Impact of Skin Decontamination Wipe Solutions on the Percutaneous Absorption of Polycyclic Aromatic Hydrocarbons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Skin DecontaminationWipe Materials
2.2. Chemicals
2.3. Flow-Through Diffusion Cell Set Up
2.4. Dosing Procedure
2.5. Sample Analysis
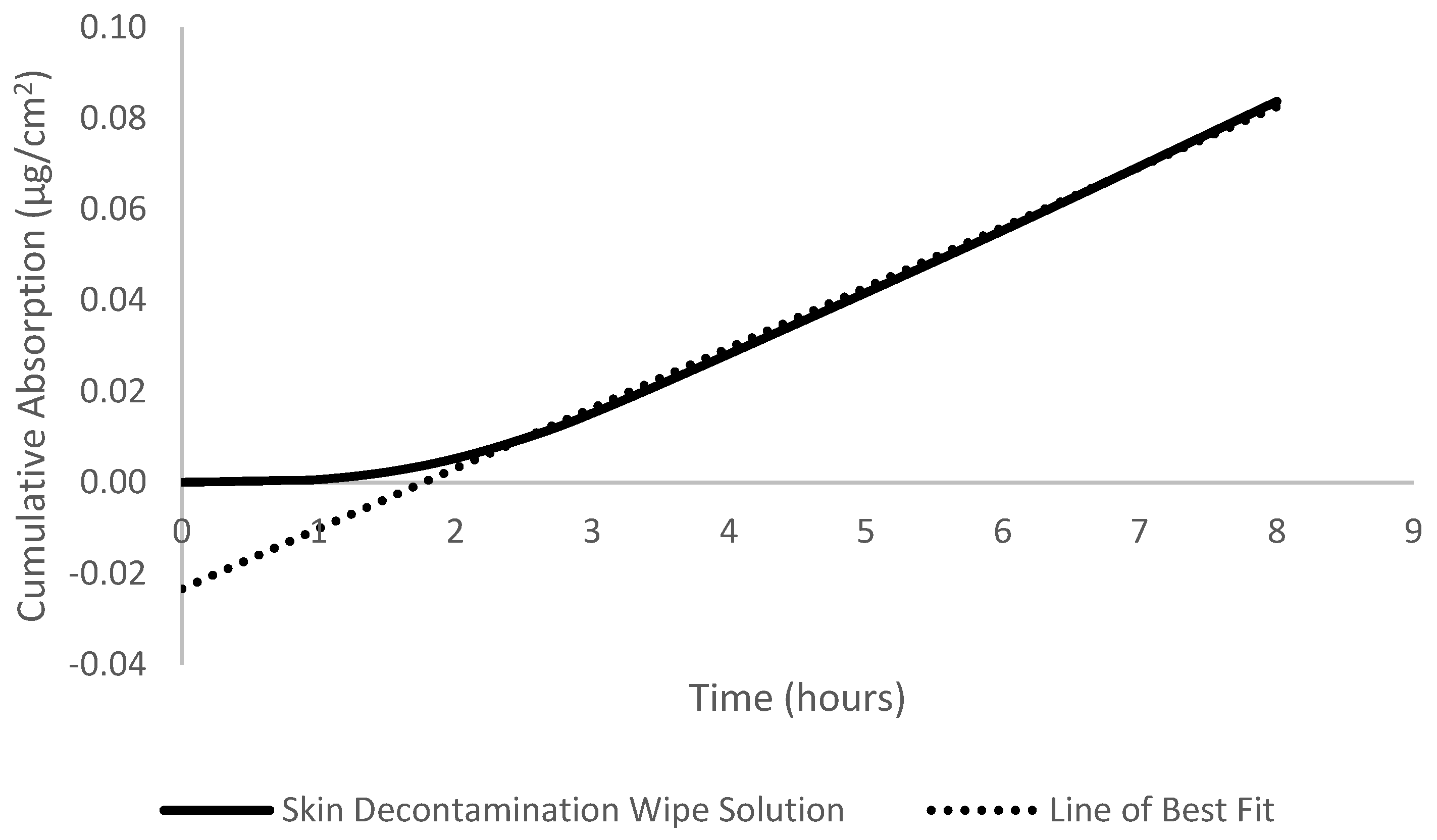
2.6. Absorption Calculations
3. Results
