New Insights into the Mechanisms of Toxicity of Aging Microplastics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Microplastic and Dyes
2.2. Fourier-Transform Infrared (FTIR) Spectroscopy
2.3. In Vitro Experiment: MPs and Dyes
2.3.1. MPs and Nitroblue Tetrazolium (NBT)
2.3.2. MPs and Methylene Blue (MB)
2.4. Biological Material
2.4.1. Mitochondria Isolation
2.4.2. Hemocytes Isolation
2.5. Experiment In Vitro: The Effect of MPs on Biological Material
2.5.1. Effect of Microplastics MPs on Mitochondria
2.5.2. Effect of MPs on Hemocytes
2.6. Statistics
3. Results
3.1. Characterization of Pristine and Aging MPs
3.1.1. FTIR Spectroscopy
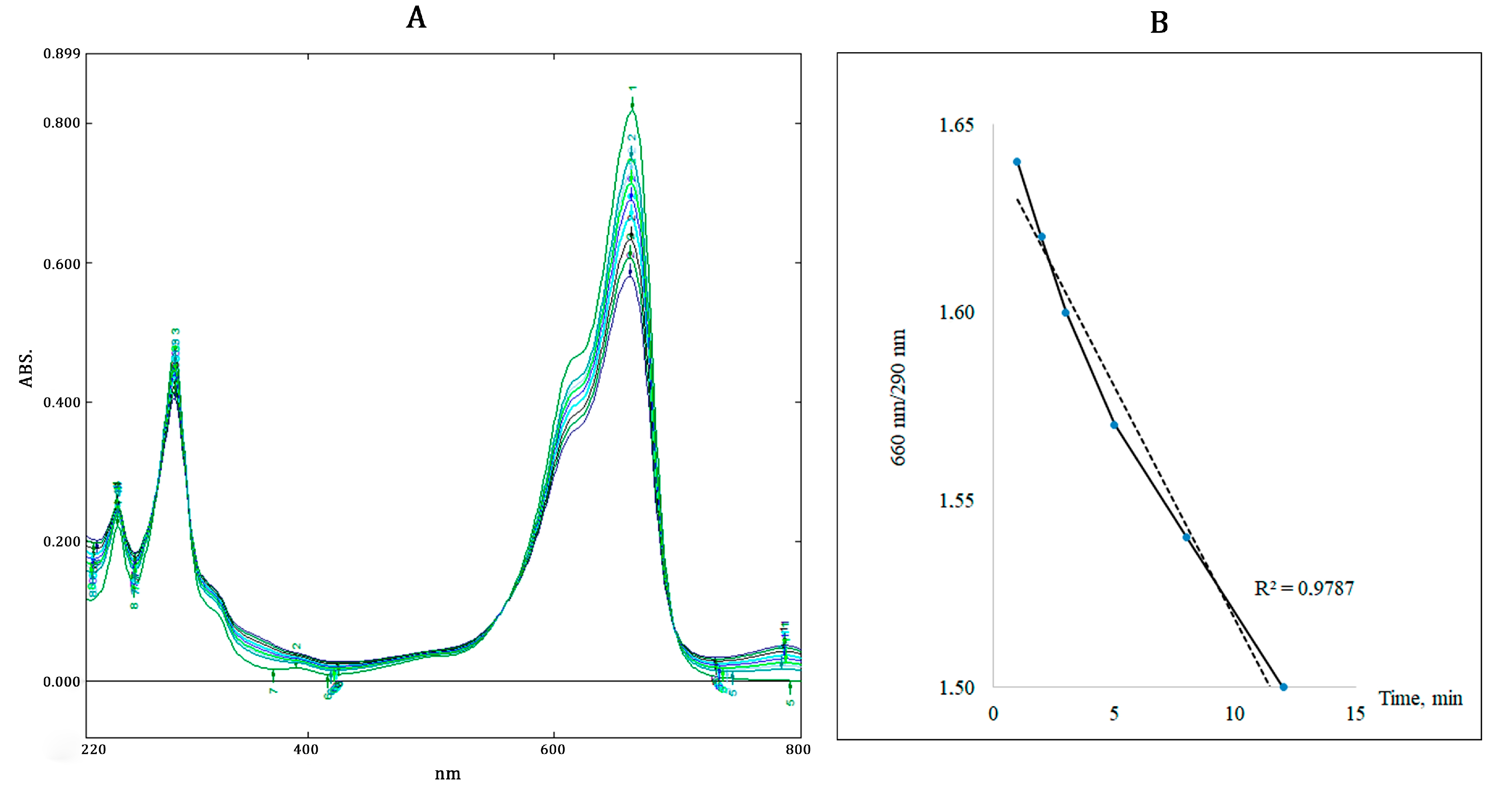
3.1.2. Sorption of Methylene Blue Dye by MPs
3.1.3. Registration of ROS Generated by MPs in Seawater
3.2. Pro-Oxidative Processes Induced by MPs Samples
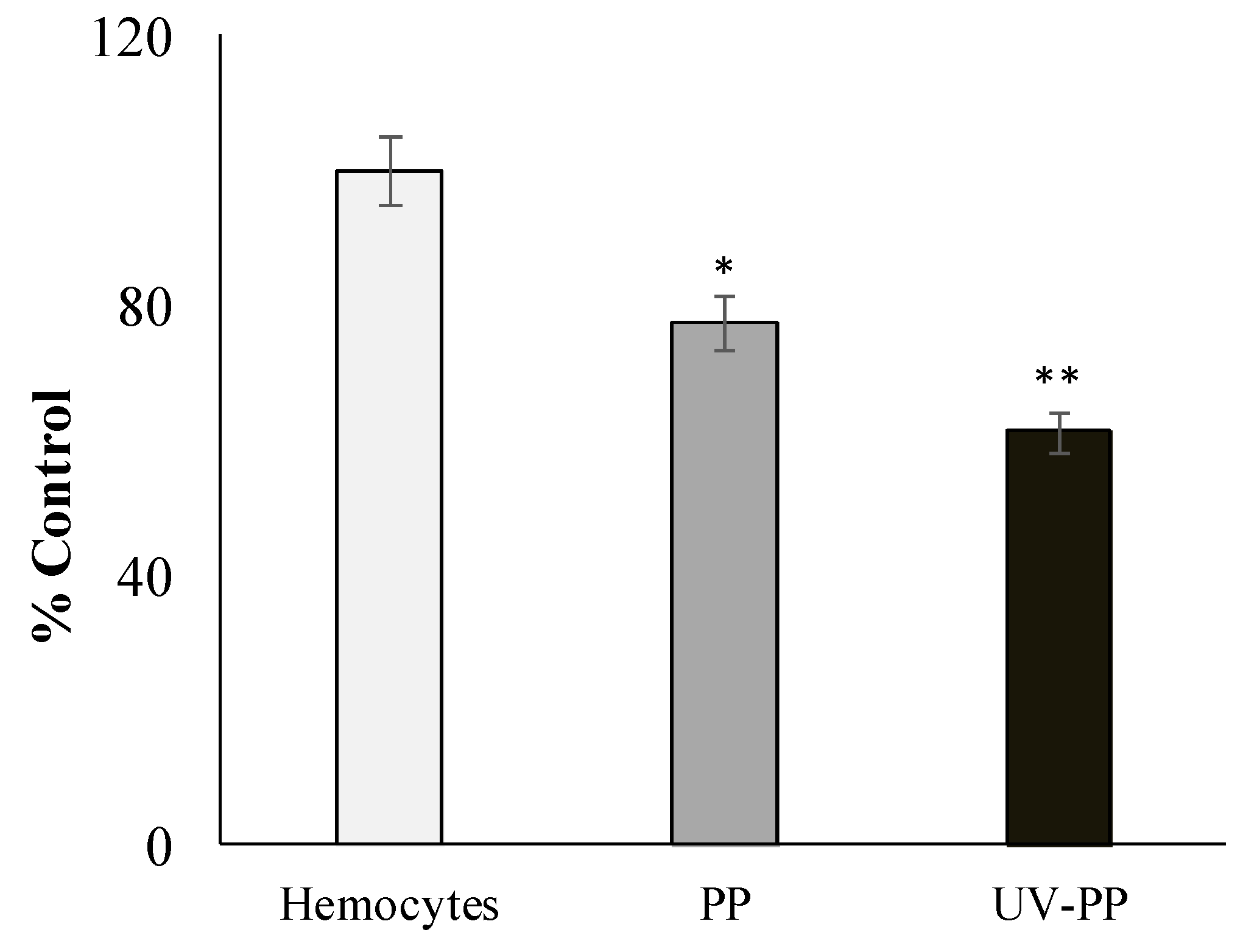
3.2.1. Lipid Peroxidation of Mitochondrial Membranes
3.2.2. Effect of MPs on the Stability of Lysosome Membranes in Mussel Hemocytes
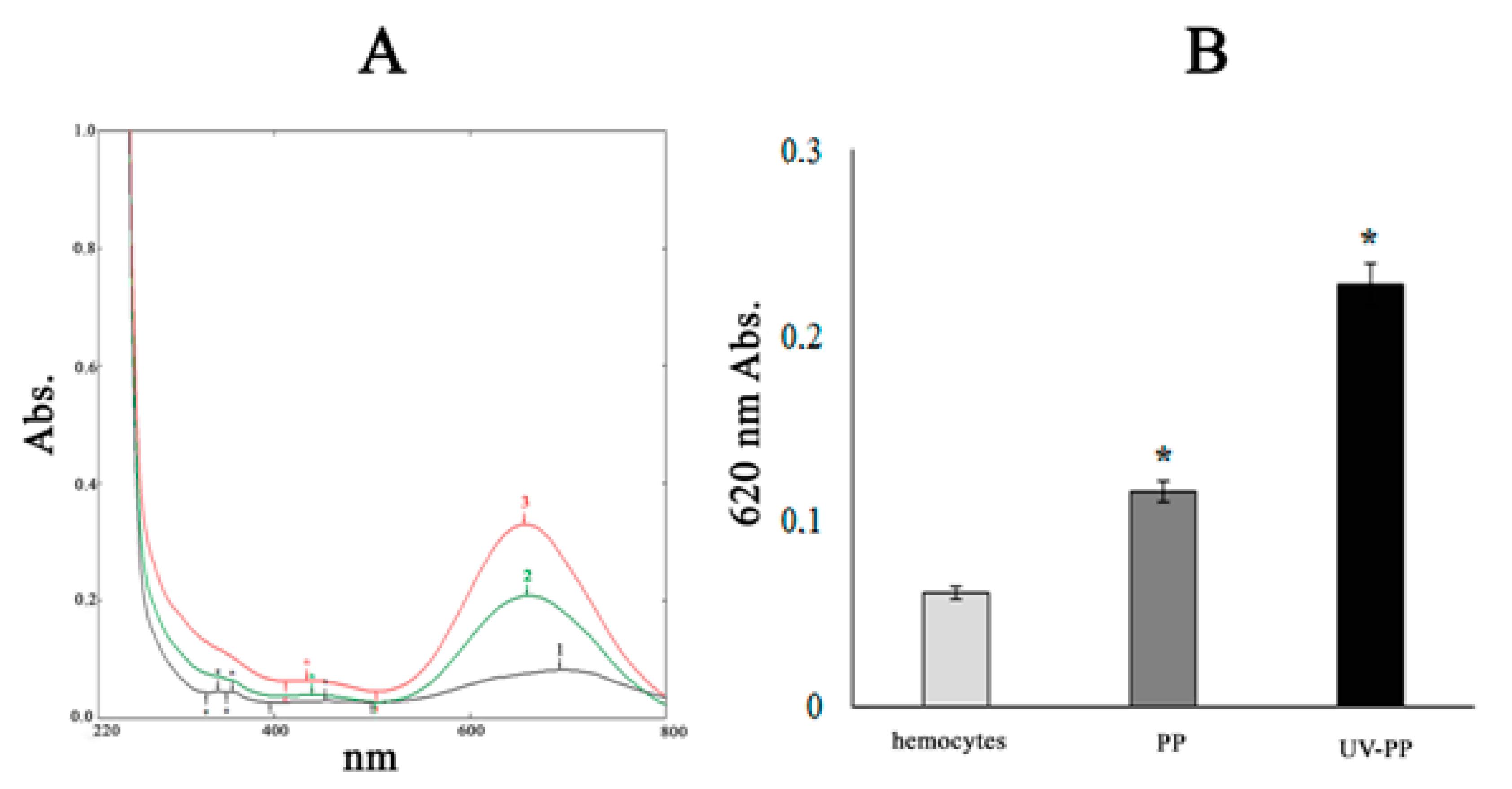
3.2.3. Formation of ROS during MPs Exposure to Hemocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, F.; Cowie, P.R. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Mar. Pollut. Bull. 2011, 62, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Janssen, C.R. Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. Pollut. 2015, 199, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lu, K.; Li, J.; Wu, X.; Qian, L.; Wang, M.; Gao, S. Effect of aging on adsorption behavior of polystyrene microplastics for pharmaceuticals: Adsorption mechanism and role of aging intermediates. J. Hazard. Mater. 2020, 384, 121193. [Google Scholar] [CrossRef]
- Vivekanand, A.C.; Mohapatra, S.; Tyagi, V.K. Microplastics in aquatic environment: Challenges and perspectives. Chemosphere 2021, 282, 131151. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Au, S.Y.; Lee, C.M.; Weinstein, J.E.; van den Hurk, P.; Klaine, S.J. Trophic transfer of microplastics in aquatic ecosystems: Identifying critical research needs. Integr. Environ. Assess. Manag. 2017, 13, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Zha, F.; Dai, J.; Han, Y.; Liu, P.; Wang, M.; Liu, H.; Guo, X. Release of millions of micro(nano)plastic fragments from photooxidation of disposable plastic boxes. Sci. Total Environ. 2023, 858 Pt 3, 160044. [Google Scholar] [CrossRef]
- Lithner, D.; Larsson, A.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Worm, B.; Lotze, H.K.; Jubinville, I.; Wilcox, C.; Jambeck, J. Plastic as a Persistent Marine Pollutant. Annu. Rev. Environ. Resour. 2017, 42, 1–26. [Google Scholar] [CrossRef]
