Mitochondrial Dysfunction Plays a Relevant Role in Heart Toxicity Caused by MeHg
Abstract
1. Introduction
2. Materials and Methods
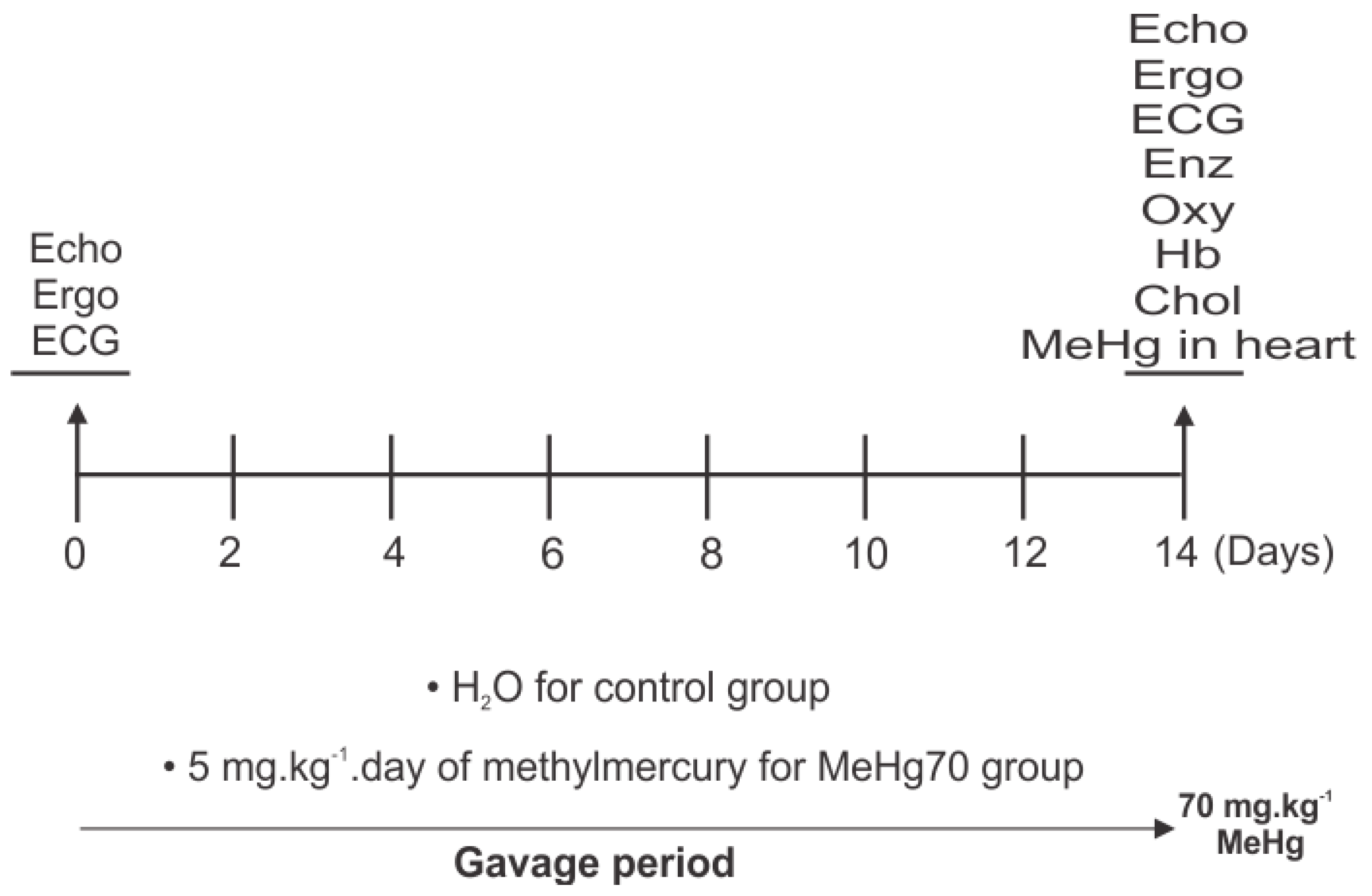
2.1. Study Design
2.2. Gas Chromatography–Atomic Fluorescence Spectrometry
2.3. RNA Isolation and Real-Time Quantitative PCR (RT-qPCR)
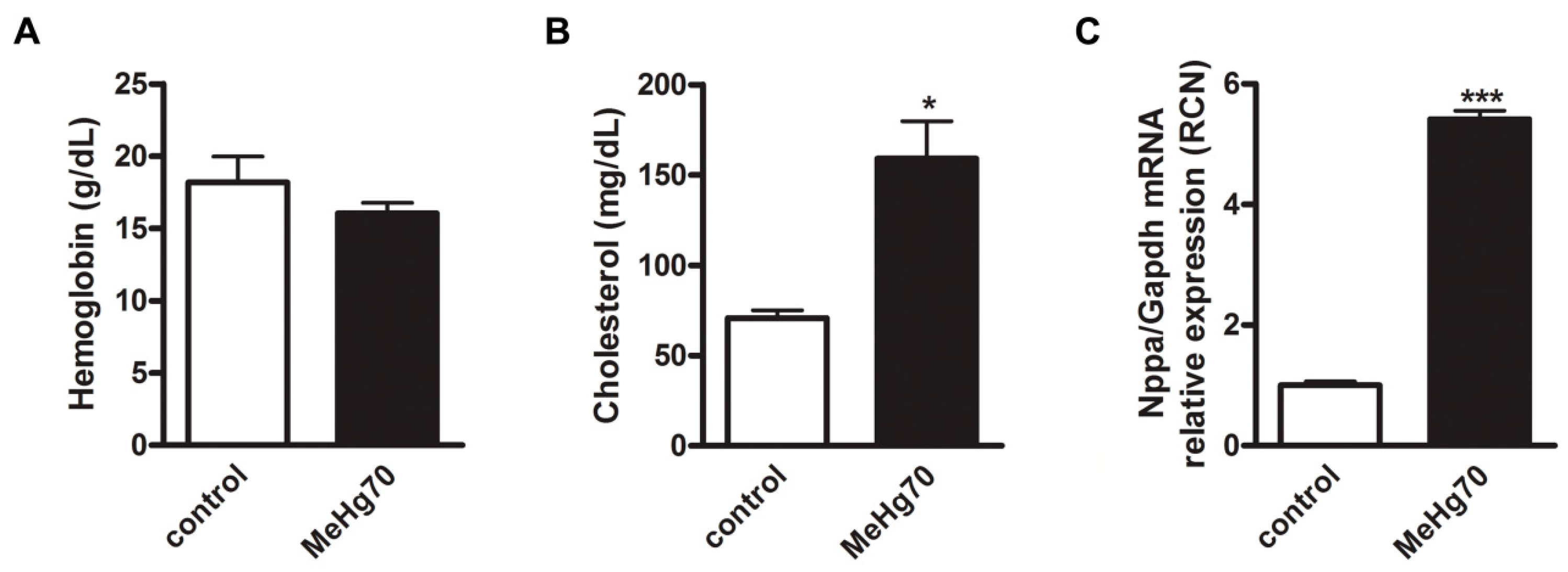
2.4. Haemoglobin and Cholesterol Measurements
2.5. Ergospirometry
2.6. Electrocardiography
2.7. Echocardiography
2.8. Mitochondrial Analysis
2.8.1. Citrate Synthase (CS) Activity Assay
2.8.2. Succinate Dehydrogenase (SDH) Activity Colorimetric Assay
2.8.3. ATPase Activity of Complex V
2.8.4. Mitochondrial Oxygen Consumption
2.8.5. Western Blot Analysis of the Heart Tissue
2.9. Statistics
3. Results
3.1. MeHg Compromised the Viability of the Mice
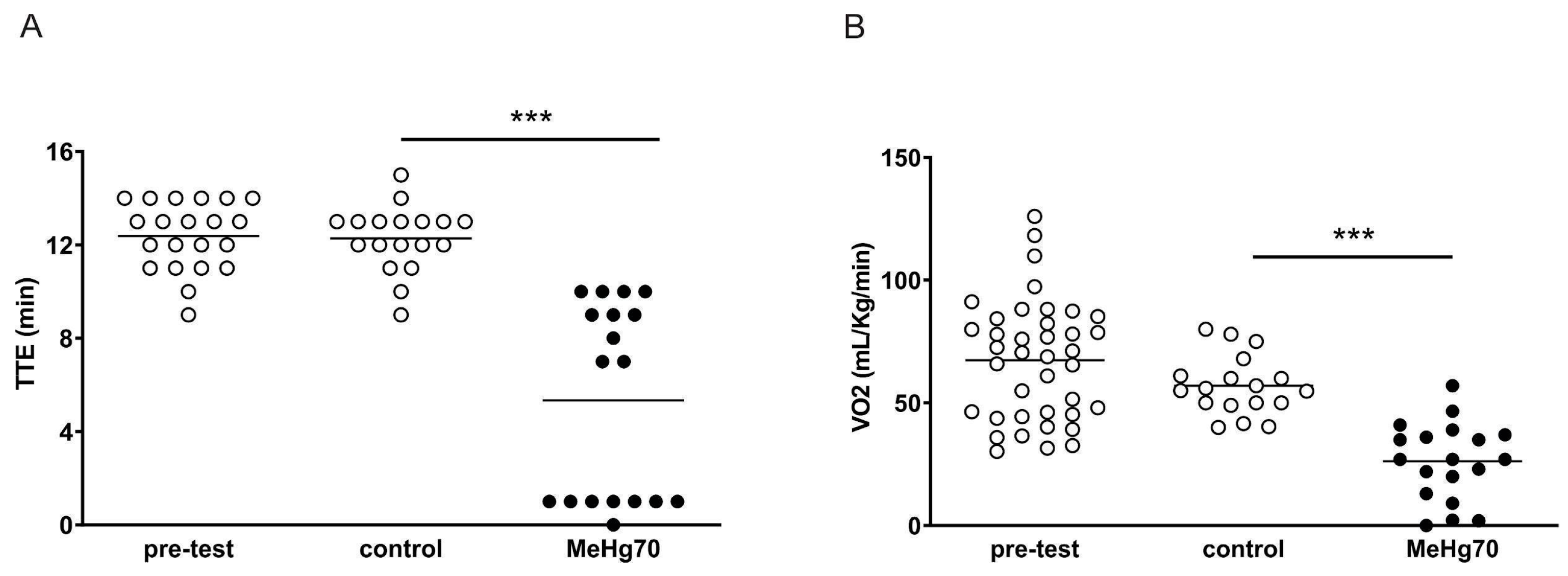
3.2. MeHg Administration Worsens Ergospirometry Performance
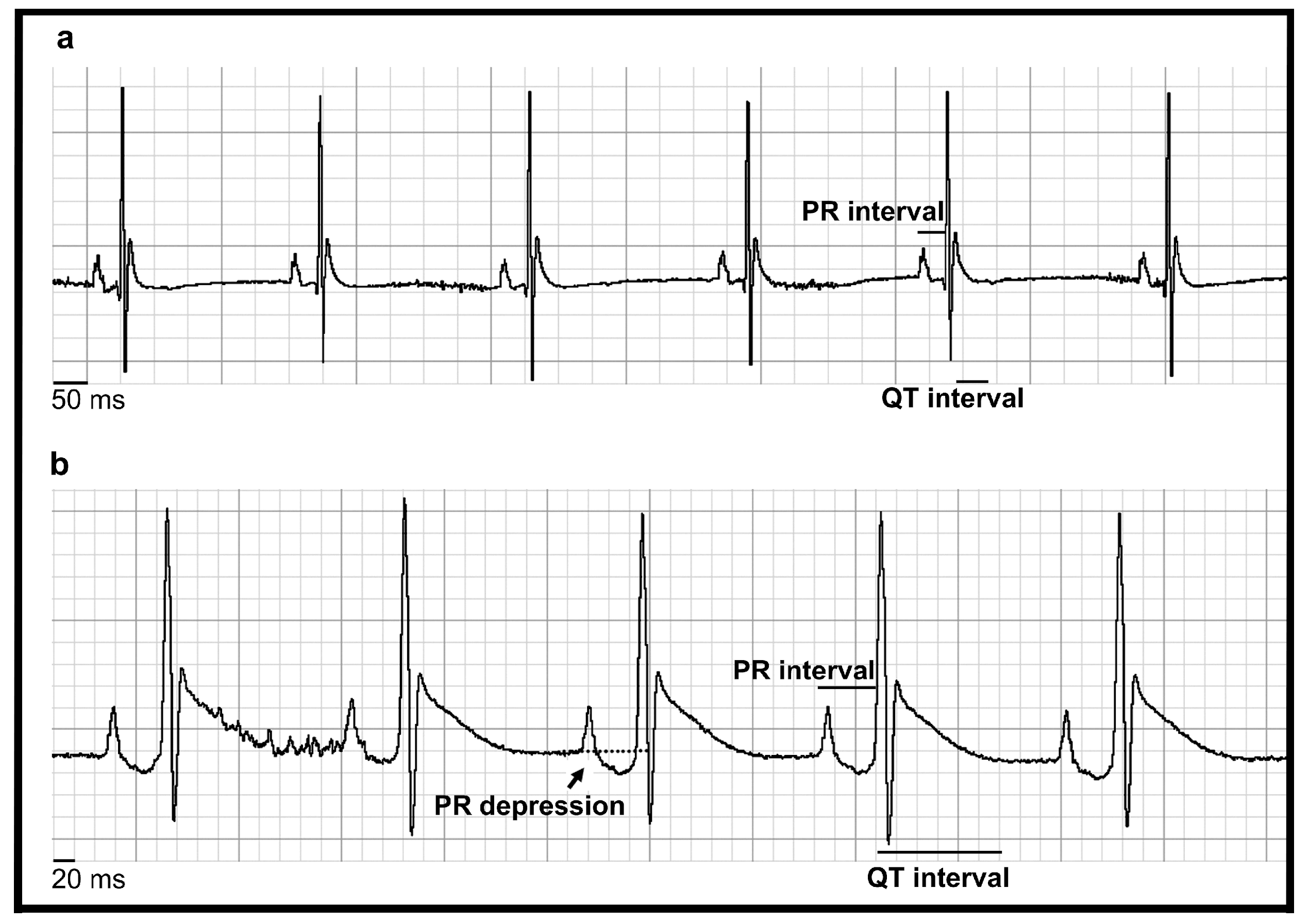
