A Chemometric Exploration of Potential Chemical Markers and an Assessment of Associated Risks in Relation to the Botanical Source of Fruit Spirits
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Spirit Samples Information
2.2. Utilization of Previously Published Data
2.3. Semiquantitative Analysis of Aromatic Compounds in Fruit Spirits
2.4. Chemometric Analysis
2.5. Compliance Evaluation and Risk Assessment
3. Results and Discussion
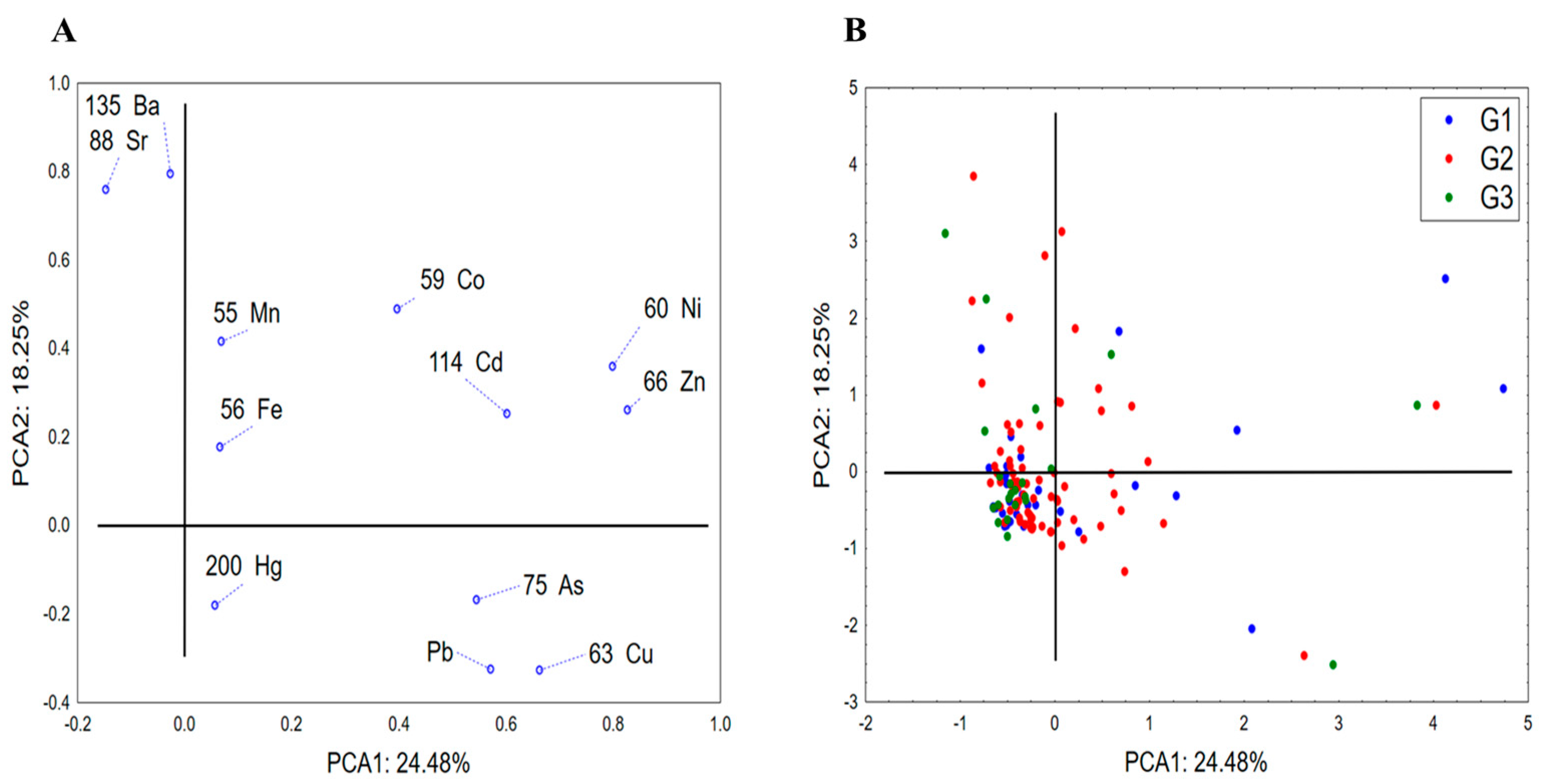
3.1. Exploration of the Patterns of Distribution of Hazardous Substances in Relation to Fruit Classes
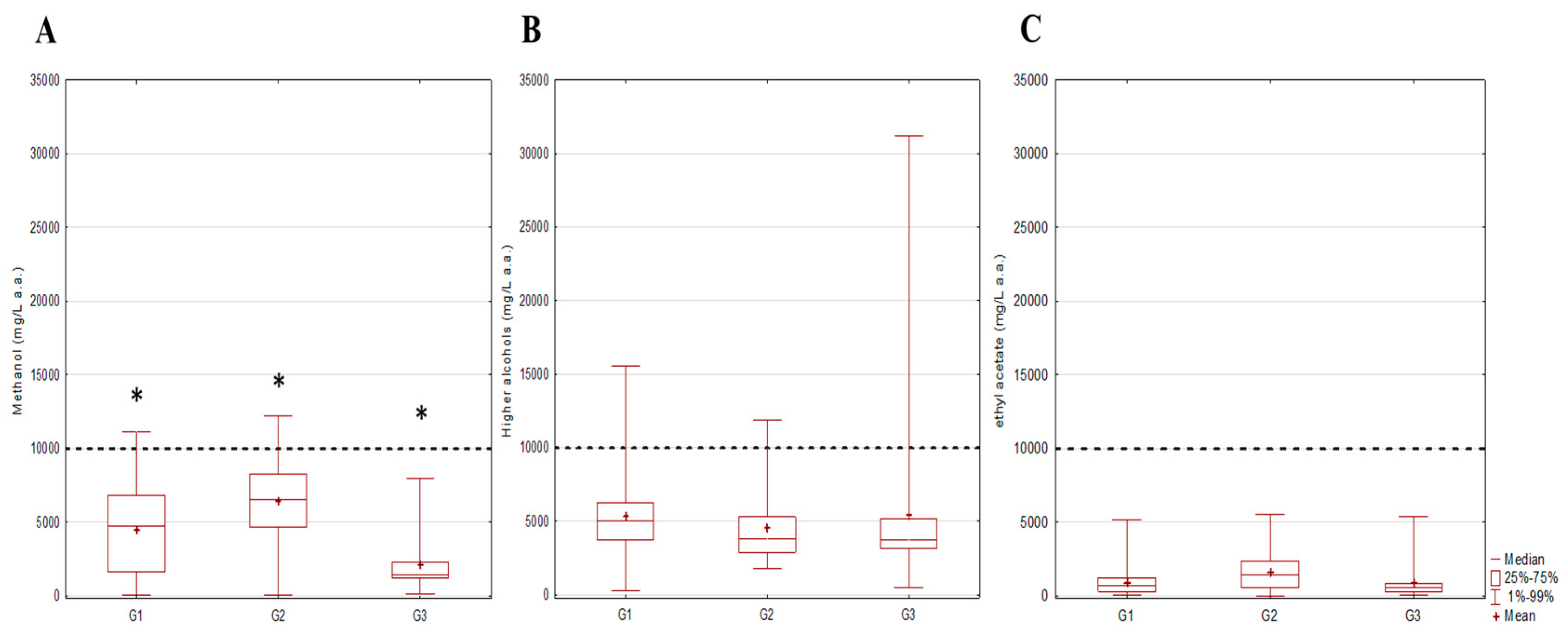
