Enhancing Europium Adsorption Effect of Fe on Several Geological Materials by Applying XANES, EXAFS, and Wavelet Transform Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rock Materials and Solid Phase Analysis
2.2. Batch Tests
2.3. Samples for XAS (X-ray Absorption Spectra) at NSRRC
2.4. Wavelet Transform
3. Results and Discussion
3.1. Distribution Coefficients (Kd) from Batch Tests
3.2. XANES for Samples and Materials
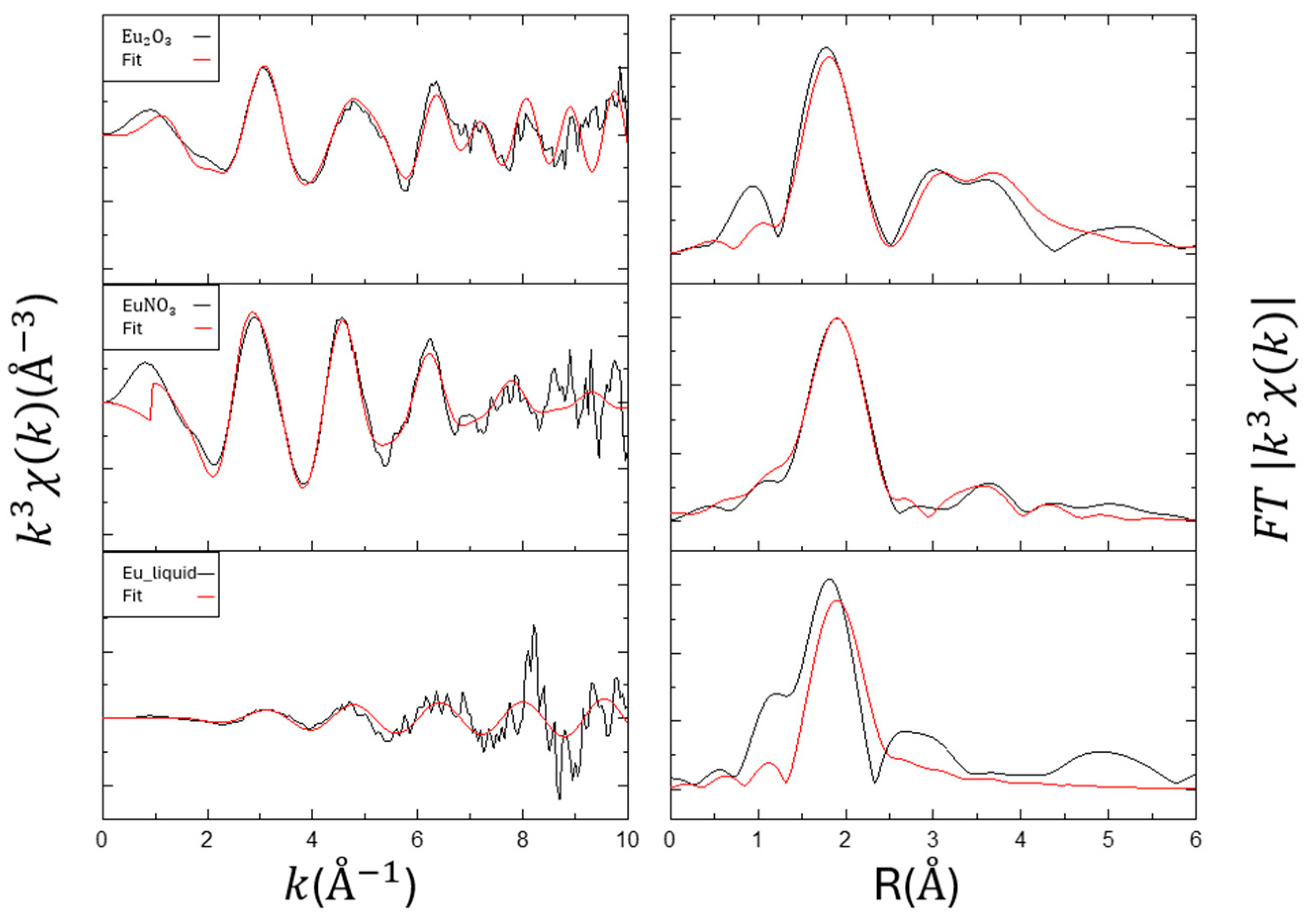
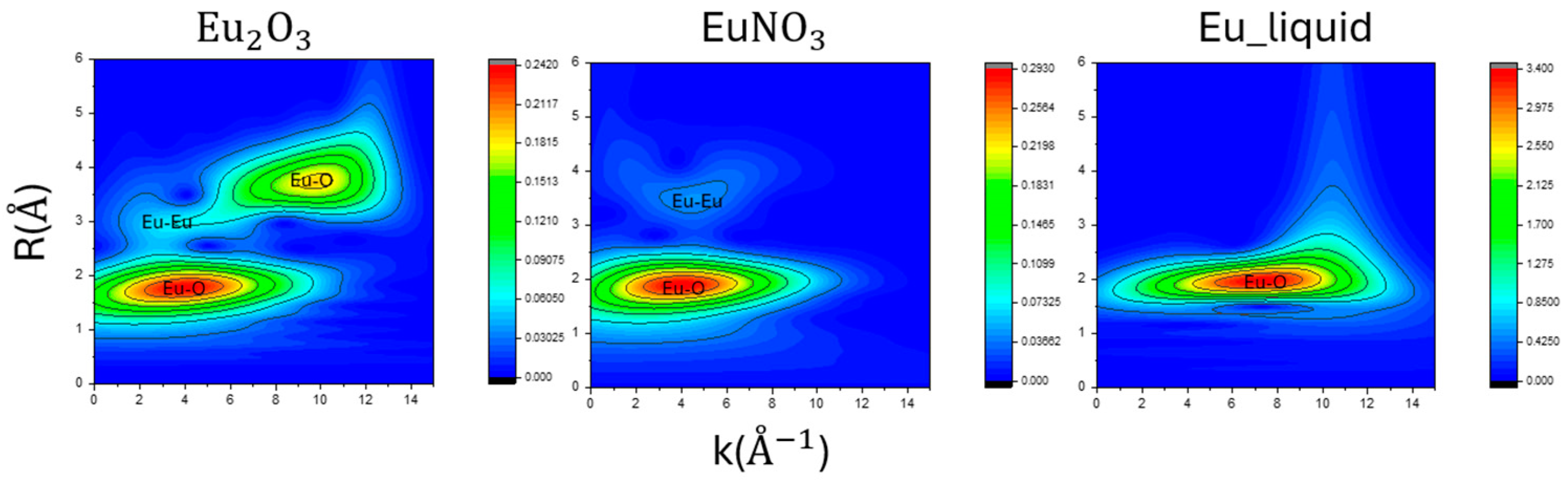
3.3. EXAFS for Eu Samples
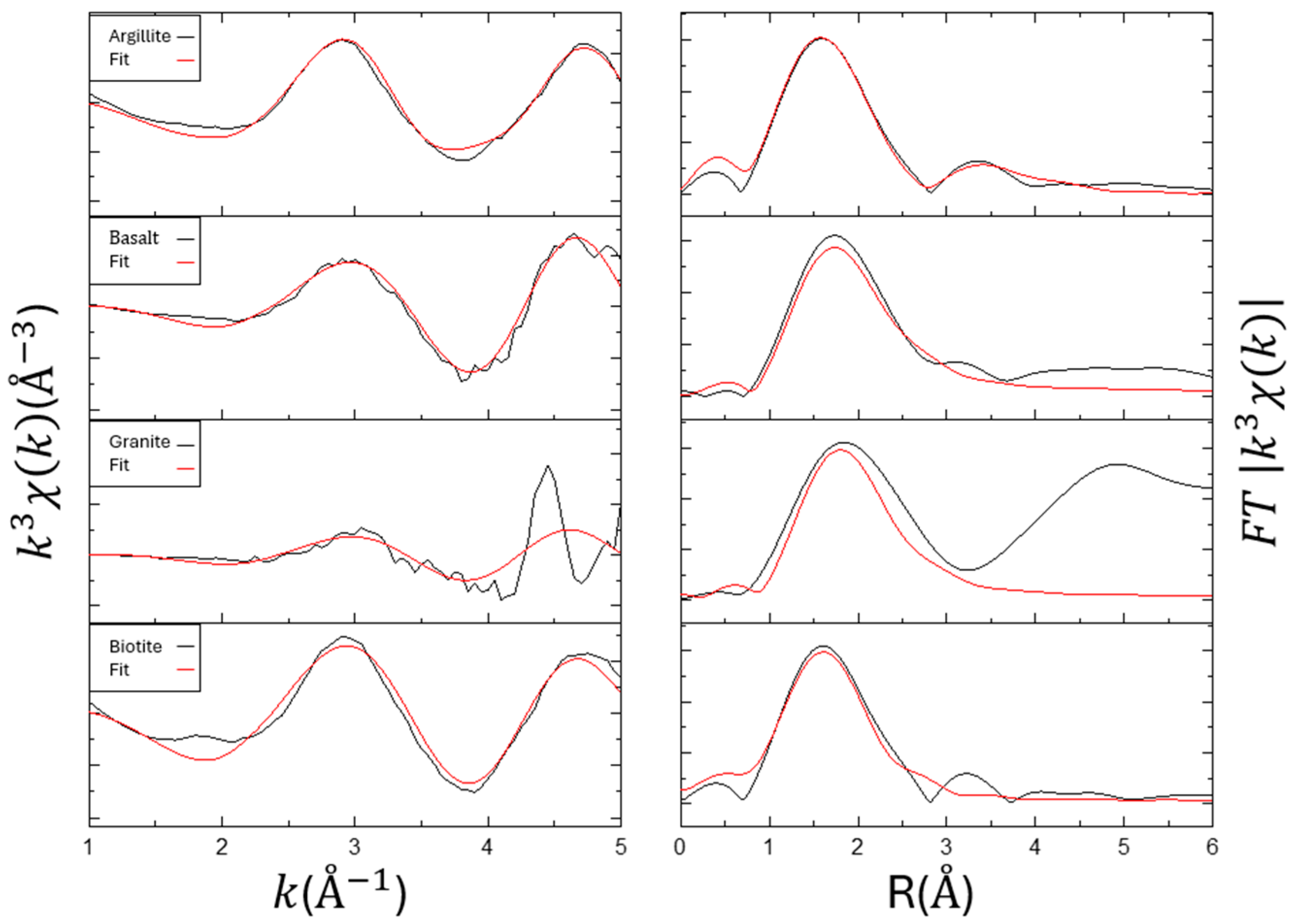
3.4. EXAFS for Materials (Effect of Fe)
3.5. EXAFS for Materials (Effect of Si)
3.6. Wavelet of EXAFS Fitting Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, C.P.; Wu, M.C.; Tsai, S.C.; Liu, C.Y.; Tsai, T.L.; Ou, L.Y. Study on sorption of Cs from aqueous solution by microcapsules enclosing natural minerals. J. Radioanal. Nucl. Chem. 2016, 307, 1743–1748. [Google Scholar] [CrossRef]
- Lee, C.P.; Tsai, S.C.; Wu, M.C.; Tsai, T.L. A study on removal of Cs and Sr from aqueous solution by bentonite-alginate microcapsules. J. Radioanal. Nucl. Chem. 2018, 318, 2381–2387. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Lee, C.P.; Luo, M.; Hua, R.; Liu, W.; Kong, J.; Hu, Y. Diffusion behavior of Se(IV) in Tamusu clayrock core by through-diffusion method. J. Radioanal. Nucl. Chem. 2021, 329, 149–158. [Google Scholar] [CrossRef]
- Sheha, R.R.; El-Shazly, E.A.; Roushdy, A.F.; Salah, B.A.; Kandil, A.E.T. Sorption and transport characteristics of europium on sandy soils. Appl. Radiat. Isot. 2023, 194, 110690. [Google Scholar] [CrossRef]