- Messinetti, S.; Mercurio, S.; Parolini, M.; Sugni, M.; Pennati, R. Effects of polystyrene microplastics on early stages of two marine invertebrates with different feeding strategies. Environ. Pollut. 2018, 237, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Paul-Pont, I.; Tallec, K.; González-Fernández, C.; Lambert, C.; Vincent, D.; Mazurais, D.; Zambonino-Infante, J.; Brotons, G.; Lagarde, F.; Fabioux, C.; et al. Constraints and Priorities for Conducting Experimental Exposures of Marine Organisms to Microplastics. Front. Mar. Sci. 2018, 5, 252. [Google Scholar] [CrossRef]
- Murano, C.; Agnisola, C.; Caramiello, D.; Castellano, I.; Casotti, R.; Corsi, I.; Palumbo, A. How sea urchins face microplastics: Uptake, tissue distribution and immune system response. Environ. Pollut. 2020, 264, 114685. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadi, A.; Papaefthimiou, C.; Genizegkini, E.; Sampsonidis, I.; Kalogiannis, S.; Feidantsis, K.; Bobori, D.C.; Kastrinaki, G.; Koumoundouros, G.; Lambropoulou, D.A. Adverse effects polystyrene microplastics exert on zebrafish heart—Molecular to individual level. J. Hazard. Mater. 2021, 416, 125969. [Google Scholar] [CrossRef]
- Yuan, Z.; Nag, R.; Cummins, E. Human health concerns regarding microplastics in the aquatic environment—From marine to food systems. Sci. Total. Environ. 2022, 823, 153730. [Google Scholar] [CrossRef]
- Von Moos, N.; Burkhardt-Holm, P.; Köhler, A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef]
- Green, D.S. Effects of microplastics on European flat oysters, Ostrea edulis and their associated benthic communities. Environ. Pollut. 2016, 216, 95–103. [Google Scholar] [CrossRef]
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef]
- Lo, H.K.A.; Chan, K.Y.K. Negative effects of microplastic exposure on growth and development of Crepidula onyx. Environ. Pollut. 2018, 233, 588–595. [Google Scholar] [CrossRef]
- Trifuoggi, M.; Pagano, G.; Oral, R.; Pavičić-Hamer, D.; Burić, P.; Kovačić, I.; Siciliano, A.; Toscanesi, M.; Thomas, P.J.; Paduano, L.; et al. Microplastic-induced damage in early embryonal development of sea urchin Sphaerechinus granularis. Environ. Res. 2019, 179, 108815. [Google Scholar] [CrossRef]
- Pan, L.; Yu, D.; Zhang, Y.; Zhu, C.; Yin, Q.; Hu, Y.; Zhang, X.; Yue, R.; Xiong, X. Polystyrene microplastics-triggered mitophagy and oxidative burst via activation of PERK pathway. Sci. Total Environ. 2021, 781, 146753. [Google Scholar] [CrossRef]
- Suckling, C.C. Responses to environmentally relevant microplastics are species-specific with dietary habit as a potential sensitivity indicator. Sci. Total Environ. 2021, 751, 142341. [Google Scholar] [CrossRef] [PubMed]
- Chelomin, V.P.; Mazur, A.A.; Slobodskova, V.V.; Kukla, S.P.; Dovzhenko, N.V. Genotoxic Properties of Polystyrene (PS) Microspheres in the Filter-Feeder Mollusk Mytilus trossulus (Gould, 1850). J. Mar. Sci. Eng. 2022, 10, 273. [Google Scholar] [CrossRef]