3.2. Evaluation of Occurrence and Compliance Assessment of Hazardous Substances in Relation to Fruit Classes
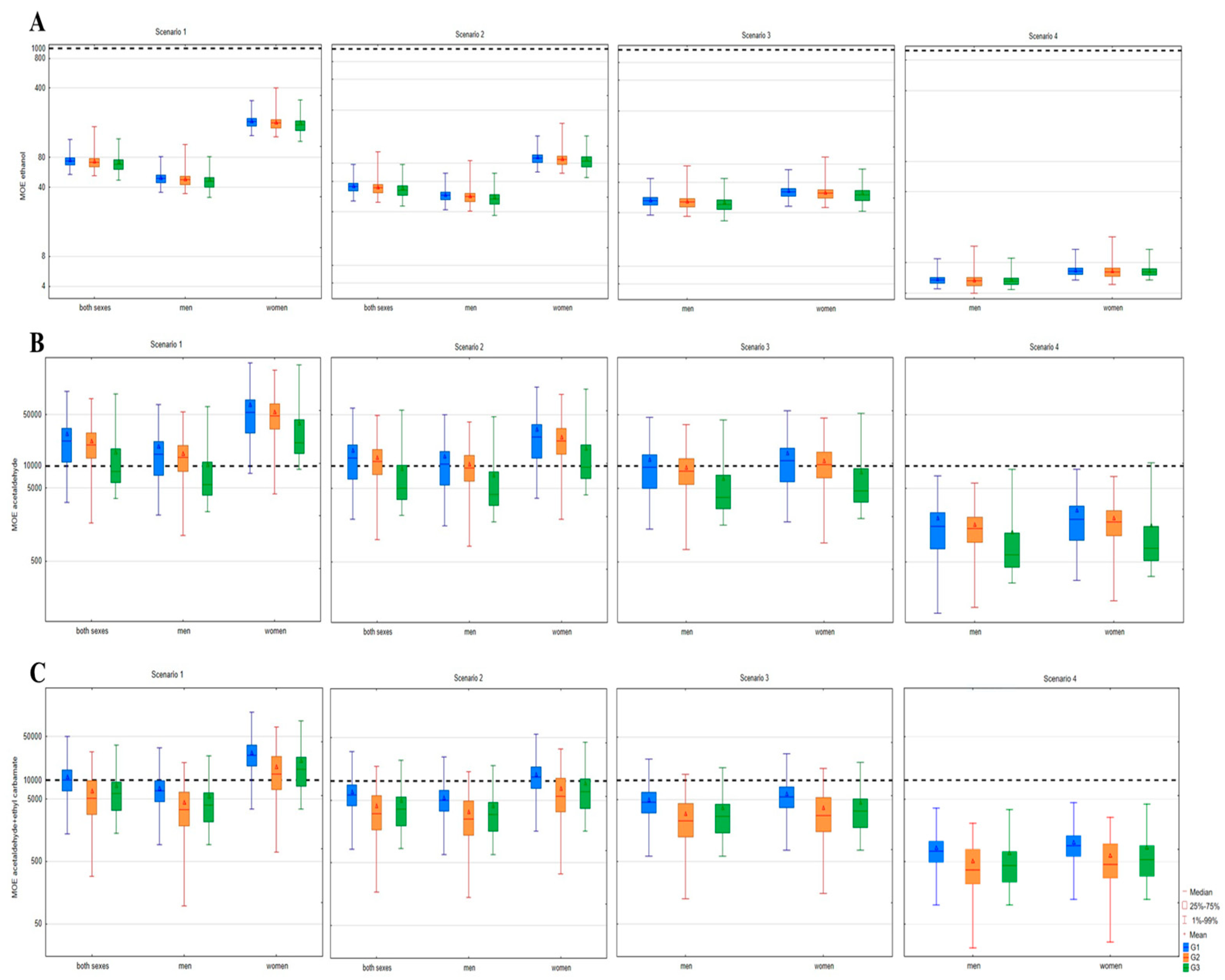
3.3. Risk Assessment of Hazardous Substances in Relation to Fruit Classes
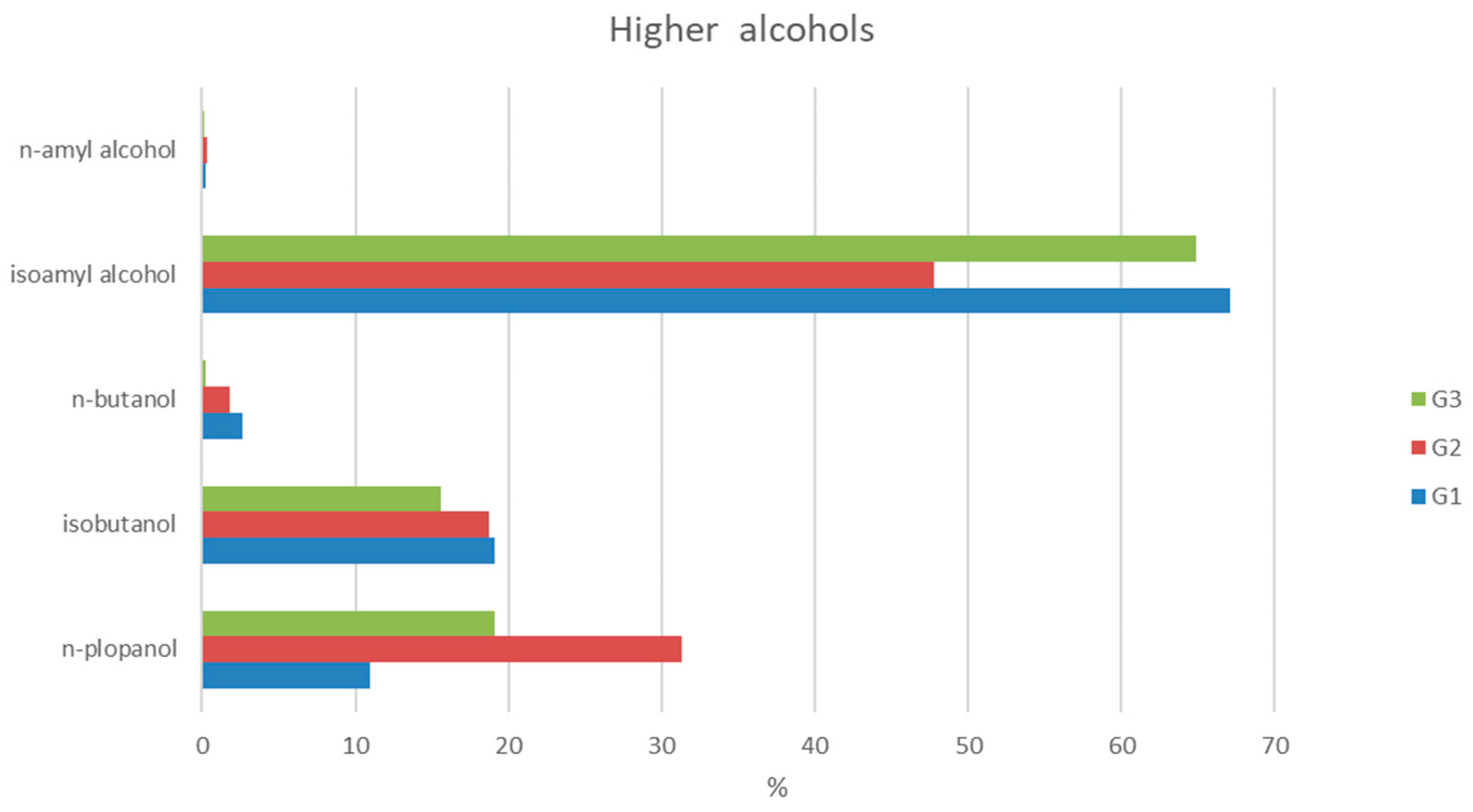
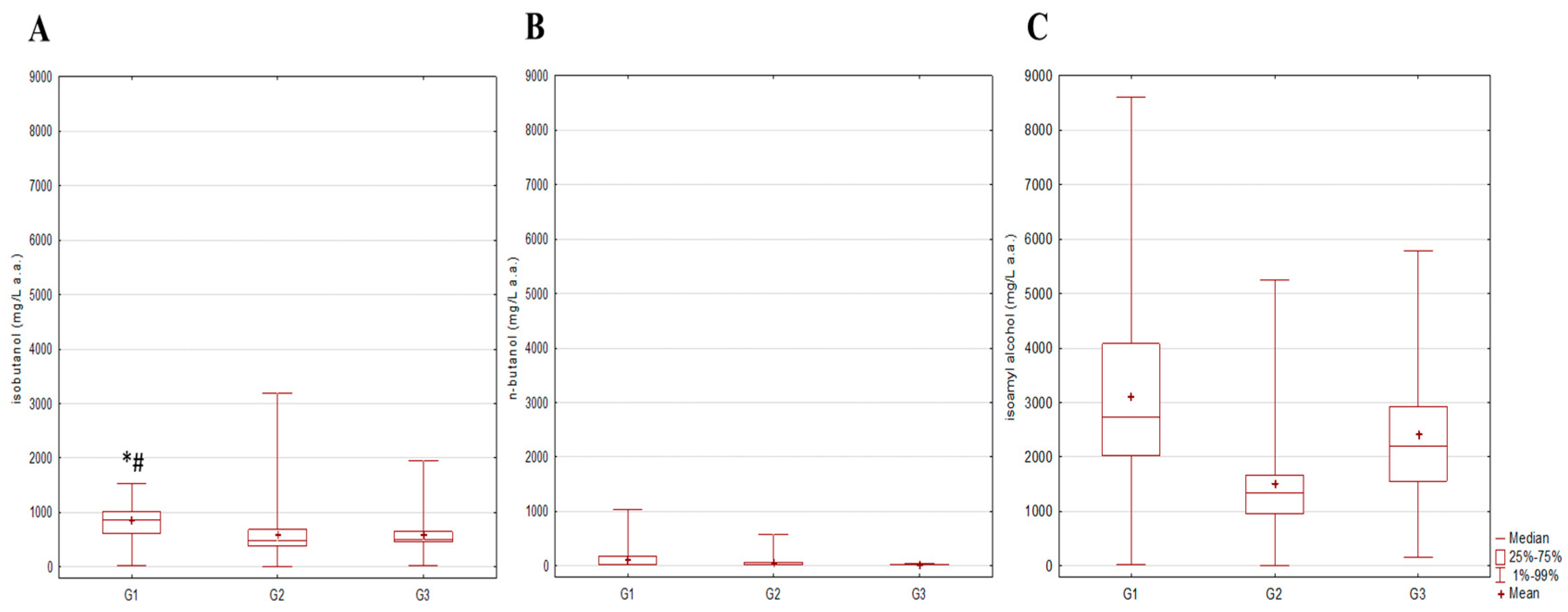
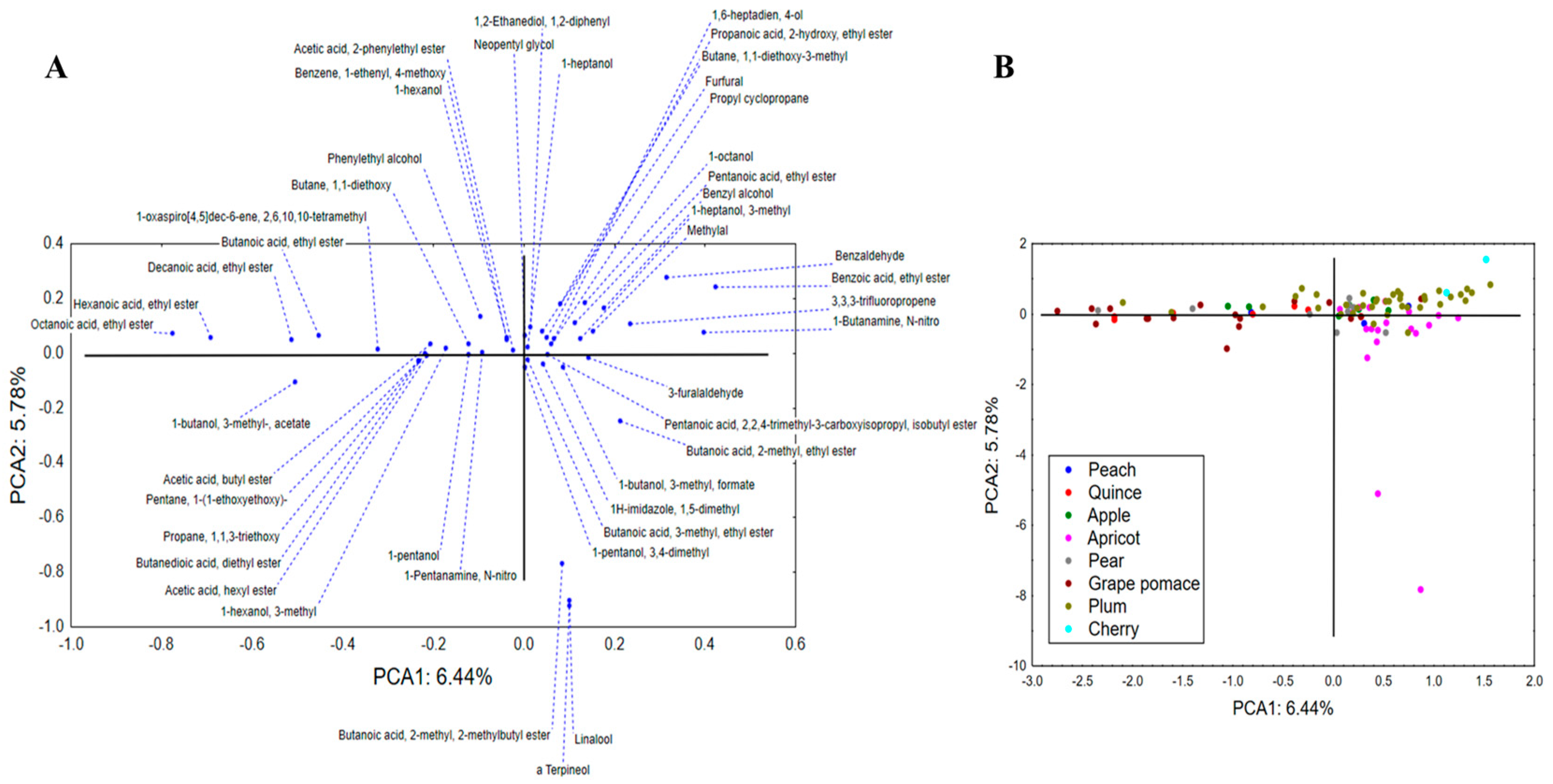
3.4. Compositional Analysis of Aromatic Compounds in Spirits in Relation to Fruit Source
3.5. Quality Markers for Differentiation of Specific Fruits Used for Spirit Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Global Status Report on Alcohol and Health and Treatment of Substance Use Disorders. Available online: https://www.who.int/publications/i/item/9789240096745 (accessed on 10 August 2024).