- Estes, S.L.; Arai, Y.; Becker, U.; Fernando, S.; Yuan, K.; Ewing, R.; Zhang, J.; Shibata, T.; Powell, B.A. A self-consistent model describing the thermodynamics of Eu (III) adsorption onto hematite. Geochim. Cosmochim. Acta 2013, 122, 430–447. [Google Scholar] [CrossRef]
- Pan, D.; Fan, F.; Wang, Y.; Li, P.; Hu, P.; Fan, Q.; Wu, W. Retention of Eu (III) in muscovite environment: Batch and spectroscopic studies. Chem. Eng. J. 2017, 330, 559–565. [Google Scholar] [CrossRef]
- Bouby, M.; Lützenkirchen, J.; Dardenne, K.; Preocanin, T.; Denecke, M.A.; Klenze, R.; Geckeis, H. Sorption of Eu (III) onto titanium dioxide: Measurements and modeling. J. Colloid Interface Sci. 2010, 350, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Shi, Y.; Li, Y.; Xian, D.; Wang, J.; Liu, C. Adsorption of Eu (III) at rutile/water interface: Batch, spectroscopic and modelling studies. Coll. Surf. A Physicochem. Eng. Asp. 2021, 611, 125811. [Google Scholar] [CrossRef]
- Tan, X.; Fang, M.; Li, J.; Lu, Y.; Wang, X. Adsorption of Eu (III) onto TiO2: Effect of pH, concentration, ionic strength and soil fulvic acid. J. Hazard. Mater. 2009, 168, 458–465. [Google Scholar] [CrossRef]
- Stumpf, T.; Bauer, A.; Coppin, F.; Fanghänel, T.; Kim, J. Inner-sphere, outer-sphere and ternary surface complexes: A TRLFS study of the sorption process of Eu(III) onto smectite and kaolinite. Radiochim. Acta 2002, 90, 345–349. [Google Scholar] [CrossRef]
- Fukushi, K.; Hasegawa, Y.; Maeda, K.; Aoi, Y.; Tamura, A.; Arai, S.; Yamamoto, Y.; Aosai, D.; Mizuno, T. Sorption of Eu (III) on granite: EPMA, LA–ICP–MS, batch and modeling studies. Environ. Sci. Technol. 2013, 47, 12811–12818. [Google Scholar] [CrossRef] [PubMed]