3.3. MeHg Altered Heart Automaticity, Atrioventricular Conduction and Ventricular Repolarisation
3.4. MeHg Poisoning Induced Left Ventricle Stress
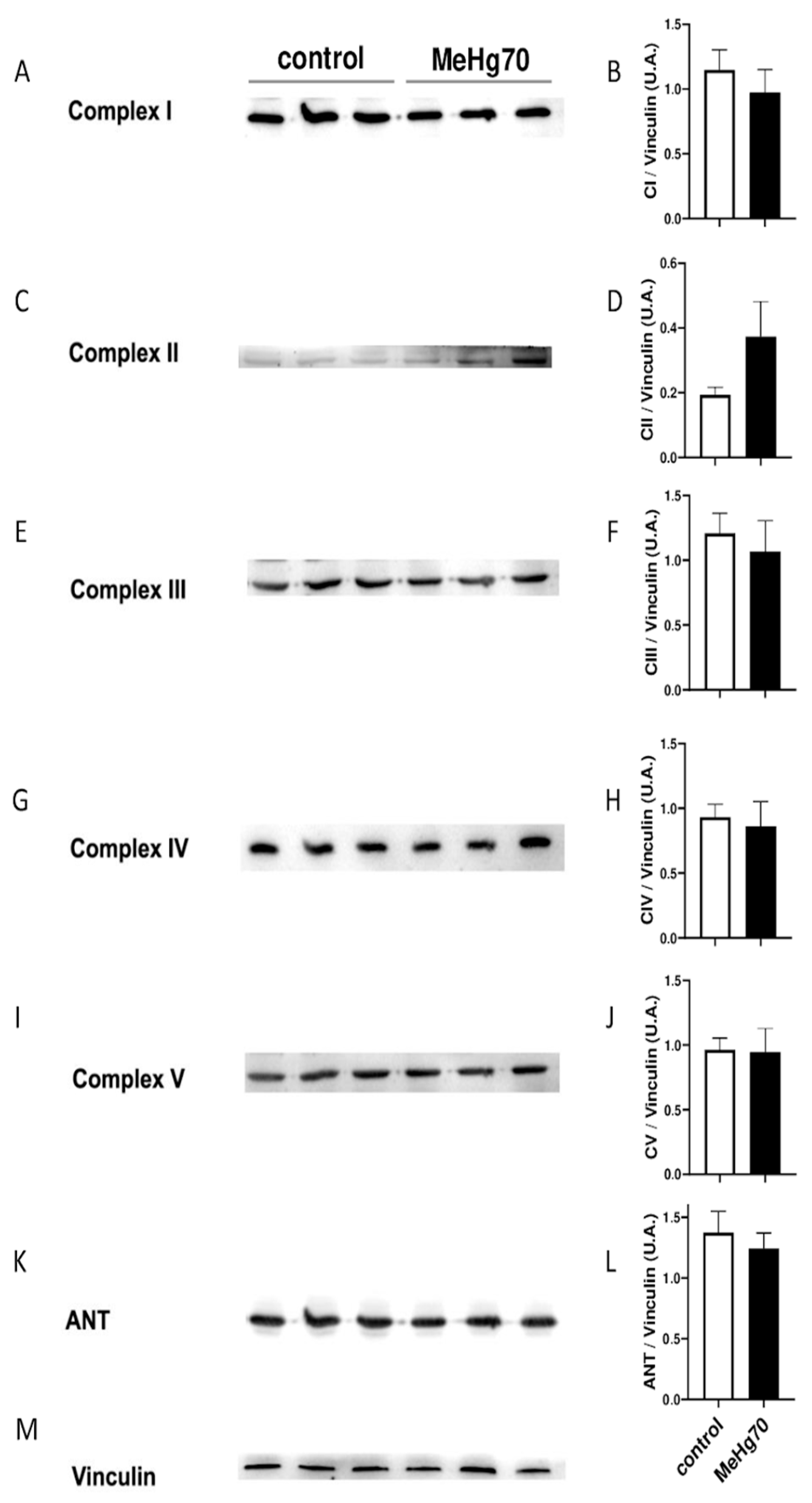
3.5. MeHg Reduced Mitochondrial Respiration without Affecting the Mitochondrial Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Top 10 Causes of Death. Available online: http://www.who.int/mediacentre/factsheets/fs310/en/ (accessed on 22 July 2024).
- Sheehan, M.C.; Burke, T.A.; Navas-Acien, A.; Breysse, P.N.; McGready, J.; Fox, M.A. Global Methylmercury Exposure from Seafood Consumption and Risk of Developmental Neurotoxicity: A Systematic Review. Bull. World Health Organ. 2014, 92, 254–269F. [Google Scholar] [CrossRef] [PubMed]
- 10 Chemicals of Public Health Concern. Available online: https://www.who.int/news-room/photo-story/photo-story-detail/10-chemicals-of-public-health-concern (accessed on 22 July 2024).