- Rovira, P.; Rehm, J. Estimation of cancers caused by light to moderate alcohol consumption in the European Union. Eur. J. Public Health 2021, 31, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.O.; Berdzuli, N.; Ilbawi, A.; Kestel, D.; Kluge, H.P.; Krech, R.; Mikkelsen, B.; Neufeld, M.; Poznyak, V.; Rekve, D. Health and cancer risks associated with low levels of alcohol consumption. Lancet Public Health 2023, 8, e6–e7. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.; Sheron, N. No level of alcohol consumption improves health. Lancet 2018, 392, 987–988. [Google Scholar] [CrossRef] [PubMed]
- Korotayev, A.; Khaltourina, D.; Meshcherina, K.; Zamiatnina, E. Distilled spirits overconsumption as the most important factor of excessive adult male mortality in Europe. Alcohol Alcohol. 2018, 53, 742–752. [Google Scholar] [CrossRef]
- Okaru, A.O.; Rehm, J.; Sommerfeld, K.; Kuballa, T.; Walch, S.G.; Lachenmeier, D.W. The threat to quality of alcoholic beverages by unrecorded consumption. In Alcoholic Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–34. [Google Scholar]
- Srdjenović-Čonić, B.; Kladar, N.; Božin, B.; Torović, L. Harmful volatile substances in recorded and unrecorded fruit spirits. Arab. J. Chem. 2022, 15, 103981. [Google Scholar] [CrossRef]
- Torović, L.; Čonić, B.S.; Kladar, N.; Lukić, D.; Bijelović, S. Elemental profile of recorded and unrecorded fruit spirits and health risk assessment. J. Food Compos. Anal. 2022, 114, 104807. [Google Scholar] [CrossRef]
- Stanzer, D.; Hanousek Čiča, K.; Blesić, M.; Smajić Murtić, M.; Mrvčić, J.; Spaho, N. Alcoholic fermentation as a source of congeners in fruit spirits. Foods 2023, 12, 1951. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. List of Classifications. Agents Classified by the IARC Monographs, Volumes 1–130. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 5 July 2024).