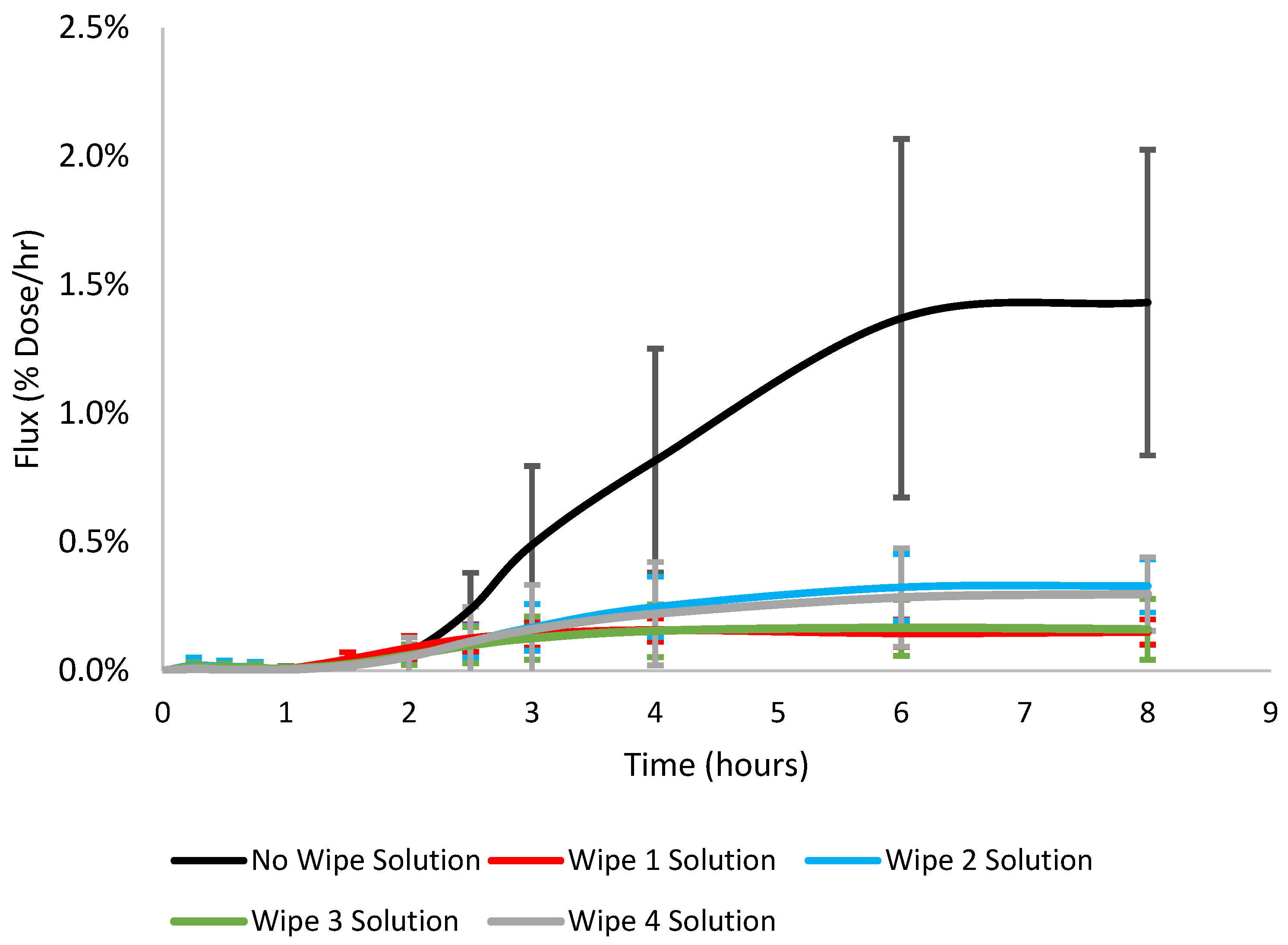
3.1. Dermal Absorption, Flux, Diffusivity, Permeability, and Lag Time
3.2. Skin Dispossition and Mass Balance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agency for Toxic Substances and Disease Registry. Toxicity of Polycyclic Aromatic Hydrocarbons; U.S. Department of Health and Human Services: Washington, DC, USA, 2009.
