Modulatory Role of Biochar Properties and Environmental Risk of Heavy Metals by Co-Pyrolysis of Fenton Sludge and Biochemical Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Sludge and Properties
2.2. Individual Sludge Pyrolysis
2.3. Co-Pyrolysis of Mixed Sludge
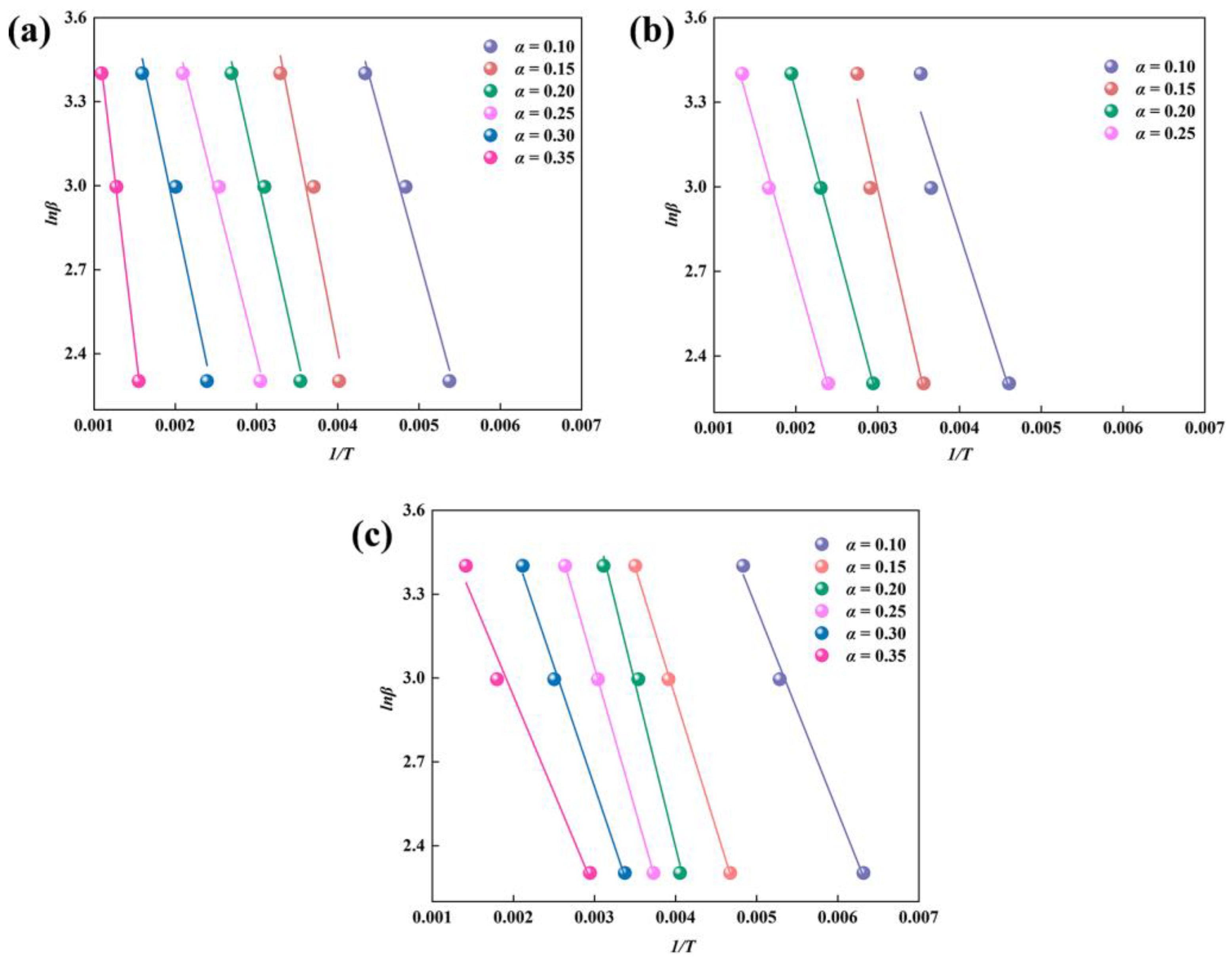
2.4. Calculation of Activation Energy
2.5. Preparation of Sludge Biochar
2.6. Characterization and Analysis of Sludge Biochar
2.7. Immobilization and Risk Assessment of HMs
3. Results and Discussion
3.1. Thermogravimetric Analysis of Sludge
3.2. Kinetic Reaction Process of Sludge Pyrolysis
3.3. Physical and Chemical Properties of Sludge and Sludge Biochar
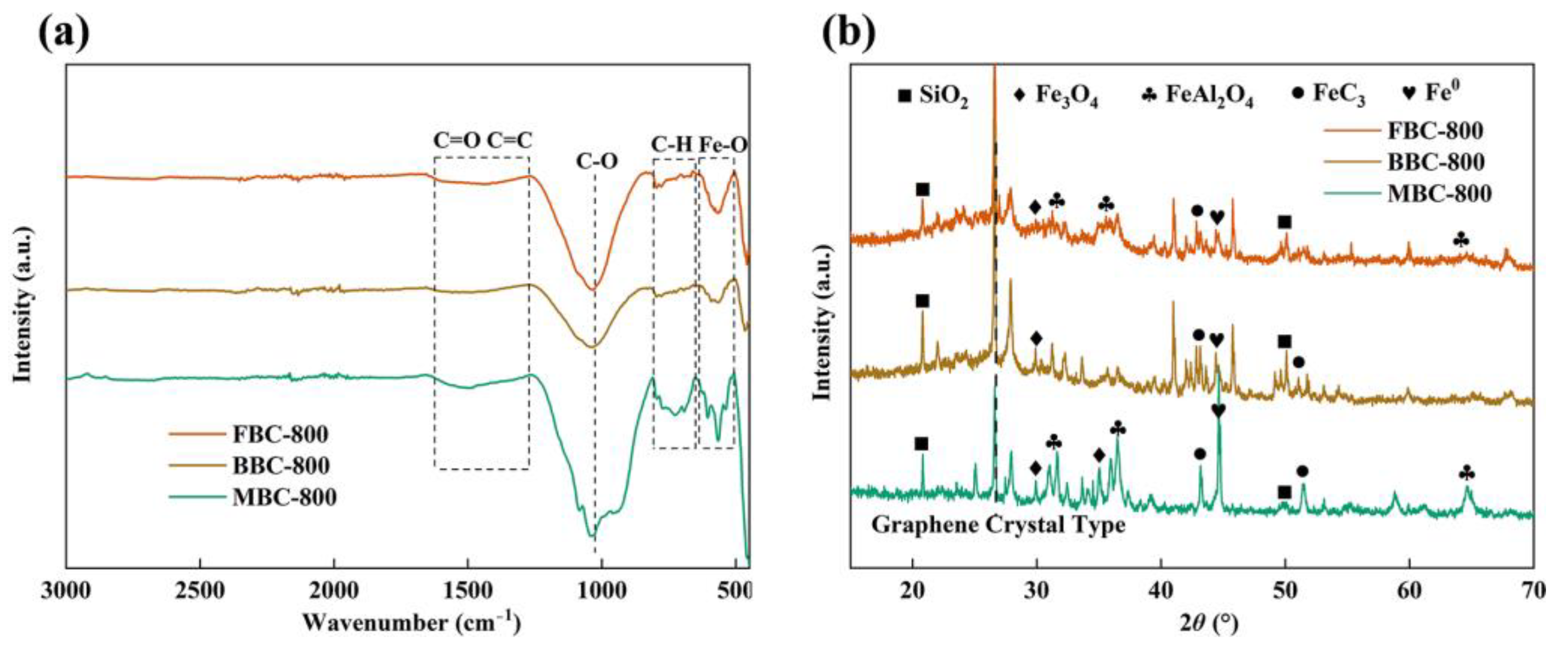
3.4. Surface Morphology and Structural Alterations of Biochar
3.5. Role of Co-Pyrolysis on the Immobilization of HMs in Sludge
3.6. Environmental Risk Assessment of HMs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caligan, C.J.A.; Garcia, M.M.S.; Mitra, J.L.; San Juan, J.L.G. Multi-objective optimization for a wastewater treatment plant and sludge-to-energy network. J. Clean. Prod. 2022, 368, 133047. [Google Scholar] [CrossRef]
- Duan, B.; Feng, Q. Comparison of the potential ecological and human health risks of heavy metals from sewage sludge and livestock manure for agricultural use. Toxics 2021, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, H.; Huang, Z.; Yuan, X.; Tan, M.; Jiang, L.; Wu, Z.; Qin, X.; Li, H. Comparison of atmospheric pressure and gas-pressurized torrefaction of municipal sewage sludge: Properties of solid products. Energy Conv. Manag. 2020, 213, 112793. [Google Scholar] [CrossRef]
- Li, D.; Shan, R.; Jiang, L.; Gu, J.; Zhang, Y.; Yuan, H.; Chen, Y. A review on the migration and transformation of heavy metals in the process of sludge pyrolysis. Resour. Conserv. Recycl. 2022, 185, 106452. [Google Scholar] [CrossRef]
- Soria-Verdugo, A.; Morato-Godino, A.; Garcia-Gutierrez, L.M.; Garcia-Hernando, N. Pyrolysis of sewage sludge in a fixed and a bubbling fluidized bed—Estimation and experimental validation of the pyrolysis time. Energy Convers. Manag. 2017, 144, 235–242. [Google Scholar] [CrossRef]