- Mrvčić, J.; Trontel, A.; Hanousek Čiča, K.; Vahčić, N.; Nikićević, N.; Spaho, N.; Mihaljević Žulj, M.; Brodski, A.; Jurak, V.; Krajnović, M. Chemical and sensorial characteristics of traditional fruit spirits from Southeast Europe. Glas. Zaštite Bilja 2021, 44, 80–89. [Google Scholar] [CrossRef]
- OEHHA. Methanol-A Fact Sheet. Available online: https://oehha.ca.gov/proposition-65/methanol-fact-sheet (accessed on 5 August 2024).
- Lal, J.J.; Sreeranjit Kumar, C.; Suresh, M.; Indira, M.; Vijayammal, P. Effect of exposure to a country liquor (Toddy) during gestation on lipid metabolism in rats. Plant Foods Hum. Nutr. 2001, 56, 133–143. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Haupt, S.; Schulz, K. Defining maximum levels of higher alcohols in alcoholic beverages and surrogate alcohol products. Regul. Toxicol. Pharmacol. 2008, 50, 313–321. [Google Scholar] [CrossRef]
- Lima, C.M.G.; Benoso, P.; Pierezan, M.D.; Santana, R.F.; de Souza Hassemer, G.; da Rocha, R.A.; Dalla Nora, F.M.; Verruck, S.; Caetano, D.; Simal-Gandara, J. A state-of-the-art review of the chemical composition of sugarcane spirits and current advances in quality control. J. Food Compos. Anal. 2022, 106, 104338. [Google Scholar] [CrossRef]
- Gowd, V.; Su, H.; Karlovsky, P.; Chen, W. Ethyl carbamate: An emerging food and environmental toxicant. Food Chem. 2018, 248, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Abt, E.; Incorvati, V.; Robin, L.P.; Redan, B.W. Occurrence of ethyl carbamate in foods and beverages: Review of the formation mechanisms, advances in analytical methods, and mitigation strategies. J. Food Prot. 2021, 84, 2195–2212. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Some anti-thyroid and related substances, nitrofurans and industrial chemicals. IARC Monogr. Eval. Carcinog. Risk Chem. Man 1974, 111–140. [Google Scholar]
- Official Journal of the European Union. Regulation (eu) 2019/787 of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32019R0787 (accessed on 15 August 2024).
- Official Journal of the European Union. Regulation (ec) no 110/2008 of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008R0110 (accessed on 10 August 2024).
- IARD. Amphora Chemical Testing Limits. Available online: https://www.iard.org/getattachment/e59844be-afe6-4ca6-a363-ef1e8944ec65/01012015toolkit-for-assessing-the-unrecorded-alcohol-market-final.pdflimits (accessed on 21 July 2024).
- Wiśniewska, P.; Śliwińska, M.; Dymerski, T.; Wardencki, W.; Namieśnik, J. The analysis of raw spirits–a review of methodology. J. Inst. Brew. 2016, 122, 5–10. [Google Scholar] [CrossRef]
- Winterová, R.; Mikulikova, R.; Mazáč, J.; Havelec, P. Assessment of the authenticity of fruit spirits by gas chromatography and stable isotope ratio analyses. Czech J. Food Sci. 2008, 26, 368–375. [Google Scholar] [CrossRef]
- Marinković, I. Alkohol kao faktor smrtnosti stanovništva u Srbiji (2016–2018). Stanovništvo 2020, 58, 89–111. [Google Scholar]
- Smailagić, A.; Zagorac, D.D.; Veljović, S.; Sredojević, M.; Relić, D.; Akšić-Fotirić, M.; Roglić, G.; Natić, M. Release of wood extractable elements in experimental spirit model: Health risk assessment of the wood species generated in Balkan cooperage. Food Chem. 2021, 338, 127804. [Google Scholar] [CrossRef]
- UNESCO. Decision of the Intergovernmental Committee: 17.COM 7.B.26. Available online: https://ich.unesco.org/en/decisions/17.COM/7.B.26 (accessed on 22 July 2024).
- Kusonić, D.; Bijelić, K.; Kladar, N.; Torović, L.; Srđenović Čonić, B. Health risk assessment of ethyl carbamate in fruit spirits. Food Addit. Contam. Part B 2023, 16, 393–403. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oils by Ion Trap Mass Spectroscopy; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- CORDIS 2013. European Commission. CORDIS EU Research Results. Final Report Summary: Alcohol Measures for Public Health Research Alliance (AMPHORA). Available online: https://cordis.europa.eu/project/id/223059/reporting (accessed on 14 August 2024).