- Junod, T.L. Gaseous Emissions and Toxic Hazards Associated with Plastics in Fire Situations—A Literature Review; National Aeronautics and Space Administration: Washington, DC, USA, 1976. [Google Scholar]
- Fent, K.W.; Alexander, B.; Roberts, J.; Robertson, S.; Toennis, C.; Sammons, D.; Bertke, S.; Kerber, S.; Smith, D.; Horn, G. Contamination of firefighter personal protective equipment and skin and the effectiveness of decontamination procedures. J. Occup. Evnironmental Hyg. 2017, 14, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.W.; Eisenberg, J.; Evans, D.; Sammons, D.; Robertson, S.; Striley, C.; Snawder, J.; Mueller, C.; Kochenderfer, V.; Pleil, J.; et al. Evaluation of Dermal Exposure to Polycyclic Aromatic Hydrocarbons in Fire Fighters; U.S. Department of Health and Human Services (DHHS): Washington, DC, USA, 2013. [Google Scholar]
- Logan, M.; Kirk, K. Structural Fire Fighting Ensembles: Accumulation and Off-gassing of Combustion Products. J. Occup. Environ. Hyg. 2015, 12, 376–383. [Google Scholar]
- Fent, K.W.; Evans, D.E.; Booher, D.; Pleil, J.D.; Stiegel, M.A.; Horn, G.; Dalton, J. Volatile Organic Compounds Off-gassing from Firefighters’ Personal Protective Equipment Ensembles after use. J. Occup. Environ. Hyg. 2015, 12, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Bolstad-Johnson, D.M.; Burgess, J.L.; Crutchfield, C.D.; Storment, S.; Gerkin, R.; Wilson, J.R. Characterization of Firefighter Exposures During Fire Overhaul. Am. Ind. Hyg. Assoc. 2000, 61, 636–641. [Google Scholar] [CrossRef]
- Kirk, K.M.; Logan, M.B. Firefighting Instructors’ Exposures to Polycyclic Aromatic Hydrocarbons During Live Fire Training Scenarios. J. Occup. Environ. Hyg. 2015, 12, 227–234. [Google Scholar] [CrossRef]
- Baxter, C.S.; Hoffman, J.D.; Knipp, M.J.; Reponen, T.; Haynes, E.N. Exposure of Firefighters to Particulates and Polycyclic Aromatic Hydrocarbons. J. Occup. Environ. Hyg. 2014, 11, D85–D91. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.W.; LaGuardia, M.; Luellen, D.; McCormick, S.; Mayer, A.; Chen, I.-C.; Kerber, S.; Smith, D.; Horn, G. Flame retardants, dioxins, and furans in air and on firefighters’ protective ensembles during controlled residential firefighting. Environ. Int. 2020, 140, 1–9. [Google Scholar] [CrossRef]
- Alexander, B.M.; Lacey, S.; Baxter, C.S. Plasticizer Contamination of Firefighter Personal Protective Clothing–a Potential Factor in Increased Health Risks in Firefighters. J. Occup. Environ. Hyg. 2014, 11, 43–48. [Google Scholar] [CrossRef]
- Fent, K.W.; Eisenberg, J.; Snawder, J.; Sammons, D.; Pleil, J.D.; Stiegel, M.A.; Mueller, C.; Horn, G.; Dalton, J. Systemic Exposure to PAHs and Benzene in Firefighters Suppressing Controlled Structure Fires. Occup. Environ. Hyg. 2014, 58, 830–845. [Google Scholar]
- Keir, J.L.A.; Akhtar, U.S.; Matschke, D.M.J.; Kirkham, T.L.; Chan, H.M.; Ayotte; White, A.; Blais, J.M. Elevated Exposures to Polycyclic Aromatic Hydrocarbosn and Other Organic Mutagens in Ottawa Firefighters Participating in Emergency, On-Shift Fire Suppression. Environ. Sci. Technol. 2017, 51, 12745–12755. [Google Scholar] [CrossRef]