- Wu, M.; Yang, Z.-H.; Jiang, T.-B.; Zhang, W.-W.; Wang, Z.-W.; Hou, Q.-X. Enhancing sludge methanogenesis with changed micro-environment of anaerobic microorganisms by Fenton iron mud. Chemosphere 2023, 341, 139884. [Google Scholar] [CrossRef] [PubMed]
- Chi, B.; Huang, Y.; Xiong, Z.; Tan, J.; Zhou, W.; Yang, Z.; Zhou, K.; Duan, X.; Chen, A.; Zha, R.; et al. Sludge lysis by thermophilic bacteria community enhances nutrient removal, sludge reduction, and modulates microbial community in anaerobic-anoxic-oxic process. J. Water Process. Eng. 2023, 56, 104385. [Google Scholar] [CrossRef]
- Chae, J.-S.; Choi, S.-A.; Kim, Y.-H.; Oh, S.-C.; Ryu, C.-K.; Ohm, T.-I. Experimental study of fry-drying and melting system for industrial wastewater sludge. J. Hazard. Mater. 2016, 313, 78–84. [Google Scholar] [CrossRef]
- Xu, Z.-X.; Song, H.; Deng, X.-Q.; Zhang, Y.-Y.; Xue-Qin, M.; Tong, S.-Q.; He, Z.-X.; Wang, Q.; Shao, Y.-W.; Hu, X. Dewatering of sewage sludge via thermal hydrolysis with ammonia-treated Fenton iron sludge as skeleton material. J. Hazard. Mater. 2019, 379, 120810. [Google Scholar] [CrossRef]
- Wang, M.; Sun, Y.; Yu, Q.; Zhao, Z.; Li, Y.; Zhang, Y. Sustainable disposal of Fenton sludge and enhanced organics degradation based on dissimilatory iron reduction in the hydrolytic acidification process. J. Hazard. Mater. 2023, 459, 132258. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Huang, Z.; Luo, X.; Ma, Y.; Chen, C.; Chen, X.; Cui, L. Activation of persulfate for tetracycline degradation using the catalyst regenerated from Fenton sludge containing heavy metal: Synergistic effect of Cu for catalysis. Chem. Eng. J. 2020, 396, 125238. [Google Scholar] [CrossRef]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Xing, X.; Zhao, H.; Zhou, L.; Wang, Y.; Chen, H.; Gao, Y.; Wang, Y.; Zhu, Y. Pyrolysis kinetics, thermodynamics of PTA sludge and product characterization of cyclic in-situ catalytic pyrolysis by using recycled char as a catalyst. Energy 2022, 251, 123821. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, D.; Hao, B.; Liu, L.; Wang, S.; Wu, Z. Thermochemical methods for the treatment of municipal sludge. J. Clean. Prod. 2021, 311, 127811. [Google Scholar] [CrossRef]