- ASTM C1733-21; Standard Test Method for Distribution Coefficients of Inorganic Species by Batch Method. ASTM: West Conshohocken, PA, USA, 2021.
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Funke, H.; Chukalina, M.; Scheinost, A.C. A new FEFF-based wavelet for EXAFS data analysis. J. Synchrotron Radiat. 2007, 14, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Funke, H.; Scheinost, A.C.; Chukalina, M. Wavelet analysis of extended X-ray absorption fine structure data. Phys. Rev. B—Condens. Matter Mater. Phys. 2005, 71, 094110. [Google Scholar] [CrossRef]
- Wavelet Transform for EXAFS. Available online: https://www.esrf.fr/UsersAndScience/Experiments/CRG/BM20/Software/Wavelets (accessed on 15 July 2024).
- Tan, X.L.; Wang, X.K.; Geckeis, H.; Rabung, T.H. Sorption of Eu (III) on humic acid or fulvic acid bound to hydrous alumina studied by SEM-EDS, XPS, TRLFS, and batch techniques. Environ. Sci. Technol. 2008, 42, 6532–6537. [Google Scholar] [CrossRef]
- Rabung, T.; Pierret, M.C.; Bauer, A.; Geckeis, H.; Bradbury, M.H.; Baeyens, B. Sorption of Eu (III)/Cm (III) on Ca-montmorillonite and Na-illite. Part 1: Batch sorption and time-resolved laser fluorescence spectroscopy experiments. Geochim. Cosmochim. Acta 2005, 69, 5393–5402. [Google Scholar] [CrossRef]
- Tsai, S.C.; Wang, T.H.; Li, M.H.; Wei, Y.Y.; Teng, S.P. Cesium adsorption and distribution onto crushed granite under different physicochemical conditions. J. Hazard. Mater. 2009, 161, 854. [Google Scholar] [CrossRef]