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a Global Pollutant: Sources, Pathways, and Effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.M.; Nislow, K.H.; Folt, C.L. Bioaccumulation Syndrome: Identifying Factors That Make Some Stream Food Webs Prone to Elevated Mercury Bioaccumulation: Bioaccumulation Syndrome. Ann. N. Y. Acad. Sci. 2010, 1195, 62–83. [Google Scholar] [CrossRef] [PubMed]
- Bastos, W.R.; Gomes, J.P.O.; Oliveira, R.C.; Almeida, R.; Nascimento, E.L.; Bernardi, J.V.E.; de Lacerda, L.D.; da Silveira, E.G.; Pfeiffer, W.C. Mercury in the Environment and Riverside Population in the Madeira River Basin, Amazon, Brazil. Sci. Total Environ. 2006, 368, 344–351. [Google Scholar] [CrossRef]
- Bastos, W.R.; Dórea, J.G.; Bernardi, J.V.E.; Lauthartte, L.C.; Mussy, M.H.; Hauser, M.; Dória, C.R.d.C.; Malm, O. Mercury in Muscle and Brain of Catfish from the Madeira River, Amazon, Brazil. Ecotoxicol. Environ. Saf. 2015, 118, 90–97. [Google Scholar] [CrossRef]
- Canela, T.A.; Monteiro, L.C.; Cabral, C.d.S.; Ximenes, F.d.S.; Oliveira, I.A.d.S.; Bernardi, J.V.E.; Almeida, R.d.; Bastos, W.R. Mercury in Fish and Human Hair and Estimated Dietary Intake in a Riverside Community of the Madeira River Basin in the Brazilian Amazon. Toxics 2024, 12, 208. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Costa, B.G.B.C.; Lopes, D.N.; Oliveira, K.; Bezerra, M.F.; Bastos, W.R. Mercury in Indigenous, Introduced and Farmed Fish from the Semiarid Region of the Jaguaribe River Basin, NE Brazil. Bull. Environ. Contam. Toxicol. 2014, 93, 31–35. [Google Scholar] [CrossRef]
- Rodríguez Martín-Doimeadios, R.C.; Berzas Nevado, J.J.; Guzmán Bernardo, F.J.; Jiménez Moreno, M.; Arrifano, G.P.F.; Herculano, A.M.; do Nascimento, J.L.M.; Crespo-López, M.E. Comparative Study of Mercury Speciation in Commercial Fishes of the Brazilian Amazon. Environ. Sci. Pollut. Res. Int. 2014, 21, 7466–7479. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Available online: https://www.atsdr.cdc.gov (accessed on 22 July 2024).