- Long, X.; Zhang, R.; Rong, R.; Wu, P.; Chen, S.; Ao, J.; An, L.; Fu, Y.; Xie, H. Adsorption characteristics of heavy metals Pb2+ and Zn2+ by magnetic biochar obtained from modified AMD sludge. Toxics 2023, 11, 590. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, X.; Zhang, J.; Ma, X.; Sun, P.; Zhao, L. Sewage sludge–coconut fiber co-pyrolysis biochar: Mechanisms underlying synergistic heavy metal stabilization and ciprofloxacin adsorption. J. Clean. Prod. 2022, 375, 134149. [Google Scholar] [CrossRef]
- Li, Z.; Yu, D.; Liu, X.; Wang, Y. The fate of heavy metals and risk assessment of heavy metal in pyrolysis coupling with acid washing treatment for sewage sludge. Toxics 2023, 11, 447. [Google Scholar] [CrossRef]
- Alharbi, H.A.; Alotaibi, K.D.; El-Saeid, M.H.; Giesy, J.P. Polycyclic aromatic hydrocarbons (PAHs) and metals in diverse biochar products: Effect of feedstock type and pyrolysis temperature. Toxics 2023, 11, 96. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, S.; Cheng, P.; Zhang, S.; Sun, Y. Effects of soil amendments on heavy metal immobilization and accumulation by maize grown in a multiple-metal-contaminated soil and their potential for safe crop production. Toxics 2020, 8, 102. [Google Scholar] [CrossRef]
- Liu, L.; Liu, X.; Wang, D.; Lin, H.; Huang, L. Removal and reduction of Cr(VI) in simulated wastewater using magnetic biochar prepared by co-pyrolysis of nano-zero-valent iron and sewage sludge. J. Clean. Prod. 2020, 257, 120562. [Google Scholar] [CrossRef]
- Khan, R.; Shukla, S.; Kumar, M.; Zuorro, A.; Pandey, A. Sewage sludge derived biochar and its potential for sustainable environment in circular economy: Advantages and challenges. Chem. Eng. J. 2023, 471, 144495. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Ruan, R.; Bilal, M.; Khan, N.A.; Awasthi, M.K.; Amer, M.A.; Leng, L.; Hamouda, M.A.; Vo, D.V.N.; Li, J. Co-pyrolysis of sewage sludge and biomass for stabilizing heavy metals and reducing biochar toxicity: A review. Environ. Chem. Lett. 2023, 21, 1231–1250. [Google Scholar] [CrossRef]