- Pandi, P.; Madhuvandhi, J.; Priya, K.K.; Thiagarajan, R.; Gopalakrishnan, S.; Elumalai, S.; Thilagam, H. Weathered polyethylene microplastics exposure leads to modulations in glutathione-S-transferase activity in fish. Front. Mar. Sci. 2022, 9, 990351. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Slobodskova, V.V.; Kukla, S.P.; Mazur, A.A.; Dovzhenko, N.V.; Zhukovskaya, A.F.; Karpenko, A.A.; Karpenko, M.A.; Odintsov, V.S. Dietary Exposure to Particles of Polytetrafluoroethylene (PTFE) and Polymethylmethacrylate (PMMA) Induces Different Responses in Periwinkles Littorina brevicula. Int. J. Mol. Sci. 2023, 24, 8243. [Google Scholar] [CrossRef]
- Kokalj, A.; Kuehnel, D.; Puntar, B.; Žgajnar Gotvajn, A.; Kalčikova, G. An exploratory ecotoxicity study of primary microplastics versus aged in natural waters and wastewaters. Environ. Pollut. 2019, 254, 112980. [Google Scholar] [CrossRef] [PubMed]
- Rummel, C.D.; Escher, B.I.; Sandblom, O.; Plassmann, M.M.; Arp, H.P.H.; MacLeod, M.; Jahnke, A. Effects of Leachates from UV-Weathered Microplastic in Cell-Based Bioassays. Environ. Sci. Technol. 2019, 53, 9214–9223. [Google Scholar] [CrossRef]
- Gewert, B.; MacLeod, M.; Breitholtz, M. Variability in Toxicity of Plastic Leachates as a Function of Weathering and Polymer Type: A Screening Study with the Copepod Nitocra spinipes. Biol. Bull. 2021, 240, 191–199. [Google Scholar] [CrossRef]
- Hariharan, G.; Purvaja, R.; Anandavelu, I.; Robin, R.S.; Ramesh, R. Accumulation and ecotoxicological risk of weathered polyethylene (wPE) microplastics on green mussel (Perna viridis). Ecotoxicol. Environ. Saf. 2021, 208, 111765. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Y.; Wang, C.; Hua, X.; Li, H.; Xie, D.; Xiang, M.; Yu, Y. Reproductive toxicity of UV-photodegraded polystyrene microplastics induced by DNA damage-dependent cell apoptosis in Caenorhabditis elegans. Sci. Total Environ. 2022, 811, 152350. [Google Scholar] [CrossRef]
- Hariharan, G.; Purvaja, R.; Anandavelu, I.; Robin, R.S.; Ramesh, R. Ingestion and toxic impacts of weathered polyethylene (wPE) microplastics and stress defensive responses in whiteleg shrimp (Penaeus vannamei). Chemosphere 2022, 300, 134487. [Google Scholar] [CrossRef]
- Luo, H.; Liu, C.; He, D.; Sun, J.; Li, J.; Pan, X. Effects of aging on environmental behavior of plastic additives: Migration, leaching, and ecotoxicity. Sci. Total Environ. 2022, 849, 157951. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, W.; Wegener, S.; Schertzinger, G.; Pannekens, H.; Schweyen, P.; Dierkes, G.; Klein, K.; Ternes, T.A.; Oehlmann, J.; Dopp, E. Chemical and toxicological assessment of leachates from UV-degraded plastic materials using in-vitro bioassays. PeerJ. 2023, 11, 15192. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, H.; Zhao, J.; Luo, X.; Wang, Z.; Xing, B. Photodegradation Elevated the Toxicity of Polystyrene Microplastics to Grouper (Epinephelus moara) through Disrupting Hepatic Lipid Homeostasis. Environ. Sci. Technol. 2020, 54, 6202–6212. [Google Scholar] [CrossRef]