- Basu, N.; Bastiansz, A.; Dórea, J.G.; Fujimura, M.; Horvat, M.; Shroff, E.; Weihe, P.; Zastenskaya, I. Our Evolved Understanding of the Human Health Risks of Mercury. Ambio 2023, 52, 877–896. [Google Scholar] [CrossRef]
- Bakir, F.; Rustam, H.; Tikriti, S.; Al-Damluji, S.F.; Shihristani, H. Clinical and Epidemiological Aspects of Methylmercury Poisoning. Postgrad. Med. J. 1980, 56, 1–10. [Google Scholar] [CrossRef]
- Wells, E.M.; Herbstman, J.B.; Lin, Y.H.; Hibbeln, J.R.; Halden, R.U.; Witter, F.R.; Goldman, L.R. Methyl mercury, but not inorganic mercury, associated with higher blood pressure during pregnancy. Environ. Res. 2017, 154, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Lee, S.; Basu, N.; Franzblau, A. Associations of Blood and Urinary Mercury with Hypertension in U.S. Adults: The NHANES 2003-2006. Environ. Res. 2013, 123, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.K.; Voutilainen, S.; Rissanen, T.H.; Mursu, J.; Tuomainen, T.-P.; Korhonen, M.J.; Valkonen, V.-P.; Seppänen, K.; Laukkanen, J.A.; Salonen, J.T. Mercury, Fish Oils, and Risk of Acute Coronary Events and Cardiovascular Disease, Coronary Heart Disease, and All-Cause Mortality in Men in Eastern Finland. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-S.; Kim, Y.-M.; Lee, K.-E. Methylmercury Exposure and Health Effects. J. Prev. Med. Public Health 2012, 45, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Tajik, B.; Tuomainen, T.-P.; Kurl, S.; Salonen, J.; Virtanen, J.K. Serum Long-Chain Omega-3 Fatty Acids, Hair Mercury and Exercise-Induced Myocardial Ischaemia in Men. Heart 2019, 105, 1395–1401. [Google Scholar] [CrossRef]
- Dahhan, S.S.; Orfaly, H. Electrocardiographic Changes in Mercury Poisoning. Am. J. Cardiol. 1964, 14, 178–183. [Google Scholar] [CrossRef]
- Jalili, M.A.; Abbasi, A.H. Poisoning by Ethyl Mercury Toluene Sulphonanilide. Br. J. Ind. Med. 1961, 18, 303–308. [Google Scholar] [CrossRef][Green Version]
- Grandjean, P.; Murata, K.; Budtz-Jørgensen, E.; Weihe, P. Cardiac Autonomic Activity in Methylmercury Neurotoxicity: 14-Year Follow-up of a Faroese Birth Cohort. J. Pediatr. 2004, 144, 169–176. [Google Scholar] [CrossRef]
- Karita, K.; Iwata, T.; Maeda, E.; Sakamoto, M.; Murata, K. Assessment of Cardiac Autonomic Function in Relation to Methylmercury Neurotoxicity. Toxics 2018, 6, 38. [Google Scholar] [CrossRef]
- Newsholme, E.; Leech, A. Mary Board Functional Biochemistry in Health and Disease, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2011; ISBN 9780471931652. [Google Scholar]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 8th ed.; W. H. Freeman: New York, NY, USA, 2021; ISBN 9781319228002. [Google Scholar]
- Ren, H.; Hu, W.; Jiang, T.; Yao, Q.; Qi, Y.; Huang, K. Mechanical Stress Induced Mitochondrial Dysfunction in Cardiovascular Diseases: Novel Mechanisms and Therapeutic Targets. Biomed. Pharmacother. 2024, 174, 116545. [Google Scholar] [CrossRef]
- Watson, W.D.; Arvidsson, P.M.; Miller, J.J.J.; Lewis, A.J.; Rider, O.J. Mitochondrial dysfunction is linked aetiologically to many heart diseases that have a common final progression to heart failure. Cardiovasc. Drugs Ther. 2024. [Google Scholar] [CrossRef]
- Roos, D.; Seeger, R.; Puntel, R.; Vargas Barbosa, N. Role of Calcium and Mitochondria in MeHg-Mediated Cytotoxicity. J. Biomed. Biotechnol. 2012, 2012, 248764. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.L.; Posser, T.; Dunkley, P.R.; Dickson, P.W.; Mattos, J.J.; Martins, R.; Bainy, A.C.D.; Marques, M.R.; Dafre, A.L.; Farina, M. Methylmercury Neurotoxicity Is Associated with Inhibition of the Antioxidant Enzyme Glutathione Peroxidase. Free Radic. Biol. Med. 2009, 47, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Shinkai, Y.; Kumagai, Y. S-Mercuration of Cellular Proteins by Methylmercury and Its Toxicological Implications. J. Toxicol. Sci. 2014, 39, 687–700. [Google Scholar] [CrossRef]