- Chen, X.; Ma, R.; Luo, J.; Huang, W.; Fang, L.; Sun, S.; Lin, J. Co-microwave pyrolysis of electroplating sludge and municipal sewage sludge to synergistically improve the immobilization of high-concentration heavy metals and an analysis of the mechanism. J. Hazard. Mater. 2021, 417, 126099. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Dey, A. P-Nitrophenol -Bioremediation using potent Pseudomonas strain from the textile dye industry effluent. J. Environ. Chem. Eng. 2020, 8, 103830. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhang, Z.; He, X.; Chen, J.; Liu, C. Pyrolysis kinetics of manganese carbonate. J. Therm. Anal. Calorim. 2022, 147, 10801–10813. [Google Scholar] [CrossRef]
- Urych, B.; Smoliński, A. Kinetics of sewage sludge pyrolysis and air gasification of its chars. Energy Fuels 2016, 30, 4869–4878. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Xie, S.; Zhang, G.; Pan, L.; Wang, R.; Wang, G.; Pan, X.; Wang, Y.; Angelidaki, I. Enhancement of heavy metal immobilization in sewage sludge biochar by combining alkaline hydrothermal treatment and pyrolysis. J. Clean. Prod. 2022, 369, 133325. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, W.; Geng, Z.; Bai, J.; Dong, B.; Zhao, J.; Zhuang, X.; Shih, K. Immobilization of heavy metals in biochar by co-pyrolysis of sludge and CaSiO3. J. Environ. Manag. 2023, 326, 116635. [Google Scholar] [CrossRef]
- Li, J.; Hu, J.; Wang, T.; Gan, J.; Xie, J.; Shui, Y.; Liu, J.; Xue, Y. Thermogravimetric analysis of the co-combustion of residual petrochemical sludge and municipal sewage sludge. Thermochim. Acta 2019, 673, 60–67. [Google Scholar] [CrossRef]
- Guo, Z.; Bai, G.; Huang, B.; Cai, N.; Guo, P.; Chen, L. Preparation and application of a novel biochar-supported red mud catalyst: Active sites and catalytic mechanism. J. Hazard. Mater. 2021, 408, 124802. [Google Scholar] [CrossRef]
- Magdziarz, A.; Werle, S. Analysis of the combustion and pyrolysis of dried sewage sludge by TGA and MS. Waste Manag. 2014, 34, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, X.; Liang, S.; Guan, R.; Li, H.; Chen, Y.; Liu, B.; Song, J.; Yu, W.; Xiao, K.; et al. Enhanced hydrogen production in catalytic pyrolysis of sewage sludge by red mud: Thermogravimetric kinetic analysis and pyrolysis characteristics. Int. J. Hydrogen Energy 2018, 43, 7795–7807. [Google Scholar] [CrossRef]