- Klein, K.; Hof, D.; Dombrowski, A.; Schweyen, P.; Dierkes, G.; Ternes, T.; Schulte-Oehlmann, U.; Oehlmann, J. Enhanced in vitro toxicity of plastic leachates after UV irradiation. Water Res. 2021, 199, 117203. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, J.; Zhou, L.; Mao, Z.; Zhang, X.; Cai, J.; Huang, P. Enhanced hepatic metabolic perturbation of polystyrene nanoplastics by UV irradiation-induced hydroxyl radical generation. J. Environ. Sci. 2024, 142, 259–268. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Slobodskova, V.V.; Dovzhenko, N.V.; Mazur, A.A.; Kukla, S.P. Photoaging Elevated the Genotoxicity of Polystyrene Microplastics to Marine Mussel Mytilus trossulus (Gould, 1850). Int. J. Mol. Sci. 2024, 25, 5740. [Google Scholar] [CrossRef]
- Rouillon, C.; Bussiere, P.O.; Desnoux, E.; Collin, S.; Vial, C.; Therias, S.; Gardette, J.L. Is carbonyl index a quantitative probe to monitor polypropylene photodegradation? Polym. Degrad. Stabil. 2016, 128, 200–208. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, J.; Zhou, T.; Zhao, Y.; Yu, F.; Ma, J. Laboratory simulation of microplastics weathering and its adsorption behaviors in an aqueous environment: A systematic review. Environ. Pollut. 2020, 265 Pt B, 114864. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, S.; Li, P. Aging of plastics in aquatic environments: Pathways, environmental behavior, ecological impacts, analyses and quantifications. Environ. Pollut. 2024, 341, 122926. [Google Scholar] [CrossRef]
- Luo, H.; Xiang, Y.; Li, Y.; Zhao, Y.; Pan, X. Photocatalytic aging process of Nano-TiO2 coated polypropylene microplastics: Combining atomic force microscopy and infrared spectroscopy (AFM-IR) for nanoscale chemical characterization. J. Hazard. Mater. 2021, 404 Pt B, 124159. [Google Scholar] [CrossRef]
- Stapleton, M.J.; Ansari, A.J.; Ahmed, A.; Hai, F.I. Change in the chemical, mechanical and physical properties of plastics due to UVA degradation in different water matrices: A study on the recyclability of littered plastics. Environ. Pollut. 2023, 334, 122226. [Google Scholar] [CrossRef]
- Hu, M.; Palić, D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef]
- Xie, X.; Deng, T.; Duan, J.; Xie, J.; Yuan, J.; Chen, M. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotoxicol. Environ. Saf. 2020, 190, 110133. [Google Scholar] [CrossRef]
- Song, J.A.; Choi, C.Y.; Park, H.S. Exposure of bay scallop Argopecten irradians to micro-polystyrene: Bioaccumulation and toxicity. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 236, 108801. [Google Scholar] [CrossRef]
- Li, Z.; Chang, X.; Hu, M.; Fang, J.K.; Sokolova, I.M.; Huang, W.; Xu, E.G.; Wang, Y. Is microplastic an oxidative stressor? Evidence from a meta-analysis on bivalves. J. Hazard. Mater. 2022, 423 Pt B, 127211. [Google Scholar] [CrossRef]
- Liu, S.; Huang, W.; Yang, J.; Xiong, Y.; Huang, Z.; Wang, J.; Cai, T.; Dang, Z.; Yang, C. Formation of environmentally persistent free radicals on microplastics under UV irradiations. J. Hazard. Mater. 2023, 453, 131277. [Google Scholar] [CrossRef]