- Jung, A.; Jung, H.; Auwärter, V.; Pollak, S.; Farr, A.; Hecser, L.; Schiopu, A. Volatile congeners in alcoholic beverages: Analysis and forensic significance. Rom. J. Leg. Med. 2010, 18, 265–270. [Google Scholar] [CrossRef]
- Dimitrov, D.; Ivanova, S. Aromatic profile of Bulgarian grape and fruit (plum) brandies. Ann. Food Sci. Technol 2016, 17, 387–393. [Google Scholar]
- Lachenmeier, D.W.; Frank, W.; Kuballa, T. Application of tandem mass spectrometry combined with gas chromatography to the routine analysis of ethyl carbamate in stone-fruit spirits. Rapid Commun. Mass Spectrom Int. J. Devoted Rapid Dissem. Up-Minute Res. Mass Spectrom. 2005, 19, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, P.; Steger, M.C.; Einfalt, D.; Rieke-Zapp, J.; Quintanilla Bellucci, A.; Sommerfeld, K.; Schwarz, S.; Lachenmeier, D.W. Methanol mitigation during manufacturing of fruit spirits with special consideration of novel coffee cherry spirits. Molecules 2021, 26, 2585. [Google Scholar] [CrossRef]
- Spaho, N. Distillation techniques in the fruit spirits production. Distill.-Innov. Appl. Model. 2017, 129–152. [Google Scholar]
- Šekerić, A.; Blesić, M.; Drkenda, P.; Spaho, N. Effect of the alcohol content on sensory perception of the fruit spirits. Acta Agric. Slov. 2023, 119, 1–7. [Google Scholar] [CrossRef]
- Popović, B.; Gavrilović-Damnjanović, J.; Mitrović, O.; Ogasanović, D.; Nikićević, N.; Tesević, V. Major volatile components and sensory characteristics of plum brandies produced from plum cultivars developed in Čačak. In Proceedings of the I Balkan Symposium on Fruit Growing 825, Plovdiv, Bulgaria, 11 November 2007; pp. 575–582. [Google Scholar]
- Kokkinakis, M.; Tsakiris, I.; Tzatzarakis, M.; Vakonaki, E.; Alegakis, A.; Papachristou, S.; Karzi, V.; Kokkinaki, A.; Goumenou, M.; Kallionakis, M. Carcinogenic, ethanol, acetaldehyde and noncarcinogenic higher alcohols, esters, and methanol compounds found in traditional alcoholic beverages. A risk assessment approach. Toxicol. Rep. 2020, 7, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Cortés, S.; Fernández, E. Differentiation of Spanish alcoholic drinks, Orujo, obtained from red and white grape pomace distillation: Volatile composition. Int. J. Food Prop. 2011, 14, 1349–1357. [Google Scholar] [CrossRef]
- Wu, Y.; Bao, H.; Yao, S.; Rodriguez-Saona, L.E. Authentication and Quality Control of Distilled Spirits by Vibrational Spectroscopy. In Chemistry of Alcoholic Beverages; ACS Publications: Washington, DC, USA, 2023; pp. 101–133. [Google Scholar]
- López, F.; Rodríguez-Bencomo, J.; Orriols, I.; Pérez-Correa, J. Fruit brandies. In Science and Technology of Fruit Wine Production; Elsevier: Amsterdam, The Netherlands, 2017; pp. 531–556. [Google Scholar]
- Spaho, N.; Đukic-Ratković, D.; Nikićević, N.; Blesić, M.; Tešević, V.; Mijatović, B.; Smajić Murtić, M. Aroma compounds in barrel aged apple distillates from two different distillation techniques. J. Inst. Brew. 2019, 125, 389–397. [Google Scholar] [CrossRef]
- Spaho, N.; Blesić, M. The influence of distillation process on the quality of slivovica. Rad. Poljopr. Fak. Univ. U Sarajev. 2005, 50, 125–135. [Google Scholar]
- WHO. Global Status Report on Alcohol and Health 2018. 2018. Available online: https://apps.who.int/iris/handle/10665/274603 (accessed on 14 August 2024).





| Abundance (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| G1—Pome Fruit | G2—Stone Fruit | G3—Grape Pomace | ||||||
| Apple | Pear | Quince | Plum | Peach | Apricot | Cherry | Grape | |
| COMPOUND | N = 7 | N = 10 | N = 7 | N = 38 | N = 3 | N = 19 | N = 2 | n = 20 |
| TOTAL ALCOHOLS | ||||||||
| 1-pentanol | 14 | 20 | 14 | 5 | 0 | 11 | 0 | 15 |
| 1-hexanol | 86 | 40 | 29 | 13 | 0 | 32 | 0 | 30 |
| 1-octanol | 57 | 30 | 43 | 32 | 33 | 32 | 50 | 35 |
| Phenylethyl alcohol | 29 | 20 | 43 | 11 | 0 | 16 | 0 | 25 |
| 1-hexanol, 3-methyl | 14 | 0 | 14 | 0 | 0 | 0 | 0 | 0 |
| 1-heptanol | 14 | 10 | 0 | 0 | 0 | 5 | 0 | 0 |
| Neopentyl glycol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1-pentanol, 3,4-dimethyl | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1,6-heptadien, 4-ol | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| Benzyl alcohol | 0 | 0 | 0 | 3 | 0 | 0 | 50 | 0 |