- Stec, A.A.; Dickens, K.E.; Salden, M.; Hewitt, F.E.; Watts, D.; Houldsworth, E.; Martin, F.L. Occupational Exposure to Polycyclic Aromatic Hydrocarbons and Elevated Cancer Incidence in Firefighters. Sci. Rep. 2018, 8, 2476. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.; Teixeira, J.; Delerue-Matos, C.; Sarmento, B.; Morais, S.; Wang, X.; Rodrigues, F.; Oliveira, M. Exposure to PAHs during Firefighting Activities: A Review on Skin Levels, In Vitro/In Vivo Bioavailability, and Health Risks. Internaltional J. Environ. Res. Public Health 2022, 19, 12677. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, N. Jourenkova and Gustavsson. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control 1997, 3, 444–472. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H.G.; Saber, A.T.; Clausen, A.; Pedersen, J.E.; Lohr, M.; Kermanizadeh, A.; Loft, S.; Ebbehoj, N.; Hansen, A.M.; Pedersen, B.; et al. Association between polycyclic aromatic hydrocarbon exposure and peripheral blood mononuclear cell DNA damage in human volunteers during fire extinction exercises. Mutagen. 2018, 33, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polyccyclic Aromatic Hydrocarbons: From Metabolism to Lung Cancer. Toxcological Sci. 2015, 145, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Hadgraft, J.; Guy, R.H. Physicochemical Models for Percutaneous Absorption. In Controlled-Release Technology; American Chemical Society: Washington, DC, USA, 1987; pp. 84–97. [Google Scholar]
- Chilcott, R.; Wilkinson, S.C.; Birch-Machin, M.A.; Brain, K.R.; Pugh, W.J.; Wakefield, J.C.; Pendlington, R.U.; Basketter, D.A.; Jones; Taylor, H.; et al. Principles and Practices of Skin Toxicology; Chilcott, R., Price, S., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2008. [Google Scholar]
- Osseiran, S.; Cruz, J.D.; Jeong, S.; Wang, H.; Fthenakis, C.; Evans, C.L. Characterizing stratum corneum structure, barrier function, and chemical content of human skin with coherent Ramam scattering imaging. Biomed. Opt. Express 2018, 9, 6425–6443. [Google Scholar] [CrossRef]
- Baynes, R.E.; Hodgson, E. Absorption and Distribution of Toxicants. In A Textbook of Modern Toxicology; John Wiley & Sons, Inc.: New York, NY, USA, 2010; pp. 79–114. [Google Scholar]
- VanRooij, J.; Roos, J.D.; Bodelier-Bade, M.; Jongeneelen, F. Absorption of polycyclic aromatic hydrocarbons through human skin: Differences between anatomical sites and individuals. J. Toxicol. Environ. Health 2010, 38, 355–368. [Google Scholar] [CrossRef]
- Dankovic, D.A.; Wright, C.W.; Zangar, R.C.; Springer, D.L. Complex Mixture Effects on the Dermal Absorption of Benzo[a]pyrene and other Polycyclic Aromatic Hydrocarbons from Mouse Skin. J. Appl. Toxicol. 1989, 9, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Wester, R.C.; Maibach, H.I.; Bucks, D.A.; Sedik, L.; Melendres, J.; Liao, C.; Dizio, S. Percutaneous Absorption of [14C]DDT and [14C]Benzo[a]pyrene from Soil. Toxicol. Sci. 1990, 15, 510–516. [Google Scholar] [CrossRef]