- Wu, X.; Liu, P.; Wang, H.; Huang, H.; Shi, Y.; Yang, C.; Gao, S. Photo aging of polypropylene microplastics in estuary water and coastal seawater: Important role of chlorine ion. Water Res. 2021, 202, 117396. [Google Scholar] [CrossRef]
- Ding, R.; Ouyang, Z.; Bai, L.; Zuo, X.; Xiao, C.; Guo, X. What are the drivers of tetracycline photolysis induced by polystyrene microplastic? Chem. Eng. J. 2022, 435, 134827. [Google Scholar] [CrossRef]
- Duan, J.; Li, Y.; Gao, J.; Cao, R.; Shang, E.; Zhang, W. ROS-mediated photoaging pathways of nano- and micro-plastic particles under UV irradiation. Water Res. 2022, 216, 118320. [Google Scholar] [CrossRef]
- Cao, H.; Ding, P.; Li, X.; Huang, C.; Li, X.; Chen, X.; Zhang, L.; Qi, J. Environmentally persistent free radicals on photoaged microplastics from disposable plastic cups induce the oxidative stress associated toxicity. J. Hazard. Mater. 2024, 464, 132990. [Google Scholar] [CrossRef]
- Liu, S.; Li, L.; Liu, S.; Liu, L.; Xiao, X.; Zhou, D.; Zhu, C.; She, X. Reactive oxygen species-induced microplastics aging: Implications for environmental fate and ecological impact. TrAC 2024, 173, 117648. [Google Scholar] [CrossRef]
- Campanale, C.; Savino, I.; Massarelli, C.; Uricchio, V.F. Fourier transform infrared spectroscopy to assess the degree of alteration of artificially aged and environmentally weathered microplastics. Polymers 2023, 15, 911. [Google Scholar] [CrossRef]
- Giannapas, M.; Karnis, L.; Dailianis, S. Generation of free radicals in haemocytes of mussels after exposure to low molecular weight PAH components: Immune activation, oxidative and genotoxic effects. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 155(2), 182–189. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Markwell, M.; Haas, S.; Bieber, L.; Tolbert, N. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 82, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Yang, F.; Hui, Y.; Xu, Y.; Lu, Y.; Liu, J. Cytotoxicity and apoptosis induction on RTG-2 cells of 2,2’,4,4’-tetrabromodiphenyl ether (BDE-47) and decabrominated diphenyl ether (BDE-209). Toxicol. In Vitro 2010, 24, 1190–1196. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, Z.; Miao, J.; Tian, Y.; Pan, L. Effects of benzo[a]pyrene exposure on oxidative stress and apoptosis of gill cells of Chlamys farreri in vitro. Environ. Toxicol. Pharmacol. 2022, 93, 103867. [Google Scholar] [CrossRef]
- Martinez-Gomez, C.; Bignell, J.; Lowe, D. Lysosoma membrane stability in mussels. ICES Tech. Mar. Environ. Sci. 2015, 56, 41. [Google Scholar] [CrossRef]
- Hirai, H.; Takada, H.; Ogata, Y.; Yamashita, R.; Mizukawa, K.; Saha, M.; Kwan, C.; Moore, C.; Gray, H.; Laursen, D.; et al. Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Mar. Pollut. Bull. 2011, 62, 1683–1692. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Enhanced desorption of persistent organic 421 pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014, 185, 16–23. [Google Scholar] [CrossRef]