- Lee, C.P.; Tsai, S.C.; Jan, Y.L.; Wei, Y.Y.; Teng, S.P.; Hsu, C.N. Sorption and diffusion of HTO and cesium in crushed granite compacted to different lengths. J. Radioanal. Nucl. Chem. 2008, 275, 371–378. [Google Scholar] [CrossRef]
- Lee, C.P.; Wu, M.C.; Tsai, T.L.; Wei, H.J.; Meng, L.J.; Lin, T.Z. Comparative study on retardation behavior of Cs in crushed and intact rocks: Two potential repository host rocks in the Taiwan area. J. Radioanal. Nucl. Chem. 2012, 293, 579–586. [Google Scholar] [CrossRef]
- Lee, C.P.; Tsai, S.C.; Wu, M.C.; Tsai, T.L.; Tu, Y.L.; Kang, L.J. A comparative study on sorption and diffusion of Cs in crushed argillite and granite investigated in batch and through-diffusion experiment. J. Radioanal. Nucl. Chem. 2017, 311, 1155–1162. [Google Scholar] [CrossRef]
- Shi, Y.; Lee, C.P.; Yu, H.; Hu, Y.; Liu, H.; Tien, N.C.; Wang, Y.; Liu, W.; Kong, J. Study on Advection-Dispersion Behavior for Simulation of HTO and Se Transport in crushed granite. J. Radioanal. Nucl. Chem. 2021, 328, 1329–1338. [Google Scholar] [CrossRef]
- Fan, Q.H.; Tan, X.L.; Li, J.X.; Wang, X.K.; Wu, W.S.; Montavon, G. Sorption of Eu(III) on attapulgite studied by batch XPS, and EXAFS techniques. Environ. Sci. Technol. 2009, 43, 5776–5782. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kar, A.S.; Tomar, B.S.; Bhattacharyya, D. X-ray absorption fine structure spectroscopy study of Eu (III) sorption products onto amorphous silica and γ-alumina: Effect of pH and substrate. Polyhedron 2012, 33, 33–40. [Google Scholar] [CrossRef]