- Yang, J.J.; Roy, T.A.; Mackerer, C.R. Percutaneous Absorption of Benzo[a]Pyrene in the Rat: Comparison of in Vivo and in Vitro Results. Toxicol. Ind. Health 1986, 2, 409–416. [Google Scholar] [CrossRef]
- Ng, K.; Chu, I.; Bronaugh, R.; Franklin, C.; Somers, D. Percutaneous absorption and metabolism of pyrene, benzo[a]pyrene, and di(2-ethylhexyl) phthalate: Comparison of in vitro and in vivo results in the hairless guinea pig. Toxicol. Appl. Pharmacol. 1992, 115, 216–223. [Google Scholar] [CrossRef]
- Hodgson, E.; Rose, R.L. Metabolism of Toxicants. In A Textbook of Modern Toxicology; Hodgson, E., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 115–156. [Google Scholar]
- Harrison, T.R.; Muhamad, J.W.; Yang, F.; Morgan, S.E.; Talavera, E.; Caban-Martinex, A.; Kobetz, E. Firefighter attitudes, norms, beliefs, barriers, and behaviors toward post-fire decontamination processes in an era of increased cancer risk. J. Occup. Environ. Hyg. 2018, 15, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Macy, G.B.; Hwang, J.; Tayler, R.; Golla, V.; Cann, C.; Gates, B. Examining Behvaiors Related to Retirement, Cleaning, and Storage of Turnout Gear Among Rural Firefighters. Workplace Health Saf. 2020, 68, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.A.; Treichel, A.; Myers, J.B.; Bourn, S.S.; Crowe, R.; Gardner, B. Evaluating Firefighter On-Scene Decontamination Practices Using a National Fire Records Management System. J. Occup. Environ. Med. 2023, 65, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Hero Wipes. Benzopyrene Study. Available online: https://cdn.shopify.com/s/files/1/1994/4039/files/Benzopyrene_Part_1-2_Report_non-confidential.pdf?3372616680490246062 (accessed on 26 September 2024).
- Hero Wipes. Evaluation of Hero Wipes Reduction of Surface Lead (Pb) Contaminants. Available online: https://cdn.shopify.com/s/files/1/1994/4039/files/FOR_PUBLIC_RELEASE_-_Hero_Wipes_Fire_Lead_Removal_Study.pdf?455725101656256215 (accessed on 26 September 2024).
- Manchester Metropolitan University. De-Wipe Investigation Report: Assessing de-Wipes Ability to Remove Dioxins and Polycyclic Aromatic Hydrocarbons from Differenet Surfaces; Manchester Metropolitan University: Manchester, UK, 2019. [Google Scholar]
- Nakashima, M.; Zhao, M.F.; Ohya, H.; Sakurai, M.; Sasaki, H.; Matsuyama, K.; Ichikawa, M. Evaluation of in-vivo transdermal absorption of cyclosporin with absorption enhancer using intradermal microdialysis in rats. J. Pharm. Pharmacol. 1996, 48, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Beneke, C.; Viljoen, A.; Hamman, J. In Vitro Drug Absorption Enhancement Effects of Aloe Vera and Aloe Ferox. Sci. Pharm. 2012, 80, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Samani, S.; Jamshidzadeh, A.; Montaseri, H.; Rangbar-Zahedani, M.; Kianrad, R. The effects of some permeability enhancers on the percutaneous absorption of lidocaine. Pak. J. Phramaceutical Sci. 2010, 23, 83–88. [Google Scholar]