- Capolupo, M.; Sørensen, L.; Jayasena, K.D.R.; Booth, A.M.; Fabbri, E. Chemical composition and ecotoxicity of plastic and car tire rubber leachates to aquatic organisms. Water Res. 2020, 169, 115270. [Google Scholar] [CrossRef]
- Coffin, S.; Lee, I.; Gan, J.; Schlenk, D. Simulated digestion of polystyrene foam enhances desorption of diethylhexyl phthalate (DEHP) and In vitro estrogenic activity in a size-dependent manner. Environ. Pollut. 2019, 246, 452–462. [Google Scholar] [CrossRef]
- Gunaalan, K.; Fabbri, E.; Capolupo, M. The hidden threat of plastic leachates: A critical review on their impacts on aquatic organisms. Water Res. 2020, 184, 116170. [Google Scholar] [CrossRef]
- Dovzhenko, N.V.; Chelomin, V.P.; Mazur, A.A.; Slobodskova, V.V.; Istomina, A.A.; Kukla, S.P. Biomarker Effects of Diesel Fuel Hydrocarbons Absorbed to PE-Plastic Debris on Mussel Mytilus trossulus. J. Mar. Sci. Eng. 2023, 11, 1446. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Brandon, J.; Goldstein, M.; Ohman, M.D. Long-term aging and degradation of microplastic particles: Comparing in situ oceanic and experimental weathering patterns. Mar. Pollut. Bull. 2016, 110, 299–308. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.; Sandblom, O.; MacLeod, M. Identification of Chain Scission Products Released toWater by Plastic Exposed to Ultraviolet Light. Environ. Sci. Technol. Lett. 2018, 5, 272–276. [Google Scholar] [CrossRef]
- Shi, Y.; Qin, J.; Tao, Y.; Jie, G.; Wang, J. Natural weathering severity of typical coastal environment on polystyrene: Experiment and modeling. Polym. Test. 2019, 76, 138–145. [Google Scholar] [CrossRef]
- Wu, X.; Liu, P.; Huang, H.; Gao, S. Adsorption of triclosan onto different aged polypropylene microplastics: Critical effect of cations. Sci. Total Environ. 2020, 717, 137033. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Jiang, R.; You, J.; Ouyang, G. New insights into the photo-degraded polystyrene microplastic: Effect on the release of volatile organic compounds. J. Hazard. Mater. 2022, 431, 128523. [Google Scholar] [CrossRef] [PubMed]
- Gijsman, P.; Meijers, G.; Vitarelli, G. Comparison of the UV-degradation chemistry of polypropylene, polyethylene, polyamide 6 and polybutylene terephthalate. Polym. Degrad. Stab. 1999, 65, 433–441. [Google Scholar] [CrossRef]
- De Paoli, M.A. Degradacao e Estabilizacao de Polímeros; Artliber Editora Ltda: Sao Paulo, Brazil, 2008. [Google Scholar]
- Bandow, N.; Will, V.; Wachtendorf, V.; Simon, F.G. Contaminant release from aged microplastic. Environ. Chem. 2017, 14, 394–405. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Jayanthi, B.; Fauziah, S.H. Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 2018, 127, 15–21. [Google Scholar] [CrossRef]
- De Bomfim, A.S.C.; Maciel, M.M.D.; Voorwald, H.J.C.; Benini, K.C.C.; de Oliveira, D.M.; Cioffi, M.O.H. Effect of different degradation types on properties of plastic waste obtained from espresso coffee capsules. Waste Manag. 2019, 83, 123–130. [Google Scholar] [CrossRef]