- Cambier, S.; Bénard, G.; Mesmer-Dudons, N.; Gonzalez, P.; Rossignol, R.; Brèthes, D.; Bourdineaud, J.-P. At Environmental Doses, Dietary Methylmercury Inhibits Mitochondrial Energy Metabolism in Skeletal Muscles of the Zebra Fish (Danio Rerio). Int. J. Biochem. Cell Biol. 2009, 41, 791–799. [Google Scholar] [CrossRef]
- Truong, J.; Mailloux, R.J.; Chan, H.M. Impact of Methylmercury Exposure on Mitochondrial Energetics in AC16 and H9C2 Cardiomyocytes. Toxicol. In Vitr. 2015, 29, 953–961. [Google Scholar] [CrossRef]
- Liang, L.; Bloom, N.S.; Horvat, M. Simultaneous Determination of Mercury Speciation in Biological Materials by GC/CVAFS after Ethylation and Room-Temperature Precollection. Clin. Chem. 1994, 40, 602–607. [Google Scholar] [CrossRef]
- Martinez, C.G.; Zamith-Miranda, D.; da Silva, M.G.; Ribeiro, K.C.; Brandão, I.T.; Silva, C.L.; Diaz, B.L.; Bellio, M.; Persechini, P.M.; Kurtenbach, E. P2×7 Purinergic Signaling in Dilated Cardiomyopathy Induced by Auto-Immunity against Muscarinic M2 Receptors: Autoantibody Levels, Heart Functionality and Cytokine Expression. Sci. Rep. 2015, 5, 16940. [Google Scholar] [CrossRef]
- Silva, M.G.; Mattos, E.; Camacho-Pereira, J.; Domitrovic, T.; Galina, A.; Costa, M.W.; Kurtenbach, E. Cardiac Systolic Dysfunction in Doxorubicin-Challenged Rats Is Associated with Upregulation of MuRF2 and MuRF3 E3 Ligases. Exp. Clin. Cardiol. 2012, 17, 101–109. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Pesta, D. Mitochondrial Density in Skeletal and Cardiac Muscle. Mitochondrion 2024, 75, 101838. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, F.M.; Laurindo, F.R.M.; Kowaltowski, A.J. Mild Mitochondrial Uncoupling and Calorie Restriction Increase Fasting eNOS, Akt and Mitochondrial Biogenesis. PLoS ONE 2011, 6, e18433. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Schwall, C.T.; Greenwood, V.L.; Alder, N.N. The Stability and Activity of Respiratory Complex II Is Cardiolipin-Dependent. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 1588–1596. [Google Scholar] [CrossRef]
- Pesta, D.; Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. In Mitochondrial Bioenergetics; Humana Press: Totowa, NJ, USA, 2012; pp. 25–58. ISBN 9781617793813. [Google Scholar]
- Anderson, E.J.; Rodriguez, E.; Anderson, C.A.; Thayne, K.; Chitwood, W.R.; Kypson, A.P. Increased Propensity for Cell Death in Diabetic Human Heart Is Mediated by Mitochondrial-Dependent Pathways. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H118–H124. [Google Scholar] [CrossRef]
- Ruybal, M.; Gallego, M.; Sottani, T.; Medei, E.; Casis, O.; Nascimento, J. Methylmercury Poisoning Induces Cardiac Electrical Remodeling and Increases Arrhythmia Susceptibility and Mortality. Int. J. Mol. Sci. 2020, 21, 3490. [Google Scholar] [CrossRef]
- Magos, L.; Butler, W. The Kinetics of Methylmercury Administered Repeatedly to Rats. Arch. Toxicol. 1976, 35, 25–39. [Google Scholar] [CrossRef]
- Gouvêa, A.L.; Gracindo Silva, M.; Cabral, B.; Martinez, C.G.; Lauthartte, L.C.; Rodrigues Bastos, W.; Kurtenbach, E. Progressive Resistance Exercise Prevents Muscle Strength Loss Due to Muscle Atrophy Induced by Methylmercury Systemic Intoxication. JCSM Clin. Rep. 2021, 6, 80–92. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Serpeloni, J.M.; Batista, B.L.; Souza, S.S.; Barbosa, F., Jr. Identification and distribution of mercury species in rat tissues following administration of thimerosal or methylmercury. Arch. Toxicol. 2010, 84, 891–896. [Google Scholar] [CrossRef]
- Clarkson, T.W.; Vyas, J.B.; Ballatori, N. Mechanisms of mercury disposition in the body. Am. J. Ind. Med. 2007, 50, 757–764. [Google Scholar] [CrossRef]