- Panico, A.; Serio, F.; Bagordo, F.; Grassi, T.; Idolo, A.; Giorgi, M.D.; Guido, M.; Congedo, M.; Donno, A.D. Skin safety and health prevention: An overview of chemicals in cosmetic products. J. Prev. Med. Hyg. 2019, 60, 50–57. [Google Scholar]
- Probert, C.; Nixon, E.; Ormond, R.B.; Baynes, R. Percutaneous Absorption of Fireground Contaminants: Naphthalene, Phenanthrene, and Benzo[a]pyrene in Porcine Skin in an Artificial Sweat Vehicle. Toxics 2024, 12, 588. [Google Scholar] [CrossRef] [PubMed]
- Bronaugh, R.L.; Stewart, R.F. Methods for In Vitro Percutaneous Absorption Studies IV: The Flow-Through Diffusion Cell. J. Pharm. Sci. 1995, 74, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Barbero, M.A.; Frasch, H.F. Pig and guinea pig skin as surrogates for human in vitro penetration studies: A quantitative review. Toxicol. Vitr. 2009, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Khiao In, M.; Richardson, K.C.; Loewa, A.; Hedtrich, S.; Kaessmeyer, S.; Plendl, J. Histological and functional comparisons of four anatomical regios of porcine skin with human abdominal skin. Anat. Histol. Embryol. 2019, 48, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Organisatoin for Economic Co-operation and Development. OECD guideline for the testing of chemicals. In Skin Absorption: In Vitro Method; Organisation for Economic Co-operation and Development: Paris, France, 2004. [Google Scholar]
- Moody, R.; Nadeau, B.; Chu, I. In vivo and in vitro dermal absorption of benzo[a]pyrene in rat, guinea pig, human and tissue-cultured skin. J. Dermatol. Sci. 1995, 9, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jung, E.-C.; Phuong, C.; Hui, X.; Maibach, H. Effects of soap-water wash on human epidermal penetration. J. Appl. Toxicol. 2016, 36, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Thors, L.; Wigenstam, E.; Qvarnstrom, J. Hagglund and A. Bucht. Improved skin decontamination efficacy for the nerve agent VX. Chem.-Biol. Interact. 2020, 325, 109135. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2014, 67, 473–485. [Google Scholar] [CrossRef]
- Williams, C.; Barry, B.W. Essential Oils as novel human skin penetration enhancers. Int. J. Pharm. 1989, 57, R7–R9. [Google Scholar] [CrossRef]
- Schafer, N.; Balwierz, R.; Biernat, P.; Ochędzan-Siodłak, W.; Lipok, J. Natural Ingredients of Transdermal Drug Delivery Systems as Permeation Enhancers of Active Substances through the Stratum Corneum. Mol. Pharm. 2023, 20, 3278–3297. [Google Scholar] [CrossRef]
- Fox, L.T.; Gerber, M.; Preez, J.L.D.; Plessis, J.D.; Hammna, J.H. Skin permeation enhancement effects of the gel and whole-loeaf materials of Aloe Vera, Aloe merlothii and Aloe ferox. Pharm. Pharmacol. 2014, 67, 96–106. [Google Scholar] [CrossRef]
- Nielsen, J.B. Natural Oils Affect the Human Skin Integrity and the Percutaneous Penetration of Benzoic Acid Dose-Dependently. Basic Clin. Pharmacol. Toxicol. 2006, 98, 575–581. [Google Scholar] [CrossRef]
- Abdullah, D.; Ping, Q.N.; Lui, G.L. Enhancing effect of essential oils on the penetration of 5-fluorouracil through rat skin. Acta Pharamacologica Sin. 1996, 31, 214–221. [Google Scholar]