- Li, Q.; Yang, H.; Chen, P.; Jiang, W.; Chen, F.; Yu, X.; Su, G. Investigation of catalytic co-pyrolysis characteristics and synergistic effect of oily sludge and walnut shell. Int. J. Environ. Res. Public Health 2023, 20, 2841. [Google Scholar] [CrossRef]
- Ma, Z.; Xie, J.; Gao, N.; Quan, C. Pyrolysis behaviors of oilfield sludge based on Py-GC/MS and DAEM kinetics analysis. J. Energy Inst. 2019, 92, 1053–1063. [Google Scholar] [CrossRef]
- Xu, G.; Cai, X.; Wang, L.; Zhang, Q.; Fang, B.; Zhong, X.; Yao, J. Thermogravimetric-infrared analysis and performance optimization of co-pyrolysis of oily sludge and rice husks. Int. J. Hydrogen Energy 2022, 47, 27437–27451. [Google Scholar] [CrossRef]
- Zhang, M.; He, T.; Jin, B. Effect of mineral additives on pyrolytic characteristics and heavy metal behavior during co-pyrolysis of industrial sludge and hyperaccumulator plant. J. Anal. Appl. Pyrolysis 2023, 169, 105827. [Google Scholar] [CrossRef]
- Fang, S.; Yu, Z.; Ma, X.; Lin, Y.; Chen, L.; Liao, Y. Analysis of catalytic pyrolysis of municipal solid waste and paper sludge using TG-FTIR, Py-GC/MS and DAEM (distributed activation energy model). Energy 2018, 143, 517–532. [Google Scholar] [CrossRef]
- Yang, K.; Sun, J.; Liu, H.; Yang, W.; Dong, L. Study on the Thermogravimetric Kinetics of Dehydrated Sewage Sludge Regulated by Cationic Polyacrylamide and Sawdust. Polymers 2023, 15, 2396. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Lu, T.; Huang, H.; Zhao, D.; Kobayashi, N.; Chen, Y. Influence of pyrolysis temperature on physical and chemical properties of biochar made from sewage sludge. J. Anal. Appl. Pyrolysis 2015, 112, 284–289. [Google Scholar] [CrossRef]
- Fan, J.; Li, Y.; Yu, H.; Li, Y.; Yuan, Q.; Xiao, H.; Li, F.; Pan, B. Using sewage sludge with high ash content for biochar production and Cu(II) sorption. Sci. Total Environ. 2020, 713, 136663. [Google Scholar] [CrossRef]
- Wang, J.; Wang, T.; Zhu, Q.; Zhang, S.; Shi, Q.; Chovelon, J.-M.; Wang, H. Preparation of a novel sludge-derived biochar by K2FeO4 conditioning to enhance the removal of Pb2+. Colloid Interface Sci. Commun. 2021, 42, 100417. [Google Scholar] [CrossRef]
- Shen, M.; Huang, Z.; Qiu, L.; Chen, Z.; Xiao, X.; Mo, X.; Cui, L. Recycling of Fenton sludge containing Ni as an efficient catalyst for tetracycline degradation through peroxymonosulfate activation. J. Clean. Prod. 2020, 268, 122174. [Google Scholar] [CrossRef]