- Gugumus, F. Re-examination of the role of hydroperoxides in polyethylene and polypropylene: Chemical and physical aspects of hydroperoxides in polyethylene. Polym. Degrad. Stab. 1995, 49, 29–50. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined Effects of UV Exposure Duration and Mechanical Abrasion on Microplastic Fragmentation by Polymer Type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef]
- Hüffer, T.; Weniger, A.; Hofmann, T. Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollut. 2018, 236, 218–225. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Rubin, A.E.; Zucker, I. Engineered Polystyrene-Based Microplastics of High Environmental Relevance. Environ. Sci. Technol. 2021, 55, 10491–10501. [Google Scholar] [CrossRef]
- Bhagat, K.; Barrios, A.C.; Rajwade, K.; Kumar, A.; Oswald, J.; Apul, O.; Perreault, F. Aging of microplastics increases their adsorption affinity towards organic contaminants. Chemosphere 2022, 298, 134238. [Google Scholar] [CrossRef]
- Fotopoulou, K.N.; Karapanagiot, H.K. Surface properties of beached plastic pellets. Mar. Environ. Res. 2012, 81, 70–77. [Google Scholar] [CrossRef]
- You, H.; Huang, B.; Cao, C.; Liu, X.; Sun, X.; Xiao, L.; Qiu, J.; Luo, Y.; Qian, Q.; Chen, Q. Adsorption-desorption behavior of methylene blue onto aged polyethylene microplastics in aqueous environments. Mar. Pollut. Bull. 2021, 167, 112287. [Google Scholar] [CrossRef]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; D’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Lacroix, C.; Fernández, C.G.; Hégaret, H.; Lambert, C.; Le Goïc, N. Exposure of marine mussels Mytilus spp. To polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut. 2016, 216, 724–737. [Google Scholar] [CrossRef]
- Das, A. The emerging role of microplastics in systemic toxicity: Involvement of reactive oxygen species (ROS). Sci. Total Environ. 2023, 895, 165076. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Fabbri, R.; Balbi, T.; Salis, A.; Damonte, G.; Cortese, K.; Caratto, V.; Monopoli, M.P.; Dawson, K.; et al. Interactions of cationic polystyrene nanoparticles with marine bivalve hemocytes in a physiological environment: Role of soluble hemolymph proteins. Environ. Res. 2016, 150, 73–81. [Google Scholar] [CrossRef]
- Dailianis, S.; Rouni, M.; Ainali, N.M.; Vlastos, D.; Kyzas, G.Z.; Lambropoulou, D.A.; Bikiaris, D.N. New insights into the size-independent bioactive potential of pristine and UV-B aged polyethylene microplastics. Sci. Total Environ. 2024, 918, 170616. [Google Scholar] [CrossRef]
- Mazur, A.A.; Chelomin, V.P.; Zhuravel, E.V.; Kukla, S.P.; Slobodskova, V.V.; Dovzhenko, N.V. Genotoxicity of Polystyrene (PS) Microspheres in Short-Term Exposure to Gametes of the Sand Dollar Scaphechinus mirabilis (Agassiz, 1864) (Echinodermata, Echinoidea). J. Mar. Sci. Eng. 2021, 9, 1088. [Google Scholar] [CrossRef]