- SAmin; Kohli, K.; Khar, R.K.; Mir, S.R.; Pillai, K.K. Mechanism of in vitro percutaaneous absorption enhancement of carvedilol by penetration enhancers. Pharm. Dev. Technol. 2008, 13, 533–539. [Google Scholar]
- Buist, H.; Craig; Dewhurst, I.; Bennekou, S.H.; Kneuer, C.; Machera, K.; Pieper, C.; Marques, D.C.; Guillot, G.; Ruffo, F.; et al. Guidance on Dermal Absorption. Eur. Food Saf. Auth. J. 2017, 15, e04873. [Google Scholar]
- Aoki, M.; Ogai, K.; Matsumoto, M.; Susa, H.; Yamada, K.; Yamatake, T.; Kobayashi, M.; Sugama, J. Comparison of wiping methods for the removal of cleaning agent residue from hair folicles. Ski. Res. Technol. 2019, 25, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Moffet, M.; Baker, B.L.; Kang, C.S.; Johnson, M.S. Evaluation of Time Required for Water-Only Decontamination of an Oil-Based Agent. Mil. Med. 2010, 175, 185–187. [Google Scholar] [CrossRef]
- Keir, J.L.; Kirkham, T.L.; Aranda-Rodriguez, R.; White, A.; Blais, J.M. Effectiveness of dermal cleaning interventions for reducing firefighters’ exposure to PAHs and genotoxins. J. Occup. Environ. Hyg. 2023, 20, 84–94. [Google Scholar] [CrossRef]
- Loke, W.-K.; U, S.-H.; Lau, S.-K.; Lim, J.-S.; Tay, G.-S.; Koh, C.-H. Wet decontamination-induced stratum corneum hydration-effects on the skin barrier function to diethylmalonate. J. Appl. Toxicol. 1999, 19, 285–290. [Google Scholar] [CrossRef]
- Forsberg, E.; Oberg, L.; Artursson, E.; Wigenstam, E.; Bucht, A.; Thors, L. Decontamination efficacy of soapy water and water washing following exposure of toxic chemicals on human skin. Cutan. Adn Ocul. Toxicol. 2020, 39, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Schenk, L.; Rauma, M.; Fransson, M.N.; Johanson, G. Percutaneous absorption of thrity-eight organic solvents in vitro using pig skin. PLoS ONE 2018, 13, 1–16. [Google Scholar] [CrossRef]
- Moody, R.; Akram, M.; Dickson, E.; Chu, I. In Vitro Dermal Absorption of Metyl Salicylate, Ethyl Parathion, and Malthion: First Responder Safety. J. Toxicol. Environ. Health 2007, 71, 985–999. [Google Scholar] [CrossRef]
- Thors, L.; Koch, M.; Wigenstam, E.; Koch, B.; Hagglund, L.; Bucht, A. Comparison of skin decontamination efficacy of commercial decontamination products follwoing exopsure to VX on human skin. Chem.-Biol. Interact. 2017, 273, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Howes, D.; Guy, R.; Hadgraft, J.; Heylings, J.; Hoeck, U.; Kemper, F.; Maibach, H.; Marty, J.-P.; Merk, H.; Parra, J.; et al. Methods for Assessing Percutaneous Absorption. Altern. Lab. Anim. 1996, 24, 81–106. [Google Scholar] [CrossRef]
| Wipe 1 | Wipe 2 | Wipe 3 | Wipe 4 |
|---|---|---|---|
| Water (Aqua) | Water | Deionized Water | Water |
| Hexylene Glycol | Propanediol | Gluconolactone | Phenoxyethanol |
| Glycerin | Aloe Barbadensis Leaf Extract | Decyl Glucoside | Decyl glucoside |
| Sodium Hydroxymethylglycinate | Chamomila Recutia (Matricaria) Flower Extract | Sodium Benzoate | Tetrasodium Glutamate Diacetate |