- Sultana, S.; Hossain, M.B.; Choudhury, T.R.; Yu, J.; Rana, M.S.; Noman, M.A.; Hosen, M.M.; Paray, B.A.; Arai, T. Ecological and human health risk assessment of heavy metals in cultured shrimp and aquaculture sludge. Toxics 2022, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhou, X.; Peng, Z.; Wan, S.; Gao, Z.F.; Deng, S.; Tong, L.; Han, W.; Chen, X. Co-pyrolysis technology for enhancing the functionality of sewage sludge biochar and immobilizing heavy metals. Chemosphere 2023, 317, 137929. [Google Scholar] [CrossRef] [PubMed]
- Chanaka Udayanga, W.D.; Veksha, A.; Giannis, A.; Lisak, G.; Chang, V.W.C.; Lim, T.-T. Fate and distribution of heavy metals during thermal processing of sewage sludge. Fuel 2018, 226, 721–744. [Google Scholar] [CrossRef]
- Song, F.; Gu, L.; Zhu, N.; Yuan, H. Leaching behavior of heavy metals from sewage sludge solidified by cement-based binders. Chemosphere 2013, 92, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Yu, S.; Deng, S.; Mikulčić, H.; Tan, H.; Wang, X. Enrichment characteristics and environmental risk assessment of heavy metals in municipal sludge pyrolysis biochar. J. Energy Inst. 2023, 111, 101417. [Google Scholar] [CrossRef]



| Level of Evaluation | Exponential Value |
|---|---|
| None | RAC ≤ 1% |
| Low | 1% < RAC ≤ 10% |
| Moderate | 10% < RAC ≤ 30% |
| High | 30% < RAC ≤ 50% |
| Very high | RAC > 50% |
| Types of Sludge | Heating Rates (°C/min) | Maximum Weight Loss (%) | Temperatures at Maximum Weight Loss Rates (°C) | Maximum Weight Loss Rates (%/°C) |
|---|---|---|---|---|
| Fenton Sludge | 30 | 35.83 | 306.35 | 0.08 |
| 20 | 35.79 | 282.64 | 0.16 | |
| 10 | 37.84 | 274.31 | 0.15 | |
| Biochemical Sludge | 30 | 26.64 | 354.05 | 0.06 |
| 20 | 27.95 | 302.69 | 0.08 | |
| 10 | 38.26 | 275.62 | 0.10 | |
| Mixed Sludge | 30 | 37.75 | 304.41 | 0.13 |
| 20 | 37.00 | 277.90 | 0.15 | |
| 10 | 42.84 | 256.97 | 0.23 |
| Types of Sludge | α | FWO | |||
|---|---|---|---|---|---|
| Fitted Equations | R2 | Eα (kJ/mol) | E (kJ/mol) | ||
| Fenton Sludge | 0.10 | y = −1062.54x + 8.05 | 0.984 | 8.40 | 11.59 |
| 0.15 | y = −1489.45x + 8.37 | 0.948 | 11.78 | ||