- Sheng, G.; Yang, Q.; Peng, F.; Li, H.; Gao, X.; Huang, Y. Determination of colloidal pyrolusite, Eu (III) and humic substance interaction: A combined batch and EXAFS approach. Chem. Eng. J. 2014, 245, 10–16. [Google Scholar] [CrossRef]
- Tan, X.; Fan, Q.; Wang, X.; Grambow, B. Eu (III) sorption to TiO2 (anatase and rutile): Batch, XPS, and EXAFS studies. Environ. Sci. Technol. 2009, 43, 3115–3121. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Tan, X.; Wang, X. Sorption of radionuclides from aqueous systems onto graphene oxide-based materials: A review. Inorg. Chem. Front. 2015, 2, 593–612. [Google Scholar] [CrossRef]
- Fan, Q.; Yamaguchi, N.; Tanaka, M.; Tsukada, H.; Takahashi, Y. Relationship between the adsorption species of cesium and radiocesium interception potential in soils and minerals: An EXAFS study. J. Environ. Radioact. 2014, 138, 92–100. [Google Scholar] [CrossRef]
- Fan, Q.H.; Tanaka, M.; Tanaka, K.; Sakaguchi, A.; Takahashi, Y. An EXAFS study on the effects of natural organic matter and the expandability of clay minerals on cesium adsorption and mobility. Geochim. Cosmochim. Acta 2014, 135, 49–65. [Google Scholar] [CrossRef]
- Liu, W.T.; Tsai, S.C.; Tsai, T.L.; Lee, C.P.; Lee, C.H. Characteristic study for the uranium and cesium sorption on bentonite by using XPS and XANES. J. Radioanal. Nucl. Chem. 2017, 314, 2237–2241. [Google Scholar] [CrossRef]
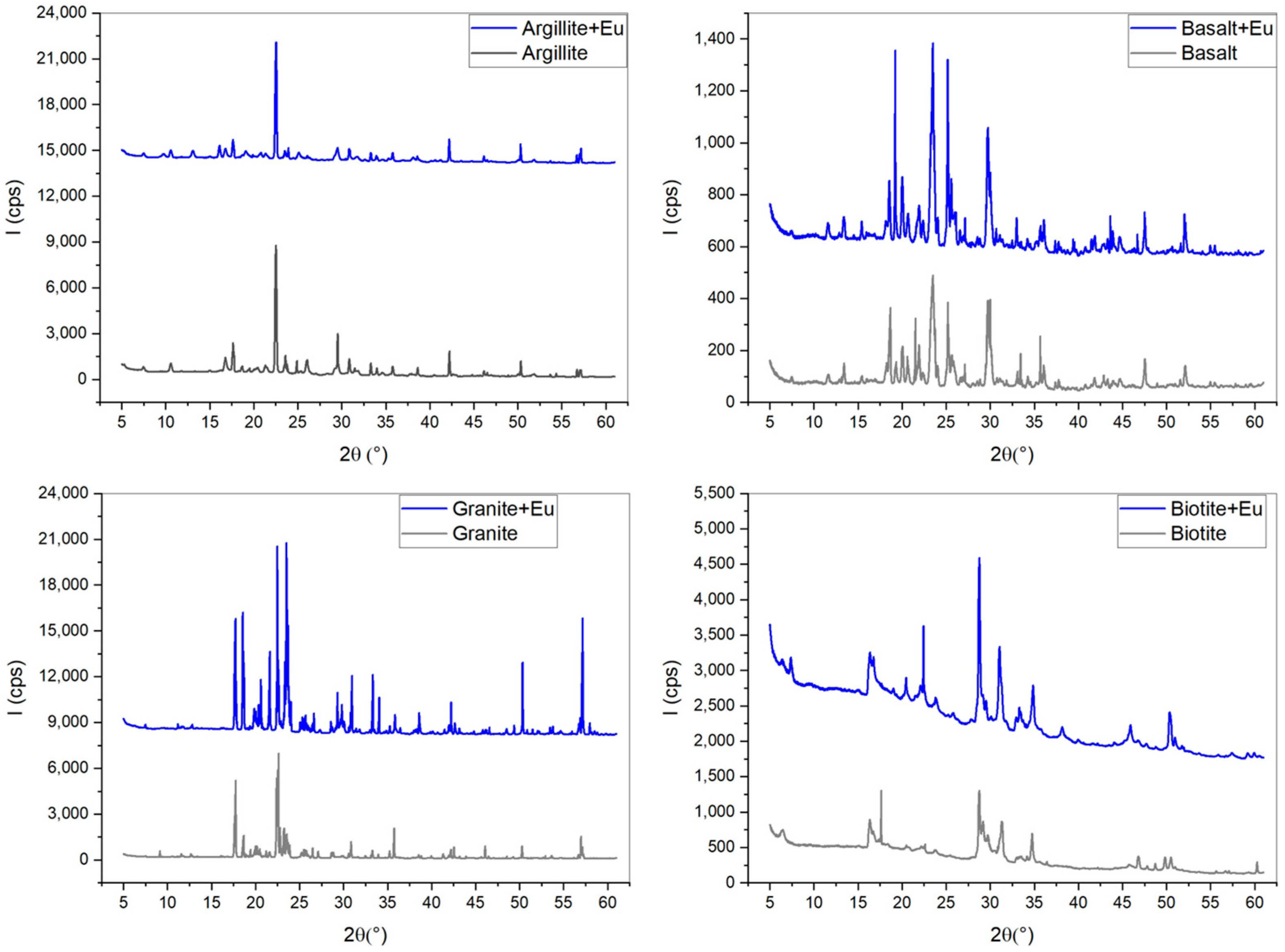



| Element | SiO2 | Al2O2 | Fe2O3 | CaO | Na2O | K2O | MnO | MgO | TiO2 | P2O2 | * L.O.I | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rocks | ||||||||||||
| Argillite | 65.99 | 15.97 | 6.74 | 0.99 | 1.26 | 2.37 | 0.09 | 2.03 | 0.87 | 0.17 | 5.39 | |