| Citric Acid | Cucumis Sativis (Cucumber) Fruit Extract | Dehydroacetic Acid | Sodium Benzoate |
| Disodium Cocoamphodiacetate | Althaea Officinalis Root Extract | Calcium Gluconate | Sodium Citrate |
| Fragrance | Avena Sativa (Oat) Kernel Extract | Caprylic/Capric Triglyceride | Citric Acid |
| Sodium Benzoate | Decyl Glucoside | Chamomilla Recrutia Extract | Sodium Bicarbonate |
| Potassium Sorbate | Polyglyceryl-10 Caprylate/Caprate | Aloe Barbadensis Extract | Glycerin |
| Disodium Cocoyl Glutamate | Coco Glucoside | Tocopheryl Acetate (Vitamin E) | Tocopheryl Acetate (Vitamin E) |
| Sodium Cocoyl Glutamate | Glyceryl Oleate | Cucumis Sativus (Cucumber) Fruit Extract | |
| Polysorbate 20 | |||
| Tetrasodium Glutamate Diacetate | |||
| Trisodium Phosphate | |||
| Citric Acid | |||
| Caprylyl glycol | |||
| Benzalkonium Chloride | |||
| Sodium Benzoate | |||
| Potassium Sorbate | |||
| Phenoxyethanol |
| Decontamination Wipe | Cumulative Absorption (µg/cm2) | Absorption Efficiency (% Dose) | Flux (µg/cm2/h) | Lag Time (minutes) | Diffusivity (cm2/h) | Permeability (cm/h) (×10−4) |
|---|---|---|---|---|---|---|
| Wipe Solution 1 | 0.09 ± 0.03 | 0.96 ± 0.31 | 0.013 ± 0.005 | 90.0 ± 17.9 | 0.63 ± 0.15 | 2.3 ± 0.8 |
| Wipe Solution 2 | 0.16 ± 0.06 | 1.76 ± 0.65 | 0.029 ± 0.010 | 157.9 ± 32.6 | 0.36 ± 0.06 | 5.1 ± 1.8 |
| Wipe Solution 3 | 0.09 ± 0.06 | 0.98 ± 0.64 | 0.015 ± 0.009 | 131.2 ± 48.1 | 0.46 ± 0.12 | 2.6 ± 1.6 |
| Wipe Solution 4 | 0.14 ± 0.09 | 1.57 ± 1.05 | 0.026 ± 0.016 | 164.3 ± 27.4 | 0.35 ± 0.06 | 4.5 ± 2.7 |
| No Wipe Solution * | 0.53 ± 0.25 | 6.80 ± 3.20 | 0.104 ± 0.045 | 183.0 ± 20.5 | 0.20 ± 0.02 | 21.0 ± 9.1 |
| Wipe | Dose (µg/cm2) | Remaining Dose (% Dose) | Stratum Corneum (% Dose) | Skin (% Dose) | Absorption (% Dose) | Total Recovery (% Dose) |
|---|---|---|---|---|---|---|
| Wipe Solution 1 | 9.03 | 86.8 ± 5.2 | 4.0 ± 4.7 | 2.9 ± 1.5 | 1.0 ± 0.3 | 94.6 ± 2.3 |
| Wipe Solution 2 | 9.03 | 81.7 ± 5.6 | 8.3 ± 9.5 | 4.0 ± 4.0 | 1.8 ± 0.6 | 95.8 ± 2.7 |
| Wipe Solution 3 | 9.03 | 75.9 ± 11.1 | 18.1 ± 14.0 | 4.0 ± 1.0 | 1.0 ± 0.6 | 99.0 ± 4.2 |
| Wipe Solution 4 | 9.03 | 82.2 ± 7.8 | 8.7 ± 10.2 | 2.8 ± 2.5 | 1.6 ± 1.1 | 95.3 ± 3.0 |
| No Wipe Solution * | 7.76 | 56.5 ± 3.5 | 2.3 ± 0.6 | 32.4 ± 5.2 | 6.8 ± 3.2 | 98.1 ± 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Probert, C.; Ormond, R.B.; Baynes, R.E. Impact of Skin Decontamination Wipe Solutions on the Percutaneous Absorption of Polycyclic Aromatic Hydrocarbons. Toxics 2024, 12, 716. https://doi.org/10.3390/toxics12100716
Probert C, Ormond RB, Baynes RE. Impact of Skin Decontamination Wipe Solutions on the Percutaneous Absorption of Polycyclic Aromatic Hydrocarbons. Toxics. 2024; 12(10):716. https://doi.org/10.3390/toxics12100716
Chicago/Turabian StyleProbert, Chandler, R. Bryan Ormond, and Ronald E. Baynes. 2024. "Impact of Skin Decontamination Wipe Solutions on the Percutaneous Absorption of Polycyclic Aromatic Hydrocarbons" Toxics 12, no. 10: 716. https://doi.org/10.3390/toxics12100716