| 0.20 | y = −1299.17x + 6.94 | 0.985 | 10.27 | ||
| 0.25 | y = −1156.67x + 5.86 | 0.987 | 9.14 | ||
| 0.30 | y = −1372.51x + 5.64 | 0.972 | 10.85 | ||
| 0.35 | y = −2415.59x + 6.06 | 0.999 | 19.10 | ||
| Biochemical Sludge | 0.10 | y = −907.84x + 6.47 | 0.931 | 7.18 | 8.50 |
| 0.15 | y = −1269.57x + 6.80 | 0.965 | 10.04 | ||
| 0.20 | y = −1094.91x + 5.53 | 0.999 | 8.66 | ||
| 0.25 | y = −1028.66x + 4.78 | 0.996 | 8.13 | ||
| Mixed Sludge | 0.10 | y = −728.21x + 6.89 | 0.995 | 5.76 | 7.11 |
| 0.15 | y = −935.29x + 6.67 | 0.999 | 7.39 | ||
| 0.20 | y = −1172.40x + 7.08 | 0.990 | 9.27 | ||
| 0.25 | y = −1009.68x + 6.07 | 0.999 | 7.98 | ||
| 0.30 | y = −860.46x + 5.19 | 0.995 | 6.80 | ||
| 0.35 | y = −691.75x + 4.32 | 0.983 | 5.47 | ||
| Sample | Major Elements (%) | HMs (mg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|
| C | H | Si | Fe | Al | Cu | Pb | Zn | |
| Fenton Sludge | 12.01 | 2.67 | 8.13 | 23.67 | 8.21 | 2158.32 | 372.41 | 1094.34 |
| Biochemical Sludge | 27.14 | 4.23 | 8.88 | 5.36 | 0.54 | 10.15 | 357.76 | 3350.14 |
| Types of Biochar | Yield (%) | pH | Contents of Elements (%) | Specific Surface Area (m2/g) | ||||
|---|---|---|---|---|---|---|---|---|
| C | H | Si | Fe | Al | ||||
| FBC-800 | 61.19 | 7.76 | 2.16 | 0.34 | 14.12 | 29.36 | 11.54 | 7.62 |
| BBC-800 | 68.61 | 6.21 | 6.37 | 0.85 | 15.73 | 10.06 | 1.48 | 64.23 |
| MBC-800 | 60.34 | 8.67 | 4.56 | 0.49 | 16.18 | 18.93 | 5.91 | 48.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Kang, M.; Wang, Y.; Bai, X.; Ye, Z. Modulatory Role of Biochar Properties and Environmental Risk of Heavy Metals by Co-Pyrolysis of Fenton Sludge and Biochemical Sludge. Toxics 2024, 12, 57. https://doi.org/10.3390/toxics12010057
Li Y, Kang M, Wang Y, Bai X, Ye Z. Modulatory Role of Biochar Properties and Environmental Risk of Heavy Metals by Co-Pyrolysis of Fenton Sludge and Biochemical Sludge. Toxics. 2024; 12(1):57. https://doi.org/10.3390/toxics12010057
Chicago/Turabian StyleLi, Yujian, Mengen Kang, Yuting Wang, Xue Bai, and Zhengfang Ye. 2024. "Modulatory Role of Biochar Properties and Environmental Risk of Heavy Metals by Co-Pyrolysis of Fenton Sludge and Biochemical Sludge" Toxics 12, no. 1: 57. https://doi.org/10.3390/toxics12010057