- Piscopo, M.; Notariale, R.; Tortora, F.; Lettieri, G.; Palumbo, G.; Manna, C. Novel Insights into Mercury Effects on Hemoglobin and Membrane Proteins in Human Erythrocytes. Molecules 2020, 25, 3278. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.L.; de Oliveira, J.; Dutra, M.F.; Santos, D.B.; Goncalves, C.A.; Goldfeder, E.M.; de Bem, A.F.; Prediger, R.D.; Aschner, M.; Farina, M. Does Methylmercury-Induced Hypercholesterolemia Play a Causal Role in Its Neurotoxicity and Cardiovascular Disease? Toxicol. Sci. 2012, 130, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Cho, S.-R.; Jeong, I.; Park, J.B.; Shin, M.-Y.; Kim, S.; Kim, J.H. Mercury Exposure and Associations with Hyperlipidemia and Elevated Liver Enzymes: A Nationwide Cross-Sectional Survey. Toxics 2020, 8, 47. [Google Scholar] [CrossRef]
- Kang, P.; Shin, H.Y.; Kim, K.Y. Association between Dyslipidemia and Mercury Exposure in Adults. Int. J. Environ. Res. Public Health 2021, 18, 775. [Google Scholar] [CrossRef] [PubMed]
- Ballard-Hernandez, J.; Sall, J. Dyslipidemia Update. Nurs. Clin. N. Am. 2023, 58, 295–308. [Google Scholar] [CrossRef]
- Gribble, M.O.; Cheng, A.; Berger, R.D.; Rosman, L.; Guallar, E. Mercury Exposure and Heart Rate Variability: A Systematic Review. Curr. Environ. Health Rep. 2015, 2, 304–314. [Google Scholar] [CrossRef]
- Gadioli, L.P.; Schmidt, A.; Maciel, B.C.; Volpe, G.J.; Simões, M.V.; Marin-Neto, J.A. Chagas Cardiomyopathy and Myocardial Sympathetic Denervation. Curr. Cardiol. Rep. 2024, 26, 635–641. [Google Scholar] [CrossRef]
- Szurhaj, W.; Leclancher, A.; Nica, A.; Périn, B.; Derambure, P.; Convers, P.; Mazzola, L.; Godet, B.; Faucanie, M.; Picot, M.-C.; et al. Cardiac Autonomic Dysfunction and Risk of Sudden Unexpected Death in Epilepsy. Neurology 2021, 96, e2619–e2626. [Google Scholar] [CrossRef]
- Vandael, E.; Vandenberk, B.; Vandenberghe, J.; Willems, R.; Foulon, V. Risk Factors for QTc-Prolongation: Systematic Review of the Evidence. Int. J. Clin. Pharm. 2017, 39, 16–25. [Google Scholar] [CrossRef]
- Porela, P.; Kytö, V.; Nikus, K.; Eskola, M.; Airaksinen, K.E.J. PR Depression Is Useful in the Differential Diagnosis of Myopericarditis and ST Elevation Myocardial Infarction: ECG in Myopericarditis. Ann. Noninvasive Electrocardiol. 2012, 17, 141–145. [Google Scholar] [CrossRef]
- Buttà, C.; Zappia, L.; Laterra, G.; Roberto, M. Diagnostic and prognostic role of electrocardiogram in acute myocarditis: A comprehensive review. Ann. Noninvasive Electrocardiol. 2020, 25, e12785. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Raja, R.; Ruiz-Velasco, A.; Liu, W. Cellular Protein Quality Control in Diabetic Cardiomyopathy: From Bench to Bedside. Front. Cardiovasc. Med. 2020, 7, 585309. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X. The Interplay between Autophagy and the Ubiquitin-Proteasome System in Cardiac Proteotoxicity. Biochim. Biophys. Acta 2015, 1852, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac Metabolism in Heart Failure: Implications beyond ATP Production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef]
- Pagel, P.S.; Tawil, J.N.; Boettcher, B.T.; Izquierdo, D.A.; Lazicki, T.J.; Crystal, G.J.; Freed, J.K. Heart Failure with Preserved Ejection Fraction: A Comprehensive Review and Update of Diagnosis, Pathophysiology, Treatment, and Perioperative Implications. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1839–1859. [Google Scholar] [CrossRef]
- Bernardi, P.; Gerle, C.; Halestrap, A.P.; Jonas, E.A.; Karch, J.; Mnatsakanyan, N.; Pavlov, E.; Sheu, S.-S.; Soukas, A.A. Identity, Structure, and Function of the Mitochondrial Permeability Transition Pore: Controversies, Consensus, Recent Advances, and Future Directions. Cell Death Differ. 2023, 30, 1869–1885. [Google Scholar] [CrossRef]
- Zhao, H.; Shi, M.; Yang, F.; Yang, X. Kearns-Sayre Syndrome with Rare Imaging Finding of SLC25A4 Mutation. Neurosciences 2022, 27, 111–115. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Woodfield, K.-Y.; Connern, C.P. Oxidative Stress, Thiol Reagents, and Membrane Potential Modulate the Mitochondrial Permeability Transition by Affecting Nucleotide Binding to the Adenine Nucleotide Translocase. J. Biol. Chem. 1997, 272, 3346–3354. [Google Scholar] [CrossRef]