| Basalt | 47.90 | 15.34 | 13.86 | 7.56 | 3.90 | 1.62 | 0.16 | 6.25 | 2.71 | 0.83 | 1.62 | |
| Granite | 77.08 | 12.44 | 1.09 | 1.16 | 3.53 | 3.06 | 0.03 | 0.16 | 0.06 | 0.01 | 0.19 | |
| Item | Rock Samples | |||
|---|---|---|---|---|
| Basalt | Argillite | Granite | Biotite | |
| pH | 7.54 ± 0.02 | 8.01 ± 0.08 | 5.86 ± 0.03 | 8.73 ± 0.07 |
| Eh (mV) | 214 ± 11 | 238 ± 4 | 299 ± 13 | 282 ± 39 |
| Q (m mol/g) | 2.76 ± 0.01 | 4.10 ± 0.03 | 0.48 ± 0.10 | 0.89 ± 0.02 |
| Kd (mL/g) | 2472 ± 38 | 4413 ± 69 | 6.16 ± 0.19 | 55.20 ± 8.19 |
| Sample | Item | R(Å) | CN | ∆E0 | Rf (%) |
|---|---|---|---|---|---|
| Eu2O3_solid | Eu-O | 2.36 | 4.00 | 0.79 | 2.14 |
| Eu-Eu Eu-O | 3.69 4.27 | 3.41 2.00 | |||
| EuNO3_solid | Eu-O | 2.45 | 5.00 | 3.33 | 1.50 |
| Eu-Eu | 4.18 | 5.00 | |||
| Eu_liquid_0.5M | Eu-O | 2.39 | 4.03 | 3.13 | 21.88 |
| First Shell (Eu–O1st) | Second Shell (Eu–O2nd/Fe) | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | R(Å) | CN | Shell | R(Å) | CN | ∆E0 | Rf(%) | CN2nd/CN1st |
| Argillite | 2.42 | 2.54 | Eu-O Eu-Fe | 2.27 3.66 | 6.68 3.00 | −3.97 | 1.31 | 2.63 |
| Basalt | 2.50 | 1.95 | Eu-O Eu-Fe | 2.31 3.17 | 7.03 5.47 | −2.99 | 2.91 | 3.61 |
| Granite | 2.52 | 2.06 | Eu-O Eu-Fe | 2.32 3.24 | 4.00 0.82 | −0.22 | 16.73 | 1.94 |
| Biotite | 2.48 | 2.91 | Eu-O Eu-Fe | 2.28 3.16 | 8.90 3.34 | −4.05 | 5.23 | 3.06 |
| First Shell (Eu–O1st) | Second Shell (Eu–O2nd/Si) | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | R(Å) | CN | Shell | R(Å) | CN | ∆E0 | Rf (%) | CN2nd/CN1st |
| Argillite | 2.42 | 7.00 | Eu-O Eu-Si | 2.20 3.85 | 2.23 6.88 | −1.66 | 6.71 | 0.32 |
