SnO2 nanoparticles modified with curcumin. Solid State Sci. 2013, 21, 91–99. [Google Scholar] [CrossRef]
- Babu, B.; Cho, M.; Byon, C.; Shim, J. One pot synthesis of Ag-SnO2 quantum dots for highly enhanced sunlight-driven photocatalytic activity. J. Alloys Compd. 2018, 731, 162–171. [Google Scholar] [CrossRef]
- Molla, M.A.I.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Solar photocatalytic decomposition of Probenazole in water with TiO2 in the presence of H2O2. Energy Sources A Recovery Util. Environ. Eff. 2018, 40, 2432–2441. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Evaluating the efficiency of nano-sized Cu doped TiO2/ZnO photocatalyst under visible light irradiation. J. Mol. Liq. 2018, 258, 354–365. [Google Scholar] [CrossRef]
- Molla, M.A.I.; Ahsan, S.; Tateishi, I.; Furukawa, M.; Katsumata, H.; Suzuki, T.; Kaneco, S. Degradation, kinetics, and mineralization in solar photocatalytic treatment of aqueous amitrole solution with titanium dioxide. Environ. Eng. Sci. 2018, 35, 401–407. [Google Scholar] [CrossRef]
- Wang, D.-P.; Yeh, M.-K. Degradation Kinetics of Metronidazole in Solution. J. Pharm. Sci. 1993, 82, 95–98. [Google Scholar] [CrossRef]
- Siuleiman, S.; Kaneva, N.; Bojinova, A.; Papazova, K.; Apostolov, A.; Dimitrov, D. Photodegradation of Orange II by ZnO and TiO2 powders and nanowire ZnO and ZnO/TiO2 thin films. J. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 408–413. [Google Scholar] [CrossRef]
- Singh, A.; Mishra, B.K. Removal of chlorhexidine digluconate from aqueous solution by heterogenous photocatalysis using sunlight-driven Ni-doped TiO2 material. Environ. Eng. Res. 2023, 28, 210546. [Google Scholar] [CrossRef]
- Molla, M.A.I.; Ahmed, A.Z.; Kaneco, S. Reaction Mechanism for Photocatalytic Degradation of Organic Pollutants. In Nanostructured Photocatalysts; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]




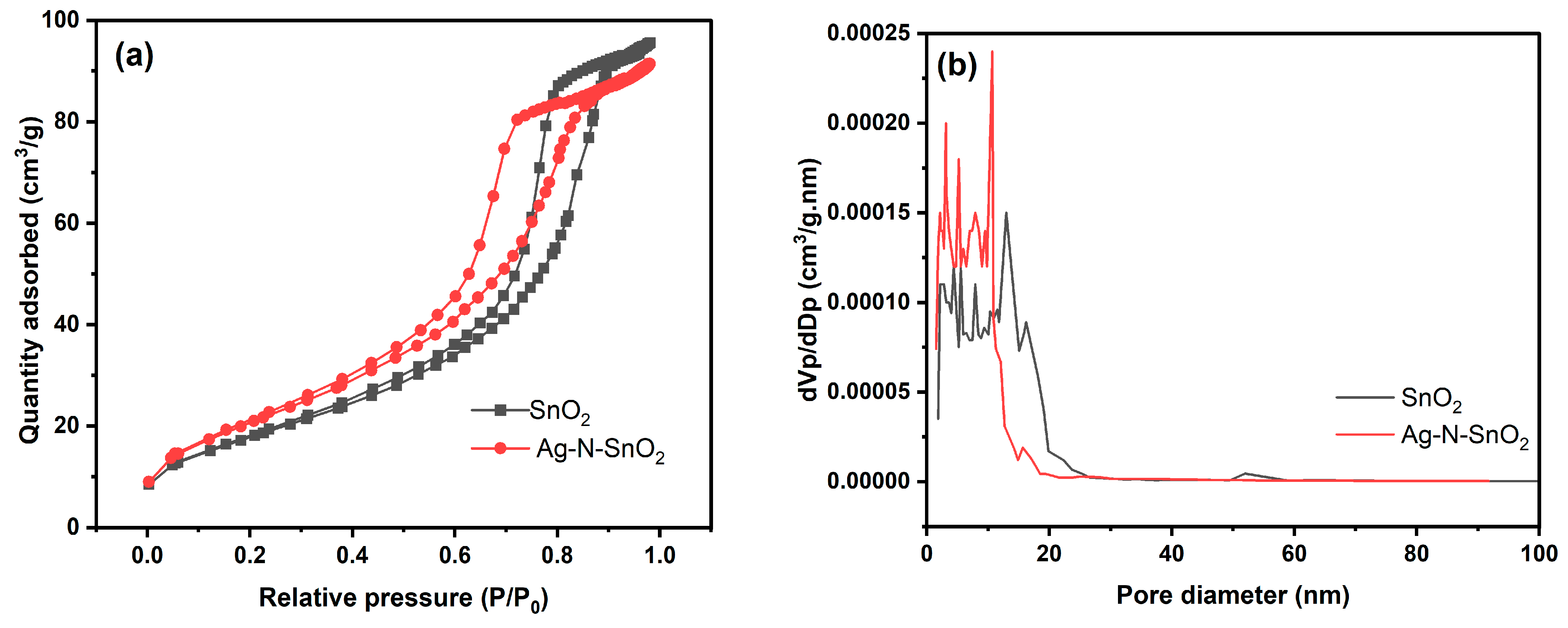



| Photocatalysts | Average Crystallite Size (nm) | BET Parameters | Band Gap Energies (eV) | ||
|---|---|---|---|---|---|
| Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) | |||
| SnO2 | 6.27 | 66.66 | 0.1472 | 8.834 | 3.61 |
| Ag-SnO2 | 7.67 | − | − | − | 2.62 |
| N-SnO2 | 7.78 | − | − | − | 2.70 |
| Ag-N-SnO2 | 6.92 | 78.24 | 0.1407 | 7.195 | 2.56 |
| Photocatalysts | Synthesis Method | Band-Gap Energies (eV) | pH | MNZ Concentration (mg/L) | Photocatalyst Dosage (g/L) | Time (min) | Light Source | Removal Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| SnO2@TiO2/ZrTiO4/ZrO2 | Hydrothermal | 3.24 | 6.5 | 20 | 0.07 | 50 | UV light | 98 | [31] |
| Fe-doped SnO2/Co3O4 | Sol-gel and precipitation | 2.30 | 6 | 30 | 2 | 15 | UV light | 98 | [32] |
| SnO2/MOF-199 | - | 2.18 | 3 | 40 | 2 | 240 | Sunlight | 80 | [33] |
| NiO-SnO2/clinoptilolite | Precipitation | 2.80 | 3 | 2 | 1.2 | 180 | Sunlight | 88 | [34] |
| Ag-N-SnO2 | Sol-gel | 2.56 | 9 | 10 | 0.4 | 120 | Natural sunlight | 97 | This study |
| Ag-N-SnO2 | Sol-gel | 2.56 | 5.8 | 10 | 0.4 | 120 | Natural sunlight | 88 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuvo, M.S.H.; Putul, R.A.; Hossain, K.S.; Masum, S.M.; Molla, M.A.I. Photocatalytic Removal of Metronidazole Antibiotics from Water Using Novel Ag-N-SnO2 Nanohybrid Material. Toxics 2024, 12, 36. https://doi.org/10.3390/toxics12010036
Shuvo MSH, Putul RA, Hossain KS, Masum SM, Molla MAI. Photocatalytic Removal of Metronidazole Antibiotics from Water Using Novel Ag-N-SnO2 Nanohybrid Material. Toxics. 2024; 12(1):36. https://doi.org/10.3390/toxics12010036
Chicago/Turabian StyleShuvo, Md. Shahriar Hossain, Rupna Akther Putul, Khandker Saadat Hossain, Shah Md. Masum, and Md. Ashraful Islam Molla. 2024. "Photocatalytic Removal of Metronidazole Antibiotics from Water Using Novel Ag-N-SnO2 Nanohybrid Material" Toxics 12, no. 1: 36. https://doi.org/10.3390/toxics12010036
APA StyleShuvo, M. S. H., Putul, R. A., Hossain, K. S., Masum, S. M., & Molla, M. A. I. (2024). Photocatalytic Removal of Metronidazole Antibiotics from Water Using Novel Ag-N-SnO2 Nanohybrid Material. Toxics, 12(1), 36. https://doi.org/10.3390/toxics12010036