- Fleury, J.B.; Baulin, V.A. Microplastics destabilize lipid membranes by mechanical stretching. Proc. Natl. Acad. Sci. USA 2021, 118, e2104610118. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Qiu, Z.; Cui, Z.; Li, N.; Li, X.; Wang, Y.; Zhang, H.; Zhao, C. Effects of polyethylene microplastics on cell membranes: A combined study of experiments and molecular dynamics simulations. J. Hazard. Mater. 2022, 429, 128323. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Zhao, S.; Xia, T.; Guo, X.; Wang, T.; Zhu, L. Formation of Environmentally Persistent Free Radicals on Microplastics under Light Irradiation. Environ. Sci. Technol. 2019, 53, 8177–8186. [Google Scholar] [CrossRef]
- Jeon, S.; Lee, D.K.; Jeong, J.; Yang, S.I.; Kim, J.S.; Kim, J.; Cho, W.S. The reactive oxygen species as pathogenic factors of fragmented microplastics to macrophages. Environ. Pollut. 2021, 281, 117006. [Google Scholar] [CrossRef]
- Satoh, A.Y.; Trosko, J.E.; Masten, S.J. Methylene blue dye test for rapid qualitative detection of hydroxyl radicals formed in a Fenton’s reaction aqueous solution. Environ. Sci. Technol. 2007, 41, 2881–2887. [Google Scholar] [CrossRef]
- Vieira, M.M.; Pereira Dornelas, A.S.; Carlos, T.D.; Pallini, A.; Gravato, C.; Pereira, D.H.; Sarmento, R.A.; Cavallini, G.S. When treatment increases the contaminant’s ecotoxicity: A study of the Fenton process in the degradation of methylene blue. Chemosphere 2021, 283, 131117. [Google Scholar] [CrossRef]
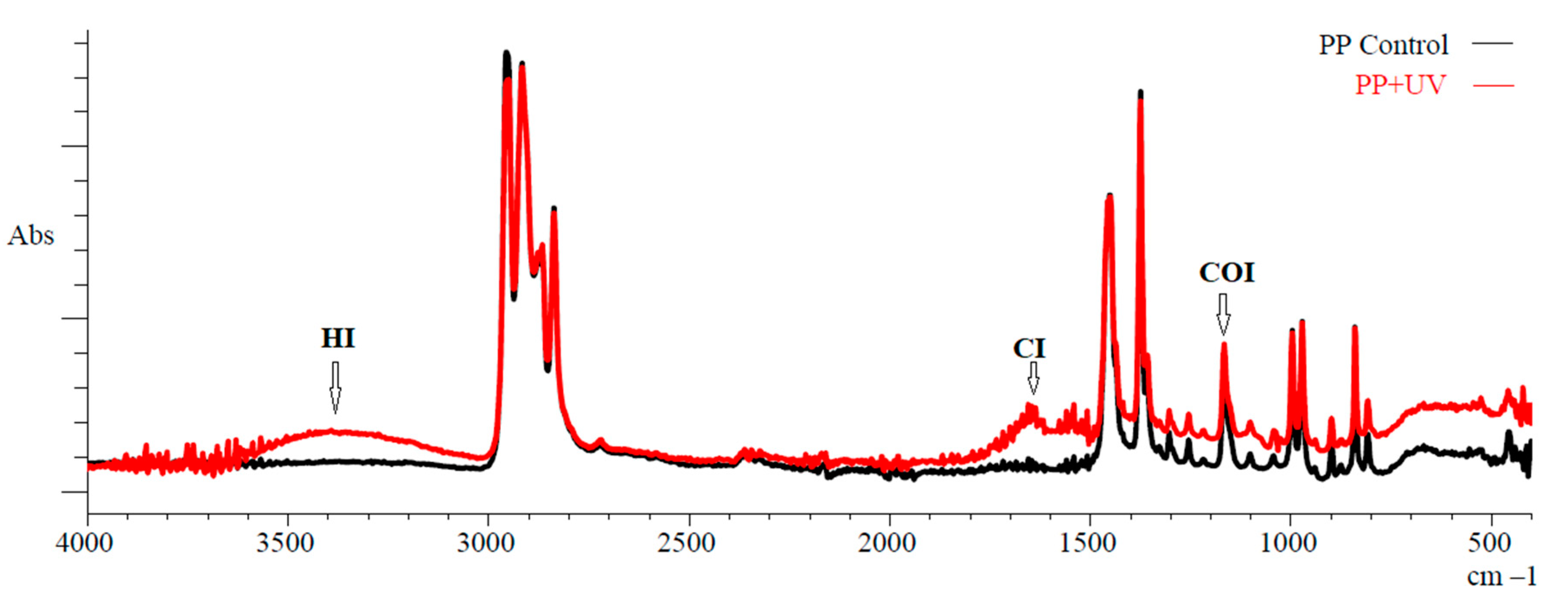

| CI | HI | COI | |
|---|---|---|---|
| Pristine PP | 0.23 ± 0.23 | 0.05 ± 0.01 | 0.32 ± 0.01 |
| Aging PP | 0.52 ± 0.35 * | 0.06 ± 0.01 | 0.34 ± 0.04 |
| Adsorption, µmol MB/cm2 | Oxidation/Reduction, % of Control | |
|---|---|---|
| Pristine PP | 0.16 | 2.9 |
| Aging PP | 0.50 | 16.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelomin, V.P.; Istomina, A.A.; Mazur, A.A.; Slobodskova, V.V.; Zhukovskaya, A.F.; Dovzhenko, N.V. New Insights into the Mechanisms of Toxicity of Aging Microplastics. Toxics 2024, 12, 726. https://doi.org/10.3390/toxics12100726
Chelomin VP, Istomina AA, Mazur AA, Slobodskova VV, Zhukovskaya AF, Dovzhenko NV. New Insights into the Mechanisms of Toxicity of Aging Microplastics. Toxics. 2024; 12(10):726. https://doi.org/10.3390/toxics12100726
Chicago/Turabian StyleChelomin, Victor Pavlovich, Aleksandra Anatolyevna Istomina, Andrey Alexandrovich Mazur, Valentina Vladimirovna Slobodskova, Avianna Fayazovna Zhukovskaya, and Nadezhda Vladimirovna Dovzhenko. 2024. "New Insights into the Mechanisms of Toxicity of Aging Microplastics" Toxics 12, no. 10: 726. https://doi.org/10.3390/toxics12100726
APA StyleChelomin, V. P., Istomina, A. A., Mazur, A. A., Slobodskova, V. V., Zhukovskaya, A. F., & Dovzhenko, N. V. (2024). New Insights into the Mechanisms of Toxicity of Aging Microplastics. Toxics, 12(10), 726. https://doi.org/10.3390/toxics12100726