| Basalt | 2.43 | 7.30 | Eu-O Eu-Si | 2.21 3.84 | 2.15 4.22 | −0.65 | 4.56 | 0.29 |
| Granite | 2.58 | 2.29 | Eu-O Eu-Si | 2.41 2.96 | 5.86 1.81 | 3.09 | 26.43 | 2.56 |
| Biotite | 2.48 | 3.50 | Eu-O Eu-Si | 2.31 3.41 | 7.00 1.31 | −1.72 | 10.86 | 2.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-W.; Chiou, Z.-S.; Lee, C.-P.; Tsai, S.-C.; Tseng, W.-H.; Wang, Y.-H.; Chen, Y.-T.; Kuo, C.-H.; Chiu, H.-M. Enhancing Europium Adsorption Effect of Fe on Several Geological Materials by Applying XANES, EXAFS, and Wavelet Transform Techniques. Toxics 2024, 12, 706. https://doi.org/10.3390/toxics12100706
Hsieh C-W, Chiou Z-S, Lee C-P, Tsai S-C, Tseng W-H, Wang Y-H, Chen Y-T, Kuo C-H, Chiu H-M. Enhancing Europium Adsorption Effect of Fe on Several Geological Materials by Applying XANES, EXAFS, and Wavelet Transform Techniques. Toxics. 2024; 12(10):706. https://doi.org/10.3390/toxics12100706
Chicago/Turabian StyleHsieh, Chi-Wen, Zih-Shiuan Chiou, Chuan-Pin Lee, Shih-Chin Tsai, Wei-Hsiang Tseng, Yu-Hung Wang, Yi-Ting Chen, Chein-Hsieng Kuo, and Hui-Min Chiu. 2024. "Enhancing Europium Adsorption Effect of Fe on Several Geological Materials by Applying XANES, EXAFS, and Wavelet Transform Techniques" Toxics 12, no. 10: 706. https://doi.org/10.3390/toxics12100706
APA StyleHsieh, C.-W., Chiou, Z.-S., Lee, C.-P., Tsai, S.-C., Tseng, W.-H., Wang, Y.-H., Chen, Y.-T., Kuo, C.-H., & Chiu, H.-M. (2024). Enhancing Europium Adsorption Effect of Fe on Several Geological Materials by Applying XANES, EXAFS, and Wavelet Transform Techniques. Toxics, 12(10), 706. https://doi.org/10.3390/toxics12100706