- Polunas, M.; Halladay, A.; Tjalkens, R.B.; Philbert, M.A.; Lowndes, H.; Reuhl, K. Role of Oxidative Stress and the Mitochondrial Permeability Transition in Methylmercury Cytotoxicity. Neurotoxicology 2011, 32, 526–534. [Google Scholar] [CrossRef]
- Nesci, S.; Trombetti, F.; Pirini, M.; Ventrella, V.; Pagliarani, A. Mercury and Protein Thiols: Stimulation of Mitochondrial F1FO-ATPase and Inhibition of Respiration. Chem. Biol. Interact. 2016, 260, 42–49. [Google Scholar] [CrossRef]



| Pretest | Control | MeHg70 | |
|---|---|---|---|
| Body weight (g) (N = 25) | 18.31 ± 0.28 | 20.41 ± 0.34 | 17.48 ± 0.65 * |
| Survival rate (%) (N = 25) | 100 | 95.23 | 46.40 |
| [MeHg] in heart tissue samples (N = 5) | unchecked | 0.01 | 23.85 ± 2.62 *** |
| Pretest (Awake) N = 33 | Anaesthetised | Awake | |||
|---|---|---|---|---|---|
| Control N = 17 | MeHg70 N = 11 | Control N = 11 | MeHg70 N = 20 | ||
| Heart rate (BPM) | 614 ± 13 | 208 ± 13 | 237 ± 8 | 634 ± 20 | 522 ± 24 ** |
| P wave duration (ms) | 11.09 ± 0.68 | 11.68 ± 1.05 | 10.75 ± 1.42 | 10.43± 0.67 | 10.37 ± 0.66 |
| PR interval (ms) | 27.80 ± 0.45 | 34.76 ± 0.49 | 44.27 ± 0.48 ** | 28.73 ± 0.73 | 28.33 ± 1.09 |
| QRS complex duration (ms) | 9.02 ± 0.28 | 9.92 ± 0.33 | 10.11 ± 0.34 | 9.19 ± 0.33 | 10.27± 0.25 * |
| QTc (ms) | 71.02 ± 1.55 | 50.51 ± 0.41 | 79.36 ± 0.75 ** | 68.96 ± 3.41 | 88.91 ± 3.32 *** |
| Pretest | Control | MeHg70 | |
|---|---|---|---|
| Heart rate (BPM) | 133 ± 13 | 130 ± 10 | 135 ± 18 |
| LVAWDT (mm) | 0.50 ± 0.0 | 0.48 ± 0.0 | 0.40 ± 0.0 ** |
| LVDD (mm) | 3.61 ± 0.01 | 3.63 ± 0.00 | 3.42 ± 0.01 |
| LVSD (mm) | 1.31 ± 0.0 | 1.37 ± 0.0 | 1.70 ± 0.0 * |
| SV (mL) | 0.05 ± 0.0 | 0.05 ± 0.0 | 0.04 ± 0.0 |
| Ao/LA | 0.92 ± 0.01 | 0.97 ± 0.01 | 0.85 ± 0.03 ** |
| EF (%) | 94.50 ± 0.8 | 94.17 ± 0.7 | 86.14 ± 1.90 *** |
| CO (mL/min) | 7.37 ± 0.75 | 6.39 ± 1.00 | 5.05 ± 1.04 |
| IVRT (ms) | unchecked | 36.83 ± 2.99 | 36.00± 6.10 |
| E/A | 4.42 ± 0.49 | 3.87 ± 0.48 | 4.84 ± 1.38 |
| Control Group | MeHg70 | |
|---|---|---|
| Citrate synthase (CS, U.mg−1.min−1), N= 4 | 1042 ± 332.1 | 1106 ± 97.9 |
| Succinate dehydrogenase (SD, [pmol.mg−1.s−1]/CS/g wet tissue), N = 6 | 14.53 ± 4.08 | 14.83 ± 5.42 |
| ATPase activity of complex V responsive to azide (ATPase, U.mg−1/CS), N = 4 | 4.45 ± 0.8 | 3.21 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.G.; Martinez, C.G.; Cavalcanti de Albuquerque, J.P.; Gouvêa, A.L.; Freire, M.M.; Lauthartte, L.C.; Mignaco, J.; Bastos, W.R.; Mattos, E.C.d.; Galina, A.; et al. Mitochondrial Dysfunction Plays a Relevant Role in Heart Toxicity Caused by MeHg. Toxics 2024, 12, 712. https://doi.org/10.3390/toxics12100712
Silva MG, Martinez CG, Cavalcanti de Albuquerque JP, Gouvêa AL, Freire MM, Lauthartte LC, Mignaco J, Bastos WR, Mattos ECd, Galina A, et al. Mitochondrial Dysfunction Plays a Relevant Role in Heart Toxicity Caused by MeHg. Toxics. 2024; 12(10):712. https://doi.org/10.3390/toxics12100712
Chicago/Turabian StyleSilva, Marcia Gracindo, Camila Guerra Martinez, Joao Paulo Cavalcanti de Albuquerque, André Luiz Gouvêa, Monica Maria Freire, Leidiane Caroline Lauthartte, Julio Mignaco, Wanderley Rodrigues Bastos, Elisabete Cesar de Mattos, Antonio Galina, and et al. 2024. "Mitochondrial Dysfunction Plays a Relevant Role in Heart Toxicity Caused by MeHg" Toxics 12, no. 10: 712. https://doi.org/10.3390/toxics12100712
APA StyleSilva, M. G., Martinez, C. G., Cavalcanti de Albuquerque, J. P., Gouvêa, A. L., Freire, M. M., Lauthartte, L. C., Mignaco, J., Bastos, W. R., Mattos, E. C. d., Galina, A., & Kurtenbach, E. (2024). Mitochondrial Dysfunction Plays a Relevant Role in Heart Toxicity Caused by MeHg. Toxics, 12(10), 712. https://doi.org/10.3390/toxics12100712