| 1,2-Ethanediol, 1,2-diphenyl | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 |
| 1-heptanol, 3-methyl | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| TOTAL ESTERS | ||||||||
| Butanoic acid, ethyl ester | 29 | 0 | 43 | 13 | 0 | 16 | 0 | 30 |
| 1-butanol, 3-methyl-, acetate | 29 | 50 | 86 | 58 | 67 | 68 | 0 | 70 |
| Hexanoic acid, ethyl ester | 57 | 70 | 71 | 50 | 0 | 16 | 50 | 70 |
| Acetic acid, hexyl ester | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 0 |
| Octanoic acid, ethyl ester | 57 | 70 | 86 | 45 | 33 | 37 | 0 | 70 |
| Decanoic acid, ethyl ester | 14 | 10 | 29 | 11 | 33 | 0 | 0 | 25 |
| Propanoic acid, 2-hydroxy, ethyl ester | 0 | 20 | 0 | 3 | 0 | 5 | 0 | 0 |
| Benzoic acid, ethyl ester | 43 | 10 | 0 | 84 | 67 | 32 | 100 | 5 |
| Acetic acid, 2-phenylethyl ester | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 0 |
| Butanoic acid, 2-methyl, ethyl ester | 29 | 50 | 0 | 37 | 67 | 84 | 0 | 10 |
| Butanedioic acid, diethyl ester | 14 | 20 | 0 | 11 | 0 | 0 | 0 | 10 |
| Pentanoic acid, ethyl ester | 0 | 0 | 0 | 16 | 0 | 5 | 0 | 0 |
| Butanoic acid, 3-methyl, ethyl ester | 14 | 10 | 0 | 13 | 67 | 5 | 0 | 5 |
| 1-butanol, 3-methyl, formate | 0 | 0 | 0 | 3 | 0 | 5 | 0 | 0 |
| Butanoic acid, 2-methyl, 2-methylbutyl ester | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 |
| Pentanoic acid, 2,2,4-trimethyl-3- carboxyisopropyl, isobutyl ester | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| Acetic acid, butyl ester | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| TOTAL CARBONYL COMPOUNDS | ||||||||
| Benzaldehyde | 100 | 60 | 43 | 82 | 33 | 26 | 100 | 10 |
| Furfural | 29 | 30 | 14 | 58 | 67 | 37 | 0 | 5 |
| 3,3,3-trifluoropropene | 0 | 0 | 14 | 11 | 0 | 5 | 0 | 0 |
| Propyl cyclopropane | 0 | 0 | 14 | 3 | 33 | 5 | 0 | 0 |
| Butane, 1,1-diethoxy-3-methyl | 0 | 0 | 0 | 3 | 0 | 0 | 50 | 10 |
| 3-furalaldehyde | 0 | 0 | 0 | 5 | 0 | 11 | 0 | 0 |
| Benzene, 1-ethenyl, 4-methoxy | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| Propane, 1,1,3-triethoxy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Butane, 1,1-diethoxy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15 |
| Pentane, 1-(1-ethoxyethoxy)- | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| TOTAL TERPENES | ||||||||
| Methylal | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| α-Terpineol | 0 | 0 | 0 | 0 | 0 | 37 | 0 | 0 |
| Linalool | 0 | 0 | 0 | 0 | 0 | 42 | 0 | 10 |
| OTHER COMPOUNDS | ||||||||
| 1-Butanamine, N-nitro | 0 | 20 | 0 | 47 | 67 | 53 | 50 | 10 |
| 1-Pentanamine, N-nitro | 43 | 20 | 14 | 21 | 33 | 11 | 0 | 15 |
| 1H-imidazole, 1,5-dimethyl | 14 | 10 | 0 | 5 | 33 | 11 | 0 | 5 |
| 1-oxaspiro[4,5]dec-6-ene, 2,6,10,10-tetramethyl | 0 | 20 | 29 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srdjenović Čonić, B.; Kladar, N.; Kusonić, D.; Bijelić, K.; Torović, L. A Chemometric Exploration of Potential Chemical Markers and an Assessment of Associated Risks in Relation to the Botanical Source of Fruit Spirits. Toxics 2024, 12, 720. https://doi.org/10.3390/toxics12100720
Srdjenović Čonić B, Kladar N, Kusonić D, Bijelić K, Torović L. A Chemometric Exploration of Potential Chemical Markers and an Assessment of Associated Risks in Relation to the Botanical Source of Fruit Spirits. Toxics. 2024; 12(10):720. https://doi.org/10.3390/toxics12100720
Chicago/Turabian StyleSrdjenović Čonić, Branislava, Nebojša Kladar, Dejan Kusonić, Katarina Bijelić, and Ljilja Torović. 2024. "A Chemometric Exploration of Potential Chemical Markers and an Assessment of Associated Risks in Relation to the Botanical Source of Fruit Spirits" Toxics 12, no. 10: 720. https://doi.org/10.3390/toxics12100720
APA StyleSrdjenović Čonić, B., Kladar, N., Kusonić, D., Bijelić, K., & Torović, L. (2024). A Chemometric Exploration of Potential Chemical Markers and an Assessment of Associated Risks in Relation to the Botanical Source of Fruit Spirits. Toxics, 12(10), 720. https://doi.org/10.3390/toxics12100720









